Abstract
Objective:
To define a group of patients with pancreatic cysts who do not require resection.
Summary Background Data:
The increased use of cross-sectional imaging has resulted in an increased identification of small, asymptomatic pancreatic cysts. Data have not been available to determine which lesions should be resected.
Methods:
All patients evaluated at our institution between January 1995 and January 2005 for the ICD-9 diagnosis of pancreatic cyst were reviewed. Analysis was performed to identify associations between patient and cyst characteristics, and selection of operative or nonoperative management.
Results:
Pancreatic cysts were evaluated in 539 patients. Initial management was operative in 170 patients (32%), and nonoperative (radiographic follow-up) in 369 patients (68%). Factors associated with initial operative management included presence of a solid component (45% vs. 6%, P < 0.001), larger size of the lesion (mean 4.8 cm vs. 2.4 cm, P = 0.001), and presence of symptoms (44% vs. 16%, P = 0.001). Malignancy was present in 18% (32 of 170) of patients initially resected. Mucinous tumors (n = 18) were the most common malignant histologic subtype. None of the invasive cancers arising from mucinous cysts was <3 cm. Median radiographic follow-up in patients initially managed nonoperatively was 24 months (range, 1–172 months). In 29 patients (8%), changes developed within the cyst that resulted in resection; malignancy was present in 11 of 39 (38%), representing 3% (11 of 369) of all patients being followed radiographically.
Conclusions:
Selected patients with cystic lesions <3 cm in diameter and without a solid component may be followed radiographically with a malignancy risk (3% this study) that approximates the risk of mortality from resection. Malignancy within mucinous tumors is associated with size, and small mucinous tumors are very unlikely to be malignant.
The increased use of high-quality cross-sectional imaging has resulted in increased identification of small, asymptomatic pancreatic cysts. In carefully selected patients, the risk of malignancy may approach the risk of mortality from resection, and expectant observation may be justified. We describe a selective approach to resection in patients with cystic lesions of the pancreas.
The appropriate management of patients with cystic lesions of the pancreas is controversial. The identification of small asymptomatic pancreatic cysts is increasing, and the natural history of these lesions is unknown.1,2 Mucinous lesions, either intraductal papillary mucinous neoplasms (IPMN) or mucinous cystadenomas, are considered premalignant lesions; however, the time course of progression from benign to malignant has not been determined. Although serous cystadenomas are considered to be benign, local growth can result in symptoms. The growth rate of these lesions, and the size at which they become symptomatic is unknown. The present ability to determine the specific histopathology of a radiographically identified pancreatic cyst remains limited. Current laboratory, radiographic, and endoscopic studies are often able to distinguish mucinous from serous lesions, but their ability to identify malignancy within the mucinous subgroup is limited.3,4 Finally, although the mortality after pancreatectomy has decreased to less than 2% in high-volume centers, reported morbidity rates remain at approximately 40%, and reported mortality continues to be as high as 15% at lower volume hospitals.5,6
Because of the unknown natural history, and diagnostic uncertainty, some authors have recommended routine resection of all pancreatic cysts.7–9 These authors argue that because preoperative differentiation between benign and malignant is unreliable, and because the potential adverse consequences of nonresectional therapy are significant, all medically fit patients should undergo resection. Although this approach provides a guarantee to patients that no premalignant or malignant lesions will be observed, it exposes patients with benign lesions to the risks of operation with unclear benefit.
Several recent reports, including a previous study from the authors’ institution, have recommended a more selective approach to resection.10–12 This approach argues that improved radiographic imaging techniques and an improved understanding of the various histologic entities allow the identification of a group of patients with an extremely low risk of malignancy. Most studies reporting selective management have recommended nonoperative management (radiographic follow-up) for patients with small, incidentally discovered cysts of the pancreas.10–12 This approach avoids the risks of operation in patients with benign lesions, but with current limitations in nonresectional diagnosis, cannot guarantee that a malignancy is not mistakenly being observed.
This study was performed to further describe and review our selective approach to resection for patients with cystic lesions of the pancreas. The authors previously reported on a series of 209 patients.12 In that study, we identified the presence of a solid component, the cyst diameter, the presence of septations, and the presence of symptoms as factors associated with the decision to perform operative resection. The current data allow further assessment of these factors, and the ability to investigate the factors involved in the decision making process.
METHODS
The pancreatic cyst database was designed to identify all patients (both operative and nonoperative) evaluated at our institution for a cystic lesion of the pancreas since 1995. Patients are included in this database if they were coded for the ICD-9 diagnosis of pancreatic cyst (577.2), had a cystic lesion of the pancreas on imaging studies, and were evaluated by a surgeon or gastroenterologist. Patients within the database who were evaluated between January 1995 and January 2005 were reviewed and included in this study. Approval for this review was obtained from Memorial Sloan-Kettering Cancer Center's Institutional Review Board.
Patient, radiographic, and treatment-related variables were reviewed. Patient variables included age, gender, history of pancreatitis, and whether or not the lesion was symptomatic at the time of presentation. A cyst was considered symptomatic if it was discovered on an imaging study performed for upper abdominal symptoms. The radiographic and endoscopic studies used in diagnosis were recorded. These included ultrasound, computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), positron emission tomography (PET), endoscopy with or without cholangiopancreatography, and endoscopic ultrasound. The cyst characteristics of septations, solid component, and cyst calcium were recorded. These factors were considered present if they were positively identified on any imaging study. When multiple cysts were identified in the pancreas, the diameter of the largest lesion was recorded.
Associations between initial resection (vs. radiographic follow-up), and various clinical and radiographic characteristics were evaluated using a 2-sample t test for continuous variables, and a Fisher exact test for categorical variables. Recursive partitioning was used to determine homogeneous subgroups with respect to the likelihood of initial resection. A decision tree was used to display the results of recursive partitioning. The optimal tree was chosen based on 10-fold cross-validation and cost-complexity pruning. Recursive partitioning was implemented using the “rpart” function in R (www.r-project.org).13
RESULTS
Patient and Cyst Data
Between January 1995 and January 2005, there were 539 patients evaluated for cystic lesions of the pancreas. Patient and cyst characteristics are presented in Table 1. The median age at the time of diagnosis was 64 years (range, 21–89 years), 65% were female, and the median diameter of the cystic lesions at the time of diagnosis was 3.2 cm (range, 0.3–20.0 cm). During the 10-year time period of the study, the annual number of patients evaluated for a cystic lesion increased (1995, n = 7 vs. 2004, n = 117), the average diameter of the lesions decreased (1995–1996, 4.5 cm vs. 2003–2004, 3.0 cm: P = 0.02), and the percentage of patients evaluated for an incidentally discovered cyst increased (1995–1996, 70% vs. 2003–2004, 85%: P = 0.003; Fig. 1).
TABLE 1. Patient, Cyst, and Treatment Characteristics of 539 Patients Evaluated for a Cystic Lesion of the Pancreas Between 1995 and 2005
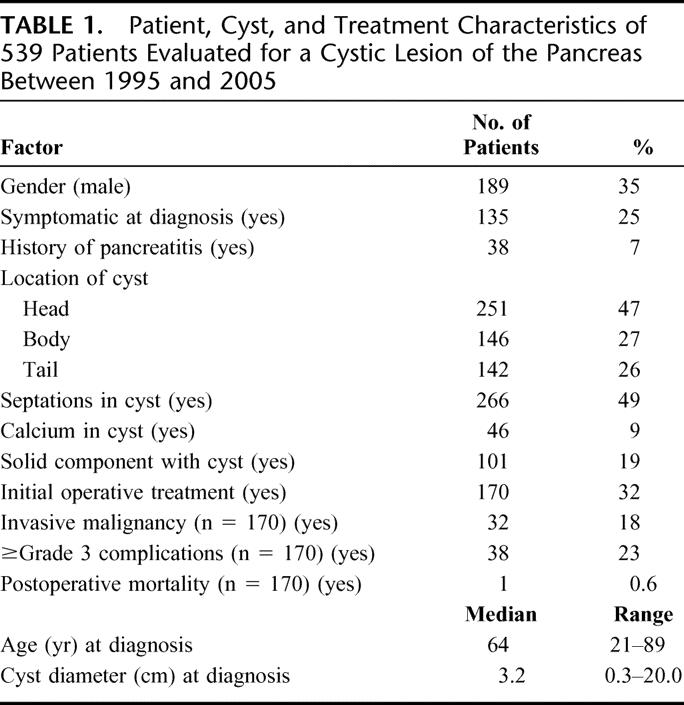
FIGURE 1. The number of patients evaluated (A), mean cyst diameter (B), and percentage of patients with symptomatic lesions (C) over the 10-year time period of the study.
Cystic lesions were located in the head or neck of the pancreas in 251 patients (47%) and in the body or tail of the pancreas in 288 patients (53%). When multiple cysts were reported, they ranged from 2 to 5 in number. The radiographic findings of septations, a solid component, and calcium were present in 49% (n = 266), 19% (n = 101), and 9% (n = 46) of patients, respectively. Extrapancreatic cysts were identified in 35% of patients. Renal cysts were identified in 121 patients (22%), and hepatic cysts were identified in 83 patients (15%).
Diagnostic Data
The most common imaging modality used in the assessment of the cystic lesions was CT. All patients except 3 underwent CT imaging. The next most common diagnostic study was MRI/MRCP, which was performed in 280 patients (52%). Endoscopy was performed in 119 patients (22%), and endoscopic ultrasound in 67 patients (11%). When endoscopic ultrasound was performed, a fine needle aspiration (FNA) was performed in 61 patients (91%), and cyst fluid analysis for CEA was performed in 35 patients.
The median cyst fluid CEA level in the 35 patients evaluated was 393 ng/mL (range, 0–17,666 ng/mL). Resection was performed in 15 of these patients (IPMN benign, n = 11; mucinous cystadenoma, n = 1; serous cystadenoma, n = 1; adenocarcinoma, n = 1; retention cyst, n = 1). The single patient resected with a serous cystadenoma had a cyst fluid CEA level of 0 ng/mL. The lowest cyst fluid CEA level in the 12 patients resected with a mucinous tumor was 53 ng/mL (range, 53–17,666 ng/mL). The single patient resected with adenocarcinoma had a cyst fluid CEA level of 83 ng/mL.
Whole body 18F-fluorodeoxyglucose (FDG) PET imaging was performed in 68 patients, and in 8 of these patients (12%) the PET scan was read as positive. The mean standard uptake value (SUV) of the 8 positive studies was 4.6 (range, 1.9–8.0). Malignancy was identified in 4 of the 8 patients with positive PET imaging (mean SUV: 5.9; range, 2.5–8.0). Resection for a benign diagnosis was performed in 2 patients with a positive PET (serous cystadenoma, n = 1, SUV = 3.0; pseudocyst, n = 1, SUV = 2.7).
Treatment and Outcome Data
The initial management was operative in 170 patients (32%), and nonoperative (radiographic follow-up) in 369 patients (68%). Within the group of 170 patients who initially underwent exploration, the operations performed included distal pancreatectomy with or without splenectomy (n = 83), pancreaticoduodenectomy (n = 61), enucleation (n = 7), central pancreatectomy (n = 3), and other nonresectional procedures (n = 16). The nonresectional procedures included biopsy for metastatic or locally advanced malignancy (n = 3), and cyst drainage for identified pseudocysts (n = 5). Complications were reported in 38 patients (22%), and a single patient (0.6%) died of complications that developed from a pancreatic leak after resection of an unsuspected pseudocyst.
The histopathologic results for the 170 patients who underwent initial operative treatment are presented in Table 2. The majority of patients who underwent initial operative treatment had a benign diagnosis (n = 138, 82%), and the most common benign cystic lesion was a serous cystadenoma (n = 72). Malignant diagnoses were present in 18% (n = 32) of patients, and the most common malignant pathology was invasive IPMN (n = 10, 10 of 170 = 6%). Ductal adenocarcinoma was identified in 4 patients, and in 2 of these patients unresectable disease was encountered at the time of operation.
TABLE 2. Histopathology of Patients Undergoing Initial Operative Management (n = 170) and Delayed Operative Management (n = 29)
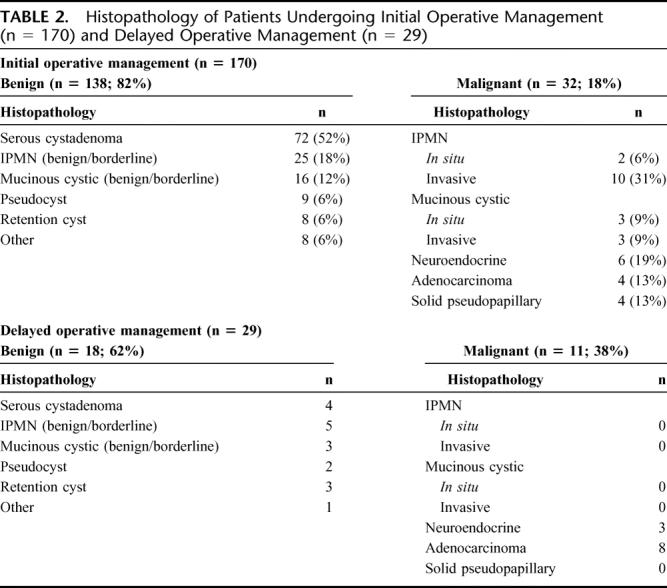
The median radiographic follow-up for the 369 patients initially managed nonoperatively was 24 months (range, 1–172 months), and the average change in cyst diameter during follow-up was 0.2 cm (range, 3.0–5.0 cm). At the time of last follow-up, changes within the cyst that prompted operative treatment occurred in 29 of the 369 patients (8%) initially selected for nonoperative management. Cyst growth was the most common reason for eventual operative resection. The pathologic diagnoses for the 29 patients who underwent delayed resection are presented in Table 2. Malignancy was identified in 11 of these 29 patients (38%). The 11 patients with malignancy represent 3% (11 of 369) of all patients who were initially managed nonoperatively. No patient with an in situ or invasive mucinous tumor underwent delayed resection.
The associations between the selection of initial operative and nonoperative management are presented in Table 3. Patients who presented with symptomatic cysts that had a solid component, septations, and a cyst diameter >2.5 cm were more likely to undergo initial operative management. Patients with lesions in the body and tail of the pancreas were more likely to undergo resection than patients with lesions located in the head and neck of the pancreas.
TABLE 3. Patient and Cyst Characteristics and Their Association With Initial Operative vs. Nonoperative Management
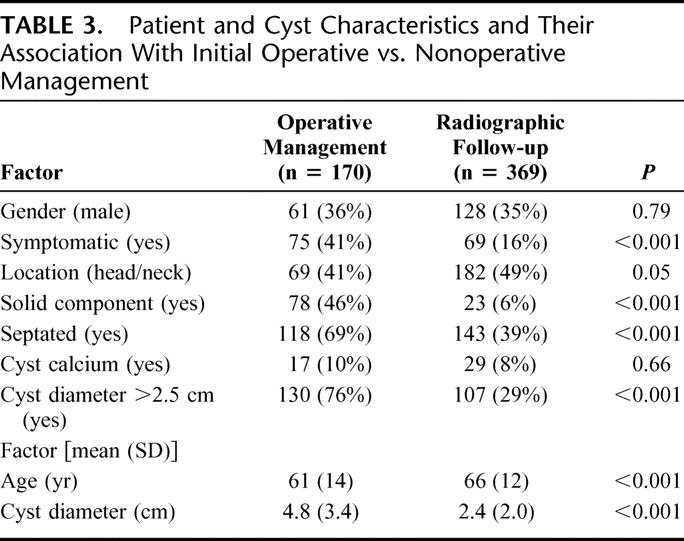
Recursive partitioning identified the presence of a solid component as the strongest predictor of initial operative management (Fig. 2). Resection was performed in 75% of patients with lesions in which a solid component was identified. Cyst size was the next most predictive factor in patients with lesions that did not have a solid component. Smaller lesions (≤2.5 cm) were more likely to be followed radiographically. In those patients with larger (>2.5 cm) lesions, and without a solid component, patient age and the presence of symptoms were found to be associated with the treatment decision. Patients <65 years old were more likely to undergo resection. The presence of symptoms was associated with the treatment decision in patients ≥65 years, with patients who had symptomatic lesions being more likely to undergo resection.
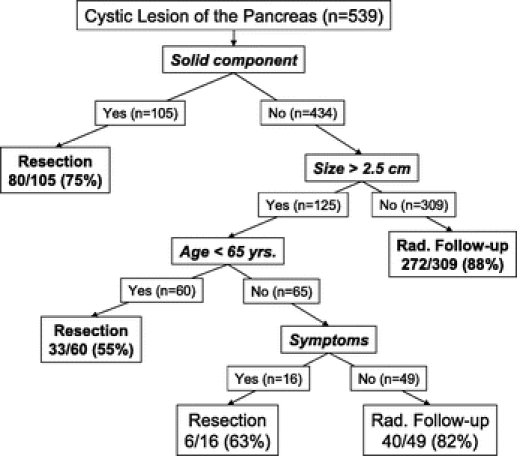
FIGURE 2. Decision tree analysis for the selection of initial operative management in the 539 patients evaluated for a pancreatic cyst.
Specific Histopathologies
Serous Cystadenoma
Serous cystadenoma was diagnosed in 76 patients who underwent resection. The average diameter of these lesions was 5.0 cm (range, 1.2–18.0 cm), and 34% of patients (n = 26) were symptomatic at the time of diagnosis. Initial operative management was performed in 72 patients. Delayed resection was performed in 4 patients after a period of 9, 13, 67, and 84 months of follow-up. An additional 6 patients who had biopsy-proven serous cystadenoma were being followed radiographically at the time of last follow-up. The growth curve for these 10 patients who were followed radiographically for pathologically proven serous cystadenoma is presented in Figure 3. Growth rate did not appear to be associated with cyst diameter in these 10 patients (Fig. 3). Median growth rates approximated 0.5 cm/year. None of the 76 patients resected for a serous cystadenoma had evidence of malignancy at the time of last follow-up. The percentage of patients resected for a serous cystadenoma decreased during the second half of the study period (first half, 56% vs. second half, 30%; P = 0.001).
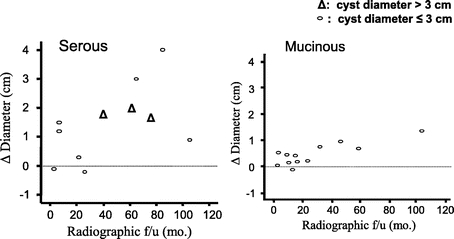
FIGURE 3. Change in cyst diameter over time in patients with serous or mucinous cysts of the pancreas (confirmed by fine needle aspiration, resection, or cyst fluid analysis).
Mucinous Tumors
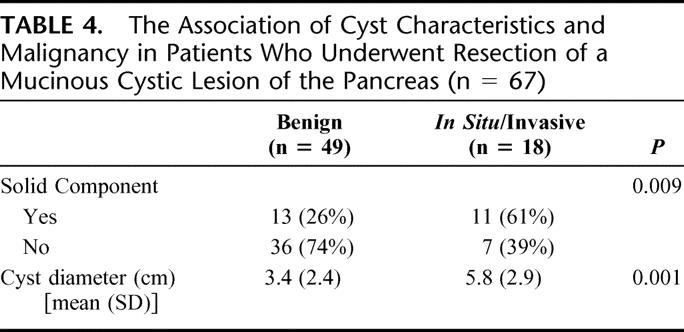
All patients with malignant mucinous tumors (IPM carcinoma and mucinous cystadenocarcinoma) were resected without delay. None of the resected patients (n = 40) with a mucinous tumor <3 cm in diameter was found to have invasive malignancy (Fig. 4). A single patient resected with a 2.7-cm mucinous cystadenoma was found to have in situ disease. Invasive and in situ malignancy was present in 49% of patients (17 of 35) who underwent resection of mucinous tumors >3 cm in diameter. The association between cyst diameter, and the presence of a solid component, with malignancy is presented in Table 4. Within the subgroup of patients with IPMN (n = 42), the presence of malignancy was also associated with increased age (mean age: 65 years without malignancy vs. 74 years with malignancy; P = 0.02).
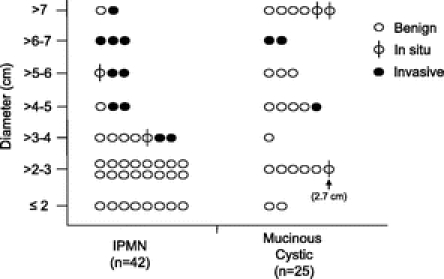
FIGURE 4. The presence of benign, in situ, and invasive disease in patients who underwent resection for a mucinous cystic tumor of the pancreas.
TABLE 4. The Association of Cyst Characteristics and Malignancy in Patients Who Underwent Resection of a Mucinous Cystic Lesion of the Pancreas (n = 67)
Nonoperative management was prescribed in 12 patients who had a cyst fluid CEA level of ≥182 ng/mL, and radiographic findings consistent with a mucinous tumor. The cyst growth curve for these 12 patients is presented in Figure 3.
Adenocarcinoma
Adenocarcinoma was discovered in 13 patients who were initially labeled as having a cystic lesion of the pancreas. Suspicious radiographic findings were present in 5 patients, and these patients underwent evaluation for immediate operative intervention. Resection was performed in 2 of these 5 patients, 2 patients were found to have metastatic disease, and the other patient was medically unfit for operation.
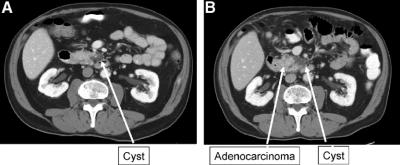
Adenocarcinoma was identified in 8 patients initially followed radiographically. The average size of the cystic lesion in these 8 patients was 2.3 cm (range, 1.0–3.8 cm), and in none of these patients did either the radiographic imaging or final pathology suggest an adenocarcinoma with central necrosis. On the contrary, all patients appeared to present with retention cysts that were immediately adjacent to the subsequently discovered malignancy (Fig. 5). At the time of operation, 3 of 8 patients were discovered to have resectable disease, 2 patients had locally advanced lesions, and 3 patients had metastatic disease. The time between initial identification of the cyst and the subsequent identification of adenocarcinoma was >48 months in 3 of the 8 patients (57, 60, and 84 months). These 3 patients had not undergone routine follow-up, and 1 of the 3 patients underwent resection. Because of the prolonged period of time and lack of interval follow-up, the relationship between cyst and malignancy is unclear. The remaining 5 patients were followed radiographically between 5 and 13 months prior to operative intervention. Radiographic changes prompted operative intervention, and resection was possible in 2 of these patients.
FIGURE 5. Representative cross section of patient with a cystic lesion initially followed radiographically (A) who 22 months later developed changes adjacent to the cyst (B) found to be adenocarcinoma at the time of resection.
DISCUSSION
This study suggests that a group of patients with pancreatic cysts can be identified who have a low risk of malignancy. The risk of malignancy approximates the risk of operative mortality in these highly selected patients, and in this group of patients radiographic follow-up may be warranted. The majority of patients (369 of 539, 68%) evaluated between 1995 and 2005 for a cystic lesion of the pancreas were managed nonoperatively. Patients who were initially followed radiographically were more likely to have asymptomatic, small (mean diameter, 2.4 cm) cysts that were without a solid component. After a median follow-up of 2 years, the average change in diameter of these lesions was 0.2 cm, and 29 patients (29 of 369, 8%) developed changes that resulted in resection. Adenocarcinoma was identified in 8 of the 369 patients (2%) initially followed radiographically, and no patient with a malignant mucinous tumor underwent a delayed resection. The risk of malignancy in patients followed radiographically in this study is lower than the reported mortality rate of pancreatectomy at all but the highest volume centers.5,6
Other institutions have reported similar results.2,11 Walsh et al11 reported on 221 patients evaluated at the Cleveland Clinic for cystic neoplasms over a 5-year time period. The majority of patients (161 of 221, 67%) in the Cleveland Clinic study were followed radiographically. Factors associated with nonoperative treatment were similar to those reported in our study, and included a lack of symptoms (0% nonoperative, 67% operative), older age (mean, 62 years nonoperative vs. 56 years operative), and a smaller cyst diameter (median, 2.4 cm nonoperative vs. 4.0 cm operative). Within the group of patients followed radiographically, 19% exhibited an increase in size (mean radiographic follow-up, 24 months), and none of the patients who underwent delayed resection was found to have a malignancy.
Similarly, Dr. Warshaw's group from the Massachusetts General Hospital (MGH) has also recommended observation for selected patients with cystic lesions of the pancreas.2,14 Fernandez-del Castillo et al reported on 212 patients evaluated at the MGH for a cystic lesion of the pancreas over a 5-year time period.2 Unlike the present study, the majority of patients in the MGH study were symptomatic, and the majority underwent resection. In patients who underwent resection with incidentally discovered small lesions (<2 cm), the incidence of malignancy was 3% (1 of 28). Mucinous lesions were identified in 50% of those patients who underwent resection for incidentally discovered small cysts. Because of the premalignant nature of mucinous lesions, the authors emphasized the need for close follow-up in patients with small mucinous tumors that are not resected. Their recommended treatment algorithm for incidentally discovered cysts was observation for those patients with lesions ≤2 cm in diameter, and in older patients with lesions >2 cm that lacked mucin and had a normal CEA level on cyst fluid analysis.
The results from these studies suggest that selective resection of cystic lesions of the pancreas is appropriate, balancing the risk of malignancy with the risk of operation. The studies noted above, as well as the data presented in this study, suggest that certain cyst characteristics are associated with the presence of malignancy. These characteristics include the presence of a solid component, increased cyst size, and symptoms, all of which should be considered when recommending a treatment plan. In the present study, the association of nonoperative management with increased patient age, and cyst location in the head of the pancreas, suggests that the increased risk associated with resection in the setting of these factors was considered in the treatment decision.
Recursive partitioning provides a statistical framework that places these factors in a hierarchical order according to the strength of their association with the treatment decision. The presence of a solid component within the cyst was the factor most predictive of initial operative management. A solid component was also associated with the presence of malignancy in patients with resected mucinous tumors. Malignancy was identified in 61% of patients who had a solid component identified within a mucinous cyst. This radiographic finding should be an indicator for resection in patients with suspected mucinous tumors. A solid component, however, can also be a common radiographic finding in patients with serous lesions; therefore, the presence of this factor must be considered in the context of other radiographic and endoscopic findings.
The diameter of the cystic lesion was the next factor identified by decision tree analysis. Increased cyst diameter was also found to be associated with the presence of malignancy in resected patients. The fact that cyst size was associated with both the treatment decision and the presence of malignancy allowed no patient with a malignant mucinous tumor to be followed radiographically. This simple factor can be used to counsel patients with suspected IPMN or mucinous cystadenoma regarding the immediate risk of malignancy. In the authors’ view, patients with larger mucinous lesions should undergo resection unless their overall health status precludes operation. Patients with small lesions may be followed radiographically. The natural history of these small lesions is unknown; therefore, the decision regarding resection of a small IPMN in a young patient is difficult. If resection is not performed in this setting, then careful, and long-term, radiographic monitoring is essential. The duration of that follow-up is undefined as of yet.
The association between cyst size and malignancy was not applicable to patients with adenocarcinoma. The average cyst diameter in the 8 patients with adenocarcinoma who were initially managed radiographically was 2.2 cm (range, 1.0–3.8 cm). Review of these patients revealed that these lesions did not represent adenocarcinoma with central necrosis, but rather retention cysts adjacent to a solid carcinoma. The relationship of the cysts to the carcinoma in these patients is unknown. Several of these patients had prolonged radiographic follow-up (57, 60, 84 months) prior to the development of the suspicious radiographic findings, and in these cases there may be no relation between the cyst and the tumor. In other cases, however, it is plausible that these small retention cysts resulted from branch duct obstruction by an adjacent carcinoma. This highlights the importance of high-quality imaging and interpretation of both what is inside the cyst and of the parenchyma adjacent to the cyst.
As diagnostic capabilities improve, treatment decisions should evolve away from ones based on radiographic characteristics to decisions based on the known natural history of the specific histopathologic entity. Current radiographic, endoscopic, and cyst fluid evaluation techniques allow the categorization of cysts as neoplastic, non-neoplastic, serous, or mucinous in the majority of cases. CT is an excellent test for the identification of cystic lesions and, in many cases, can further characterize them by visualization of septa, calcifications, and mural nodules.3,15 The CT imaging characteristics of serous (microcystic) and mucinous (macrocystic) lesions have been well described. MR imaging provides improved visualization of the cyst features and can often identify IPMN of the pancreas through identification of cyst to duct communication.16 When cystic lesions are radiographically indeterminate, endoscopic ultrasound and cyst fluid aspiration may be of benefit. Elevated CEA levels and the presence of extracellular mucin have been shown to have a positive predictive value for mucinous lesions as high as 85%.3,17,18 In a prospective study by Brugge et al of 112 patients with cystic lesions, an elevated cyst fluid CEA level (>192 ng/mL) was the best predictor of a mucinous lesion, and accurately identified these lesions in 79% of cases.19 Serous cystadenomas and retention cysts have been shown to almost uniformly have undetectable cyst fluid CEA levels.3,18,20 Alone, or in combination, these studies now allow the distinction between neoplastic and non-neoplastic, as well as serous from mucinous, in many patients with cystic lesions of the pancreas.
Routine resection has been recommended for patients with mucinous lesions because of their malignant potential. For all patients undergoing resection of IPMN tumors of the pancreas, the prevalence of malignancy is approximately 35% to 45%.21,22 Factors associated with the presence of malignancy have not been well described. Increased age has been reported to be associated with malignancy in IPMN in several studies.22,23 Because of this association, a report from Johns Hopkins concluded that the lag time from adenoma to carcinoma in IPMN was approximately 5 years.22 The current study also found that patients with IPMN adenoma were significantly younger than those with carcinoma (65 years vs. 74 years, P = 0.02). We think that this represents the course of progression from adenoma to carcinoma; however, it is unknown how long the benign lesions have been present prior to their identification on imaging studies. A 1-cm branch-duct IPMN is probably of no consequence to an 80-year-old patient. A 1-cm branch-duct IPMN identified in a 40-year-old patient may represent a lesion that will become malignant in his or her lifetime. Immediate resection in the 40-year-old patient will likely yield a benign diagnosis, and will be performed in the setting of a soft gland and small pancreatic and bile duct.
When a serous cystadenoma is identified, resection should be reserved for the symptomatic patient, or in a healthy patient in whom significant growth has been observed. The data from the present study, as well as other large single institution studies, confirm the benign nature of serous cystadenomas. However, these lesions can become symptomatic, and resection remains indicated in the presence of symptoms. Cyst size does appear to be associated with symptoms. Compagno and Oertel's study in 1978 of 34 cases of serous cystadenoma reported an average tumor diameter of 10.8 cm, and 71% were symptomatic.24 In our series, the average tumor diameter of patients with resected serous lesions was 4.9 cm, and 35% were symptomatic. Tseng et al reported on 106 patients with serous cystadenoma and found tumor size to be associated with symptoms (symptoms: <4 cm, 22% vs. ≥4 cm, 72%, P < 0.001).14
Because the exact size at which a serous lesion will become symptomatic is unknown, and because the growth rate of serous cystadenomas has not been defined, the appropriate management of the young patient with an asymptomatic serous lesion is yet to be determined. Tseng et al reported a growth rate of 0.6 cm/year for patients with serous cystadenoma.14 In Tseng's report, serial radiography was obtained for a group of 24 patients who had a median radiographic follow-up of 23 months. There was a significant difference in growth rates between patients with tumors <4 cm at presentation (0.48 cm/year) and patients with lesions ≥4 cm (1.98 cm/year). Because of the observed increased rate of growth in larger lesions, Tseng et al recommended resection for asymptomatic patients with serous cystadenomas >4 cm. Our study found a similar overall growth rate of approximately 0.5 cm/year, but did not find any association between the size of the lesion and the rate of growth. We feel that asymptomatic patients can be safely followed with the possible exception of those patients who have large lesions that are marginally resectable.
Careful radiographic follow-up of both the cyst and the surrounding parenchyma is essential for all patients with neoplastic cysts who are managed nonoperatively. During the initial evaluation, only high-quality cross-sectional pancreatic imaging (CT or MR) with contrast should be considered acceptable. When additional data is necessary to formulate a treatment recommendation (eg, 2.5–3.0 cm cyst in an elderly patient), EUS with cyst aspiration should be considered in attempt to determine whether or not a mucinous tumor is present. After the initial evaluation, our general approach has been to repeat cross-sectional imaging every 6 months for a period of 2 years, and then annually thereafter. In some patients, such as an elderly patient with a small serous cystadenoma, after a period of observation with stability, no further imaging may be necessary.
CONCLUSION
The results of this study support a selective approach to resection in patients with cystic lesions of the pancreas. The factors most associated with the selection of nonoperative management in this study included the lack of a solid component, and small cyst diameter. The malignancy risk in patients followed radiographically was 3%. Efforts should be made to improve the ability to distinguish histopathologic subtypes without resection. Serous cystadenomas should be considered benign, and in the absence of symptoms, can be safely followed radiographically. Cyst diameter is associated with the size of the lesion in patients with mucinous cysts of the pancreas. Elderly patients with small mucinous tumors should be considered for radiographic follow-up. The future challenge will be to characterize the progression of IPMN and mucinous cystic tumors in more detail, and to develop better methods for identifying the presence of in situ or invasive disease in these patients. Continued improvements in cross-sectional imaging and endoscopic techniques and further investigation into markers in the serum and cyst fluid should allow better identification of mucinous subtypes.
Discussions
Dr. Andrew L. Warshaw (Boston, Massachusetts): Dr. Allen, this is a very fine study. You and your co-authors have added a considerable experience with the 369 patients whom you observed with regard to the safety and efficacy of nonoperative observation of pancreatic cysts. This amounts to two-thirds of the patients who were referred to you with lesions that were presumed to be cystic neoplasms. You state that asymptomatic patients with cysts, which were smaller than 3 centimeters and without mural nodules were followed, but this decision was based on a personal choice of the surgeons. The study is particularly relevant in that we are all seeing more and more patients with small asymptomatic cystic lesions discovered incidentally during management of conditions involving other organs.
You demonstrate the essential safety of this strategy inasmuch as only 29 patients, or 30 patients, 8%, developed worrisome growth or other changes which precipitated resection, and frank malignancy was found in 38% of those lesions. These observations generally correlate with our own published findings in which the risk of cancers in cysts smaller than 2 centimeters was extremely low. However, 17% of our asymptomatic patients had in situ or invasive cancer and 42% had pre-malignant lesions, generally MCN or IPMN like your asymptomatic patients.
In your study you focus on established malignancy and to some extent put aside the consideration of pre-malignancy of MCN and IPMN. Most of us consider that over the course of time the development of cancer in MCN and IPMN increases over and above the number who already have cancer at the time of discovery.
Your median follow-up was only 2 years and you do not indicate how complete it was. A French study in Clinical Gastroenterology published just this month, appears to confirm that main duct IPMNs have a greater risk of high grade dysplasia and invasive cancer than do branch duct IPMNs. However, the 5-year actuarial risks of at least high-grade dysplasia or in situ carcinoma are 15% and 63% respectively. The overall 10-year actuarial risk was 49% for high-grade dysplasia and 29% for invasive cancer. Since these neoplasms are generally slow growing and evolving neoplasms with an increasing risk of malignancy, can you really be confident about your proposed guidelines?
Eight adenocarcinomas were found among your patients initially designated for observation. We don't have the follow-up cure rates in them. Was anything lost in following these patients longer than you might have?
You rarely used endoscopic ultrasound and fine needle aspiration for cytology, mucin detection, or cyst fluid CEA. Other groups, including ours, have found this useful to help to discriminate mucinous neoplasms with malignant potential from non-mucinous benign lesions such as serous cystadenomas or pseudocysts. Do you anticipate changing your practice?
Do you use either patient age or patient anxiety in your algorithm of management? Both tend to increase with time, producing to some extent a “sooner-or-later factor.”
At the end of the day, your study attests to the quality and wisdom of your past decisions. Perhaps we can infer principles for future decisions as well, but there remains a degree of uncertainty in each patient to be followed, and follow-up must be rigorous and complete.
Dr. Peter J. Allen (New York, New York): With respect to your first question, which is our confidence in our guidelines, we view this as “guidelines in evolution”. We are currently following a large number of these patients, and in some, we do have diagnostic information, specifically EUS with cyst fluid analysis for CEA levels, to suggest that many of these patients have small mucinous tumors. As you noted, these are considered to be pre-malignant lesions. And certainly what we do not want to follow somebody who has a potentially curable problem where it has developed an invasive cancer and an insolvable problem.
With the data we have now we are not seeing much change within these very small lesions. These very small lesions probably represent branched-duct IPMNs. The significance of that lesion in the 75- or 80-year-old patient we are unsure of.
I think that segues into your third question, with respect to age and anxiety. We think that probably the most difficult group of patients are the younger patients who have small mucinous tumors because they may live long enough to face the progression to malignancy in that group of patients, the anxiety factor plays a role. As you saw from our chart, over 40 patients have been resected who have mucinous lesions less than 3 centimeters in diameter. We are resecting some of these. And these tend to be patients who are younger and who have rather significant anxiety about following these radiographically.
We are using EUS more frequently. We have accepted the data that certainly your group and others have established with respect to CEA levels within the cyst fluid being predictive of a mucinous pathology. In situations where we or the patient are unsure of which treatment path we or they would like to follow can be helpful. So, yes, we are using EUS more frequently.
Dr. John L. Cameron (Baltimore, Maryland): Dr. Allen, very nice study and beautifully and clearly presented. And I compliment you on your interest in pancreatic surgery. I would like to point out, however, that the first 2 discussants of your paper by virtue of age, verified by the paper we heard yesterday, are safer pancreatic surgeons than are you! You will have to wait a considerable number of years before you become a safe pancreatic surgeon!
The criteria that you have set up I think are the criteria that all of us have used in recent years to follow these patients, with perhaps one exception. And that is the size or diameter of the pancreatic duct. Are you willing to follow somebody with a 2 centimeter pancreatic cyst in the uncinate process as a branch duct IPMN, who has a dilated pancreatic duct of, say, half a centimeter? That patient almost certainly has an IPMN. And despite the fact that the cyst size is small, are you willing to follow such a patient?
I think from a single institution we have the largest number of IPMNs treated surgically. We are now well over 200. And even though the main duct IPMNs have the highest incidence of carcinoma, 40%, our branch duct incidence is about 30%. The criteria you presented I think are the criteria that all of us are using, except perhaps for the diameter of the pancreatic duct. And should that be included amongst your criteria?
Dr. Peter J. Allen (New York, New York): With respect to the safety of pancreatic surgery, we tip our hat to you and the work from everyone at Johns Hopkins.
This is a selected group of patients. And this selected group of patients are patients that were labeled as cystic lesions of the pancreas, 577.2. So these are patients that I think most of us put into this lump category based on the radiographic findings. On review of these patients, very few represent main duct IPMNs. The majority of these patients are branch duct IPMNs. And in the lesions under 3 cm that were resected we have not seen malignancy with that subgroup.
The Mass General reported a series last month of about 40 patients with cystic lesions less than 3 cm in size, and within that group of 40 patients with mucinous tumors in that category less than 3, they also didn't find any patients with invasive cancer. There was one patient within that group of 40 that had in situ disease.
Dr. Keith D. Lillemoe (Indianapolis, Indiana): Dr. Allen, very nice paper, very well presented. I have a couple questions.
One is a follow-up with respect to Dr. Warshaw's question to the EUS. I think we all would accept that radiographic assessment of these lesions is probably too primitive in these days. You commented about cyst fluid CEA. Have you had any experience with the molecular analysis that is now commercially available? I haven't ordered it myself, but when they come back, by the time I have read the report they all sound pretty suspicious.
Point number 2 relates to your follow-up. Would you describe how you follow these patients, at what intervals, and what moves you from observation to operative management?
Three, I don't think you said it, but could you tell us the node status and the margin status of those patients that did have adenocarcinoma that were in the observed group that eventually went to surgery? This is a bad disease when it becomes cancer. I know none of us want to do a lot of resections for benign disease, but again, letting somebody slip over into a more advanced cancer stage isn't doing them any favors.
Finally, one potential flaw in your analysis, which I am afraid if it is interpreted improperly may lead to people observing these patients without getting surgeons involved. You looked at a certain ICD-9 code, which describes cystic lesions of the pancreas. I wonder how many patients were not included in your analysis that had a 2 centimeter or 1.5 centimeter in cystic IPMN that had been aspirated and diagnosed as cancer, had an operation, and were not included in this study because it was not part of your observational data from your ICD-9 code. It is possible that you may have missed in your entire database some small cystic cancers that were operated on and just weren't included in the analysis, which could alter your statistics somewhat?
Dr. Peter J. Allen (New York, New York): I will take your last question first. You are absolutely correct, the ICD-9 code is what drives patient entry into this database. Patients presenting with an IPMN that has been aspirated and diagnosed as a malignancy would not typically be entered into this database. So we could shift our denominator a bit if we included patients based on pathology. I think in general that this would change the denominator so that more patients within the immediate operative group would have a diagnosis of malignancy.
We are doing cyst fluid analysis more frequently. We are interested in looking at other markers within the cyst fluid as well. I am not aware of the commercially available DNA marker test being used at our institution. I have only seen preliminary sensitivity and specificity results of this test and they do not seem very high.
Regarding your question about follow-up, we thoroughly image the patients at their initial presentation. High-quality, dedicated, cross-sectional imaging of the pancreas is essential. Once it has been decided that follow-up will be the course of treatment we typically re-image every six months for two years, and then annually thereafter. Elderly patients with small serous lesions may be imaged less frequently.
Dr. Henry A. Pitt (Indianapolis, Indiana): First, please let me set the record straight. You quoted our paper whose subtitle was “Observe or Operate” as being in favor of a nonoperative approach. Clearly, our message was that most of these patients should undergo surgery.
Secondly, one aspect of our analysis and the literature suggests that increasing age does predict malignancy. Therefore, I would suggest that age should be near the top of a treatment algorithm and should be one of the strong factors that should drive physicians and patients toward surgery.
Thirdly, we are all seeing patients with multiple small side branch IPMNs who must have a field defect and a high likelihood of developing a malignancy. If multiple small cysts were localized to one area of the pancreas, would you observe or operate? In my opinion, surgery is indicated in these patients.
Fourth, shouldn't one of our goals be to prevent cancer? Two-thirds of these patients have premalignant or malignant tumors. Therefore, we ought to have a low threshold to operate on these patients and not wait until they develop cancer and have a worse outcome from surgery.
Finally, Dr. Warshaw's group has reported that serous cystadenomas grow slowly at first but rapidly when they get to 4 centimeters. If the patient is younger, wouldn't it be more cost-effective to do an enucleation, a central resection or a laparoscopic distal pancreatectomy early rather than multiple tests for 20–30 years and a much bigger operation later?
Dr. John M. Howard (Toledo, Ohio): How often have you seen more than one cyst and how would that influence your management?
Dr. Peter J. Allen (New York, New York): Dr. Pitt, I do not believe I characterized your paper as being in favor of observation. Nor would I characterize our paper as being in favor of observation. I would characterize both of our studies as being descriptive of a selective approach. In your paper there were many patients who did not undergo resection, and these patients were selected for observation because of characteristics suggestive of a benign etiology.
Dr. Howard, we often see patients with multiple cysts. These patients often have multiple branch duct IPMN. The only significance of this is that we feel even after resection of a dominant cyst, patients with IPMN need to be followed carefully for recurrence within the pancreatic remnant.
Footnotes
Reprints: Peter J. Allen, MD, Memorial Sloan-Kettering Cancer Center, E-mail: allenp@MSKCC.ORG.
REFERENCES
- 1.Gorin AD, Sackier JM. Incidental detection of cystic neoplasms of the pancreas. Md Med J. 1997;46:79–82. [PubMed] [Google Scholar]
- 2.Fernandez-Del CC, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. [DOI] [PubMed] [Google Scholar]
- 4.Sakorafas GH, Sarr MG. Cystic neoplasms of the pancreas: what a clinician should know. Cancer Treat Rev. 2005;31:507–535. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250–256. [PubMed] [Google Scholar]
- 7.Horvath KD, Chabot JA. An aggressive resectional approach to cystic neoplasms of the pancreas. Am J Surg. 1999;178:269–274. [DOI] [PubMed] [Google Scholar]
- 8.Siech M, Tripp K, Schmidt-Rohlfing B, et al. Cystic tumours of the pancreas: diagnostic accuracy, pathologic observations and surgical consequences. Langenbecks Arch Surg. 1998;383:56–61. [DOI] [PubMed] [Google Scholar]
- 9.Ooi LL, Ho GH, Chew SP, et al. Cystic tumours of the pancreas: a diagnostic dilemma. Aust NZ J Surg. 1998;68:844–846. [DOI] [PubMed] [Google Scholar]
- 10.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh RM, Vogt DP, Henderson JM, et al. Natural history of indeterminate pancreatic cysts. Surgery. 2005;138:665–670. [DOI] [PubMed] [Google Scholar]
- 12.Allen PJ, Jaques DP, D'Angelica M, et al. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003;7:970–977. [DOI] [PubMed] [Google Scholar]
- 13.Breiman L, Friedman J, Olson R, et al. Classification and Regression Trees. Monterey, CA: Wadsworth and Brooks, 1984. [Google Scholar]
- 14.Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minami M, Itai Y, Ohtomo K, et al. Cystic neoplasms of the pancreas: comparison of MR imaging with CT. Radiology. 1989;171:53–56. [DOI] [PubMed] [Google Scholar]
- 16.Koito K, Namieno T, Ichimura T, et al. Mucin-producing pancreatic tumors: comparison of MR cholangiopancreatography with endoscopic retrograde cholangiopancreatography. Radiology. 1998;208:231–237. [DOI] [PubMed] [Google Scholar]
- 17.Walsh RM, Henderson JM, Vogt DP, et al. Prospective preoperative determination of mucinous pancreatic cystic neoplasms. Surgery. 2002;132:628–633. [DOI] [PubMed] [Google Scholar]
- 18.Lewandrowski KB, Southern JF, Pins MR, et al. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: a comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. [DOI] [PubMed] [Google Scholar]
- 20.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383–389. [DOI] [PubMed] [Google Scholar]
- 21.D'Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvia R, Fernandez-Del CC, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol. 1978;69:289–298. [DOI] [PubMed] [Google Scholar]