Abstract
Objectives:
Although weight loss following Roux-en-Y gastric bypass is acceptable in patients with preoperative body mass index (BMI) between 35 and 50 kg/m2, results from several series demonstrate that failure rates approach 40% when BMI is ≥50 kg/m2. Here we report the first large single institution series directly comparing weight-loss outcomes in super-obese patients following biliopancreatic diversion with duodenal switch (DS) and Roux-en-Y Gastric Bypass (RYGB).
Methods:
All super-obese patients (BMI ≥50 kg/m2) undergoing standardized laparoscopic and open DS and RYGB between August 2002 and October 2005 were identified from a prospective database. Two-sample t tests were used to compare weight loss, decrease in BMI, and percentage of excess body weight loss (% EBWL) after surgery. χ2 analysis was used to determine the rate of successful weight loss, defined as achieving at least 50% loss of excess body weight.
Results:
A total of 350 super-obese patients underwent DS (n = 198) or RYGB (n = 152) with equal 30-day mortality (DS,1 of 198; RYGB, 0 of 152; P = not significant). The % EBWL at follow-up was greater for DS than RY (12 months, 64.1% vs. 55.9%; 18 months, 71. 9% vs. 62.8%; 24 months, 71.6% vs. 60.1%; 36 months, 68.9% vs. 54.9%; P < 0.05). Total weight loss and decrease in BMI were also statistically greater for the DS (data not shown). Importantly, the likelihood of successful weight loss (EBWL >50%) was significantly greater in patients following DS (12 months, 83.9% vs. 70.4%; 18 months, 90.3% vs. 75.9%; 36 months, 84.2% vs. 59.3%; P < 0.05).
Conclusions:
Direct comparison of DS to RYGB demonstrates superior weight loss outcomes for DS.
A total of 350 super-obese patients underwent duodenal switch (DS) or Roux-en-Y gastric bypass (RYGB). Weight loss, decrease in body mass index, percent excess body weight loss, and likelihood of successful weight loss were greater for the DS than RYGB.
Data from the National Health and Nutrition Examination Survey indicate that the prevalence of obesity among adults in the United States, defined as body mass index ≥30 kg/m2 [BMI; calculated as weight (kg) divided by the square of the height (m)], has increased from 13% in 1960 to 19621 to 32% in 2003 to 2004.2 In this recent estimate, 3% of men and 7% of women were severely obese (BMI ≥40 kg/m2). Bariatric surgery has been shown to be the most effective means of achieving significant and sustained weight loss in individuals with this degree of obesity.3–6 Indeed, the increased prevalence of severe obesity, improved public, and physician awareness of the burden of obesity-related medical and psychosocial morbidity, recognition of the effectiveness of bariatric surgery, and the development of laparoscopic techniques for bariatric procedures7 have all contributed to the significant increase in the number of bariatric procedures performed in the United States and worldwide.8,9
A “hidden” component of the obesity pandemic is the disproportionate increase observed at the upper end of the obesity spectrum: between 1986 and 2000, severe obesity (BMI ≥40 kg/m2) quadrupled, and the prevalence of BMI ≥50 kg/m2 quintupled, accounting for approximately 1 in 400 U.S. adults in 2000.10 This latter subgroup of severe obesity (BMI ≥50 kg/m2) was termed “super-obesity” by Mason et al in 1987.11 Hospitals and clinics face significant infrastructural challenges when caring for super-obese patients. The surgeon may face several technical challenges when operating, including massive hepatomegaly, greater intra-abdominal adiposity, and an increased abdominal wall thickness requiring significant torque when using laparoscopic instruments. Super-obesity is also associated with a greater burden of obesity-related comorbidities.12 Taken together, these challenges may contribute to increased complication rates and mortality following bariatric surgery observed in super-obese patients in some series.13–15,24
It is important to note that the concept of super-obesity initially proposed by Mason et al was based on the observation that patients with a higher degree of severe obesity undergoing vertical banded gastroplasty (VBG) often failed to achieve satisfactory weight loss after surgery.11 Indeed, the differences in weight-loss outcomes between patients with severe obesity and super-obesity initially noted following VBG has subsequently been demonstrated with other bariatric operations.15,18,19,22,42
Roux-en-Y gastric bypass (RYGB) is the most commonly performed bariatric procedure in the United States, accounting for over 80% of all bariatric procedures performed in 2002.8 In a recent meta-analysis by Buchwald et al of more than 7000 patients in 44 studies, the estimated percentage of excess body weight loss (% EBWL) following RYGB was 62%.16 Several studies, however, have demonstrated less favorable results in super-obese patients.17–22 In a series with 90% follow-up, MacLean et al reported good or excellent weight loss results in 93% of patients with severe obesity but in only 57% of super-obese patients.19 In most of these series, while there was usually greater absolute weight loss in the super-obese group, the % EBWL and final BMI achieved following surgery were often inferior. Results such as these have prompted the development of several technical modifications to the RYGB, including extension of the Roux limb (long-limb gastric bypass)17,20,23 and a malabsorptive (distal) gastric bypass,24,25 in an effort to obtain better long-term weight loss outcomes.
The increase in the prevalence of super-obesity as well as the recognition of inadequate weight loss following RYGB in super-obese patients has prompted a growing interest in the biliopancreatic diversion with duodenal switch (DS). The DS, developed by Hess26 and Marceau et al,27,28 is a hybrid operation that combines the DeMeester duodenal switch,29 initially developed for the treatment and prevention of bile reflux, with the Scopinaro biliopancreatic diversion.30 The greater technical complexity (particularly when performed laparoscopically) and perceived perioperative13 and nutritional31–35 risks of DS in comparison to RYGB, however, have limited the widespread adaptation of DS among bariatric surgeons. Nonetheless, 2 recent meta-analyses suggest that weight loss results following DS may be superior to RYGB.3,5 While several DS series have demonstrated excellent outcomes,28,36,37 there are little data directly comparing weight loss outcomes between DS and RYGB in super-obese patients. Indeed, it may be difficult to justify subjecting a patient to the increased potential risks of DS without demonstrating any significant advantage of DS over RYGB.
The University of Chicago Center for the Surgical Treatment of Obesity is a multidisciplinary program consisting of surgeons, dieticians, and psychologists that has been performing bariatric surgery since 1992. RYGB was the primary procedure performed prior to adding the DS to our program in August 2002, with our first totally laparoscopic DS performed in November 2002. The first laparoscopic adjustable gastric band placed at our institution was in March 2005. We herein report a comparison of the weight loss outcomes between DS and RYGB in super-obese patients during a 3-year time period.
METHODS
We conducted a retrospective review of an Institutional Review Board-approved, prospectively maintained database containing the demographic and anthropomorphic data of patients undergoing RYGB, biliopancreatic diversion with DS, and laparoscopic adjustable gastric banding (LAGB) between August 5, 2002 and November 10, 2005. The initial date was chosen as it corresponds to the first DS performed at our institution. Patients underwent extensive multidisciplinary preoperative evaluation by a board-certified surgeon (V.N.P. or J.C.A.), dietician, and psychologist, and were found to be appropriate candidates for bariatric surgery based on current NIH criteria [severe obesity (BMI ≥40 kg/m2 or 35–40 kg/m2 with significant obesity-related comorbidities), history of multiple previous nonsurgical weight loss attempts, adequate comprehension and support, and absence of active substance abuse or poorly controlled psychologic disorders].38 Eligibility for inclusion in this study included all consecutive patients undergoing standardized primary RYGB or DS with a preoperative BMI ≥50 kg/m2. Patients who had previous bariatric procedures or who underwent staged bariatric operations were excluded. Patients undergoing LAGB were excluded as the length of follow-up was insufficient.
Procedure Selection
The relative advantages and disadvantages of the procedures were extensively discussed with the patient by the surgeon, and a general recommendation was made based on the severity of obesity, comorbidities present, and the patient's preference. While specific mention was made of the potential advantage of the DS with regards to weight loss in super-obese patients, the final decision with regards to the procedure performed was made by the patient. In many instances, because the patient's insurance would not cover the DS, patients elected to proceed with the RYGB rather than attempt to appeal the decision of the insurance company, despite the patient's preference for DS. The patient's primary care physician was notified in writing regarding the decision by the bariatric surgery team and the patient. Any necessary preoperative testing or treatments were performed. Mandatory preoperative weight loss or special diet was not routinely required.
Surgical Technique
All patients received mechanical bowel preparation and 3 doses of perioperative antibiotics. For deep venous thrombosis prophylaxis, sequential pneumatic compression devices were placed on both lower extremities and intravenous heparin was initiated at 500 to 750 units/hr and continued until subcutaneous enoxaparin was initiated the following morning. Beginning in October 2004, selective prophylactic placement of a retrievable percutaneous inferior vena cava filter was performed by a vascular surgeon for patients with BMI >60 kg/m2, history of thromboembolic phenomenon, or severe lack of mobility, usually immediately prior to the bariatric procedure. Patients were placed in the supine position with both arms abducted and padded appropriately. A total of 346 of 350 operations (98.9%) were attempted and 318 (91.9%) successfully completed laparoscopically. Procedures were typically performed by an attending surgeon and senior surgical resident with a medical student operating the laparoscopic camera.
RYGB was performed in 152 super-obese individuals. All cases were initially started laparoscopically. A 15- to 30-mL proximal gastric pouch was created using several firings of the endoGIA 60–4.8 staplers (Autosuture, Norwalk, CT) with buttressing material. Early in the series, bovine pericardial strips were used, while more recently, a bioabsorbable poly(glycolide-trimethylene carbonate) copolymer (SeamGuard Bioabsorbable, W. L. Gore and Assoc., Flagstaff AZ) has been our preference for all gastric, jejunal, and ileal transections. Roux limb construction was performed by dividing the proximal jejunum 40 to 50 cm distal to the ligament of Treitz and creating a 100- to 150-cm Roux limb. While our preferred Roux limb length was 150 cm, 27 patients (17.9%) had shorter (100 cm) Roux limbs created so as to allow the procedure to be covered by the patient's insurer. The technique for gastrojejunostomy was based on surgeon preference and included linear stapled and circular stapled techniques. The jejunojejunostomy was created in a side-to-side functional end-to-side linear stapled manner. Mesenteric defects were routinely closed with permanent suture.
DS was performed in 198 super-obese individuals. The procedure was initiated laparoscopically in 194 cases. One surgeon used a hand-assisted laparoscopic approach while the other preferred a totally laparoscopic approach. Lateral gastrectomy beginning 6 cm proximal to the pylorus extending to the upper fundus was performed using 5 to 8 sequential firings of the linear 60–4.8 stapler with buttressing material with a transorally passed 50–60 F Bougie dilator in place to calibrate the diameter of the gastric sleeve. The duodenum was transected 2 to 5 cm distal to the pylorus using a linear 60–3.5 stapler without buttressing material. A 150 cm alimentary limb and 100 cm common channel were created by dividing the ileum 250cm proximal to the ileocecal junction using a linear 60–2.5 stapler with bioabsorbable buttressing material. The distal (alimentary) limb of ileum was anastomosed to the postpyloric duodenum in an end-to-side manner, with the technique for duodenoileostomy based on surgeon preference [circular-stapled (21 or 25 EEA), linear-stapled, and 2-layer hand-sewn techniques]. The distal anastomosis (ileoileostomy) was performed by approximating the distal biliopancreatic limb 100 cm proximal to the ileocecal junction in a side-to-side functional end-to-side manner using linear (60–2.5) or circular (21 EEA) staplers. Mesenteric defects were routinely closed with nonabsorbable suture.
Intraoperative endoscopy with Roux (RYGB) or alimentary limb (DS) occlusion and air insufflation was used to test the integrity of the staple lines of the gastric reservoir and proximal anastomosis. A single 19 Blake drain was placed near the proximal anastomosis extending up into the left upper quadrant, with removal taking place during the first postoperative visit (8–10 days postoperative). Patients were routinely admitted to the intermediate care unit with telemetry and continuous pulse-oximetry after discharge from the recovery room, and occasionally to the intensive care unit at the discretion of the surgeon and anesthesiologist. Patient-controlled intravenous narcotic analgesia was used for pain control.
Low carbohydrate clear liquids at 30 mL/hr were initiated on the morning of postoperative day 1, and enoxaparin 40 to 100 mg SQ bid was started, titrated to achieve a serum level just below therapeutic. Diet was advanced to pureed foods on postoperative day 2 as tolerated, and patients were discharged after demonstration of diet tolerance and return of bowel function. Enoxaparin was continued for 2 to 3 weeks after discharge.
Follow-up
Patients were seen 1.5 weeks postoperative for drain removal and 2.5 weeks postoperative for diet advancement and initiation of vitamin supplements (prenatal multivitamin, B12, and calcium citrate with vitamin D). Patients were seen by the surgeon and a bariatric dietician at each visit and by psychologists as needed. While the diet contents and progression were identical for both procedures, DS patients were instructed to achieve 75 to 85 g protein intake/day as opposed to 60 to 65 g protein/d for the RYGB patients. Subsequent follow-up appointments took place 1 month, 3 months, 6 months, then yearly, thereafter. Nutritional parameters, including complete blood count, complete metabolic profile, iron studies, folate, B12, intact parathyroid hormone, and vitamins A, E, and D were measured at the 3-month, 6-month, and yearly visit, with supplementation adjusted accordingly. Attempts were made by phone and by mail to contact patients who failed to keep follow-up appointments, moved, or whose insurance was no longer accepted at the University of Chicago Hospitals.
Statistical Analysis
Ideal body weight (IBW) was calculated using the formula IBW = [(2.3 × (height in inches) − 60)) + A) × 2.2], where A is 45.5 for females and 50 for males, with excess body weight (EBW) = measured weight − IBW. Comparison of the demographic data was performed using 2-tailed pooled t tests for continuous data (age, weight, BMI, EBW) except length of stay, for which the Satterthwaite t test was used due to unequal variances. χ2 analysis was used for the comparison of discrete data except when a low number of observations required Fisher exact test. Weights were recorded at the time of clinic visit or telephone conversation, and all recorded weights in the database were manually checked with the patient chart to confirm their accuracy. For purposes of analysis, weights recorded between 4 and 8 months were grouped as “6 months postoperative,” 9 to 15 months as “12 months,” 16 to 20 months as “18 months,” and 21 to 30 months as “24 months.” If more than one weight was available for an individual patient during any of these periods, the latest weight was used and the others excluded. Data were available for 169 of 198 (85.4%), 143 of 186 (76.9%), 72 of 149 (48.3%), 74 of 134 (55.2%), and 38 of 85 (44.7%) of patients in the DS group and 121 of 152 (79.6%), 81 of 140 (57.9%), 29 of 109 (26.6%), 29 of 90 (32.2%), and for 27 of 56 (48.2%) of patients in the RYGB group at 6, 12,18, 24, and 36 months, respectively. Two-sample t tests were used to compare weight loss, BMI, decrease in BMI, and percentage of excess body weight loss (% EBWL) after surgery. χ2 analysis was used to compare the rate of successful weight loss, defined as achieving at least 50% loss of excess body weight, as well as the likelihood of achieving BMI <35 and <30 kg/m2.
RESULTS
A total of 350 super-obese (BMI ≥50 kg/m2) patients underwent DS (n = 198) or RY (n = 152) over a 39-month period with equal 30-day mortality (DS, 1 of 198, 0.51%; RY, 0 of 133, P = not significant). Demographics of the 2 groups are shown in Table 1. Mean age and gender were similar in both groups, while mean preoperative weight (368.2 vs. 346.3 lb, P = 0.0002) and BMI (58.8 vs. 56.4 kg/m2, P = 0.0014) were significantly greater in the DS group compared with the RY group. A total of 346 of 350 cases were attempted laparoscopically, with 91.8% of DS and 92.1% of RYGB successfully completed laparoscopically (P = not significant). All RYGB procedures were attempted laparoscopically, while 4 DS operations (2.0%) were performed using the open technique without initial laparoscopy for the following reasons: loss of abdominal domain, extreme obesity (BMI, 96 kg/m2), severe abdominal distention following difficult intubation, and extensive previous open upper abdominal operations. Mean length of stay was 1 day longer for DS than RYGB (4.86 vs. 3.83 days, P < 0.030), although equal proportions of patients in both groups required hospitalization for greater than 4 days (24.2% vs. 19.7%, P = 0.3154). There was one 90-day mortality in the DS group (0.5%) presumed to be due pulmonary embolism 3 days after being discharged on postoperative day 3. There were no mortalities in the RYGB group. Sixty-day reoperation rates were similar for both the DS and RYGB groups when including and excluding endoscopic dilation (9.6% vs. 11.2%, P = 0.183; and 4.0% vs. 5.3%, P = 0.587, respectively).
TABLE 1. Patient Characteristics
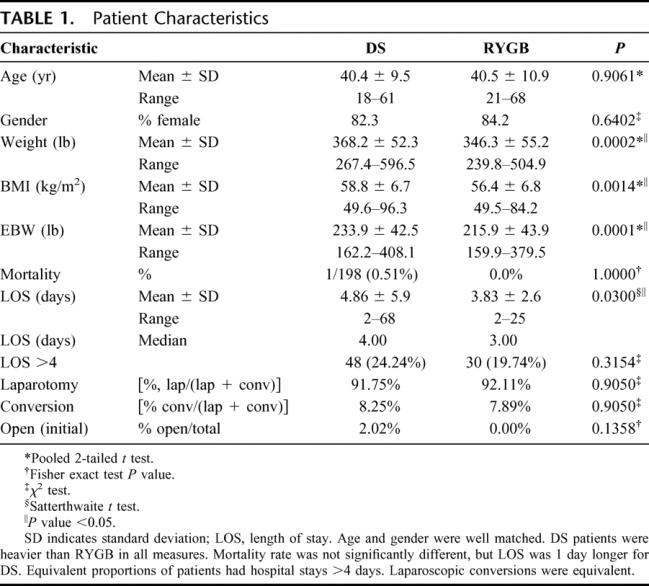
The mean absolute weight loss and percentage of excess body weight loss (% EBWL), shown in Figures 1 and 2, respectively, was significantly greater for DS compared with RYGB at all time points. Mean BMI (Fig. 3), while greater in the DS than the RYGB group initially, was less than the RYGB at 36 months (33.6 ± 6.8 vs. 37.2 ± 7.48 kg/m2, P = 0.05). Similarly, there was a greater change in BMI in the DS than the RYGB group at each time interval (Fig. 4).
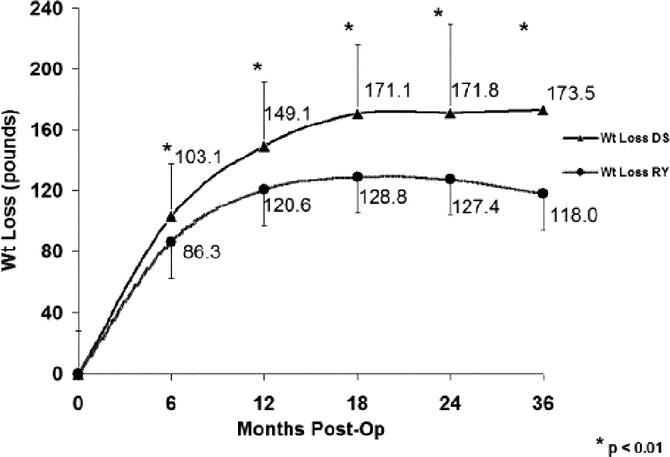
FIGURE 1. Weight loss following DS is greater than RYGB. DS, duodenal switch; RY, Roux-en-Y gastric bypass. Two-sample t tests were used for comparison. *P < 0.01.
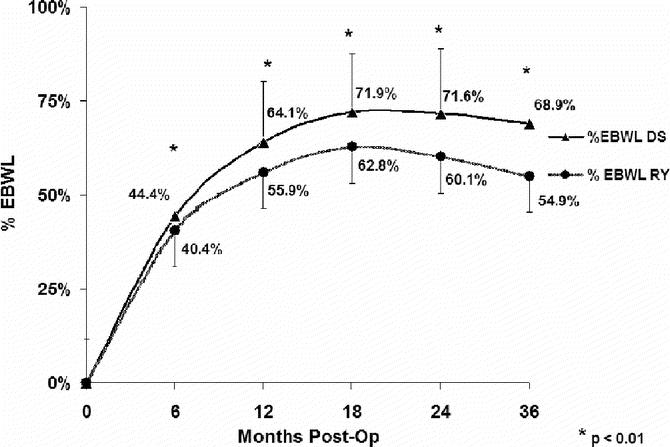
FIGURE 2. Excess body weight loss following DS is greater than RYGB. DS, duodenal switch; RY, Roux-en-Y gastric bypass. EBWL is expressed as a percentage; EBWL is calculated as: weight lost/(initial weight − ideal body weight). DS, duodenal switch; RY, Roux-en-Y gastric bypass. Two-sample t tests were used for comparison. *P < 0.01.
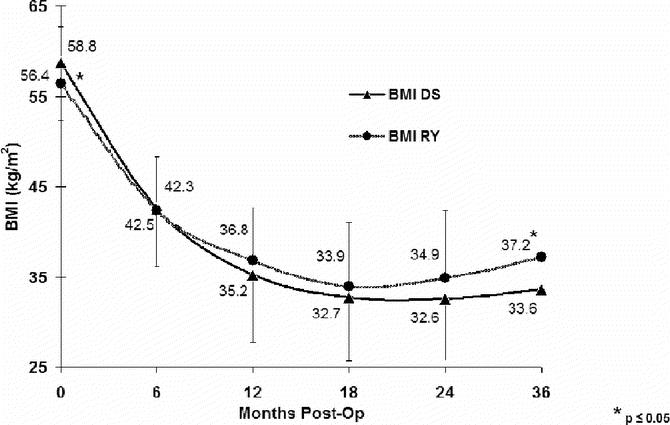
FIGURE 3. Comparison of body mass index (BMI) during follow-up. DS, duodenal switch; RY, Roux-en-Y gastric bypass. By 3 years after surgery, despite having an initially greater BMI, the mean BMI was significantly less in the DS group. Two-sample t tests were used for comparison. *P < 0.05.
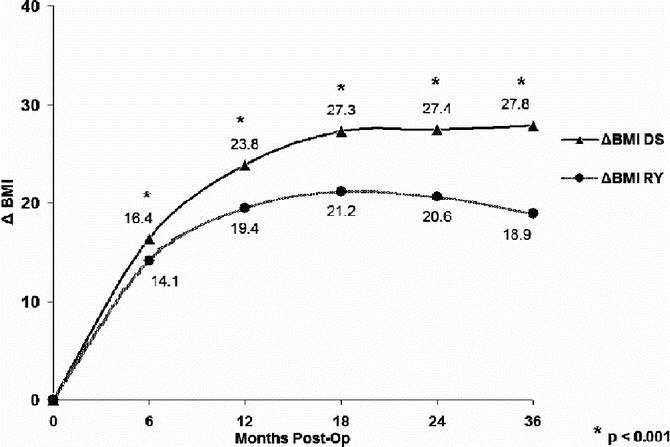
FIGURE 4. Comparison of changes in body mass index (BMI) over time. DS, duodenal switch; RY, Roux-en-Y gastric bypass. Two-sample t tests were used for comparison. *P < 0.01.
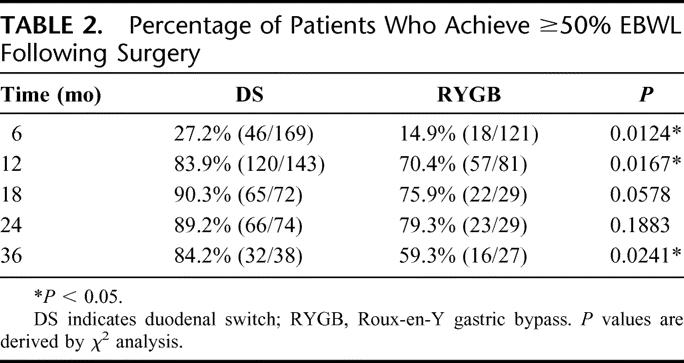
In addition to comparing the mean % EBWL after surgery, we also examined the likelihood that an individual achieved “success” as defined by % EBWL ≥50% (Table 2). At 1 year after surgery, 83.9% of DS patients versus 70% of RYGB patients (P = 0.0167) had lost at least 50% of their excess body weight. At 3 years after surgery, this proportion remained constant for the DS group (84.2%), while only 59.3% of RYGB patients maintained ≥50% EBWL (P = 0.0241).
TABLE 2. Percentage of Patients Who Achieve ≥50% EBWL Following Surgery
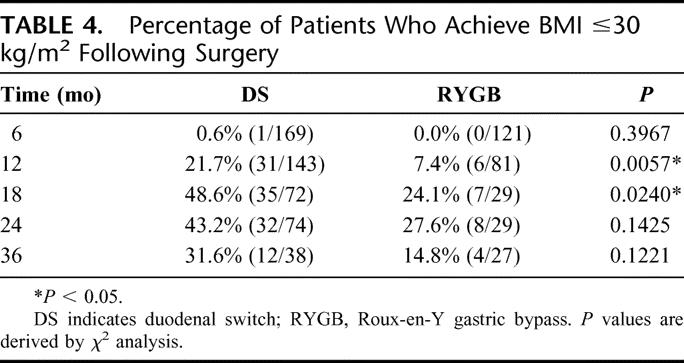
In a similar fashion, we examined the likelihood that an individual would no longer be a potential candidate for weight loss surgery (BMI ≤35 kg/m2, Table 3) as well as the likelihood that the individual would no longer be categorized as obese (BMI ≤30 kg/m2, Table 4). In each case, the likelihood appeared to be greater following DS, but at 2 years after surgery, the differences between the 2 groups were not statistically significant.
TABLE 3. Percentage of Patients Who Achieve BMI ≤35 kg/m2 Following Surgery
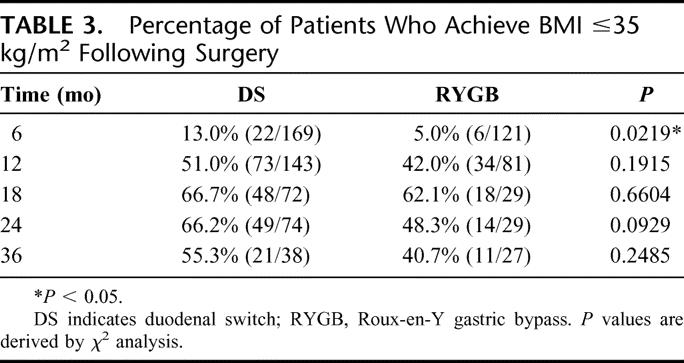
TABLE 4. Percentage of Patients Who Achieve BMI ≤30 kg/m2 Following Surgery
DISCUSSION
Given the exponential increase in the prevalence of super-obesity within the population of patients who may be potential candidates for bariatric surgery, determining the “best” surgical treatment of super-obesity is an important task facing the bariatric surgical community. The optimal procedure should have acceptably low morbidity and mortality rates, result in significant and durable weight loss, and lead to improvement or resolution of obesity-related comorbidities as well as quality of life.
The main focus of this report is the comparison of weight loss outcomes following DS and RYGB. We demonstrate that DS provides a significant advantage over RYGB when comparing weight loss, percentage of excess body weight lost, decrease in BMI, and likelihood of achieving at least 50% excess body weight loss. Some of the increased absolute weight loss observed with DS can be attributed to the higher preoperative weight of the DS group: several series have demonstrated that heavier patients tend to lose more absolute weight but a smaller % EBWL following surgery.15,22,24 In contrast, our observation that the % EBWL was greater for the DS than RYGB at all time points, however, suggests that the difference seen in weight loss is due to greater weight-loss efficacy of DS itself, rather than the greater preoperative weight of the DS group. This finding was quite different from the findings by Deveney et al who failed to demonstrate any significant differences in percentage EBWL between the 2 operations.39 That series included patients who were both severely and super-obese, which may have obscured the relatively greater differences seen between the operations in the super-obese group. Furthermore, we speculate that the exponential model used by the authors to generate the curves used for the comparison of EBWL may have resulted in a type II error, although this is not possible to confirm given the absence of recorded measurements of actual and excess body weight within the manuscript.
An alternative explanation of our findings is that, rather than demonstrating superiority of weight loss following DS, we are seeing inferior weight loss outcomes in our RYGB group. In contrast to the DS group, in which all individuals had a 100-cm common channel and 250-cm alimentary limb, there was some heterogeneity in Roux limb length in the RYGB group, with 27 of 152 patients (17.9%) having a Roux limb length of approximately 100 cm. This was necessary for individuals having surgery before January 2005, as many insurers considered a Roux limb length of greater than 100 cm to be “malabsorptive” and hence “investigational” based on the definition of the Current Procedural Terminology (CPT) code used at that time.40 Since then, the definition has been changed so as to include Roux limb lengths up to 150 cm. Importantly, in at least 2 prospective randomized trials, a short-term weight-loss benefit provided by a longer Roux limb was not sustained at 2 years after surgery.17,23 Furthermore, the EBWL and BMI changes seen in our super-obese RYGB group are comparable to the results described elsewhere in the literature.5
In addition to providing good weight loss, both operations appear to be reasonably safe with low 30-day mortality rates and equivalent and reasonable reoperation rates, particularly considering that this series includes our initial experience with the DS. Both operations can effectively be performed laparoscopically with acceptable conversion rates. Because our focus was on weight loss, we did not compare specific complications in this study. Nonetheless, the need for extended postoperative length of stay (>4 days), which may indicate the presence of a significant postoperative complication, was equivalent in both groups in our study. Parikh et al showed increased perioperative morbidity with BPD compared with RYGB (16.3% vs. 11.3%, P = 0.02) in their 4-year study of 332 super-obese patients; only 43 BPD patients were included in this review, however, and there was no comment with regards to the EBWL comparison between BPD and DS.41
Because the selection of the procedure performed was not randomized, a significant limitation of this study is the inherent presence of selection bias. We did generally recommend DS for all super-obese patients, particularly if their BMI was ≥60 kg/m2 (n = 109). Of the patients who ultimately underwent RYGB, about half did so because their insurer considered the DS to be “investigational” and they did not want to initiate a lengthy appeals process; about half did so because the DS was “too radical” or because an acquaintance or family member had a good outcome with RYGB. DS was not recommended in a few instances when patients had frequent or loose stools at baseline. Despite the lack of randomization, patient age and gender distribution did appear to be closely matched. Additionally, while there were variations in surgical technique with regards to the method of access and creation of anastomoses, the measured lengths used for small intestinal reconstruction were standardized to the extent possible. Finally, with the exception of a slightly greater daily protein requirement for DS patients, the perioperative management and follow-up regimen were purposefully kept the same for both procedures in an effort to minimize the influence that differences in postoperative care may have had on outcomes.
The loss of patients to follow-up is another factor that limits the quality of our data. While the rate of follow-up 1 year after DS and RYGB was 80% and 60%, respectively, at 3 years, the follow-up was about 50% for both groups. This disappointing follow-up may have limited our ability to assess the likelihood of reaching a BMI of less than 35 or 30 kg/m2 2 to 3 years after surgery. While our follow-up rate is less than the 80% to 99% follow-up obtained in studies performed by groups in the Canadian heathcare system.19,42 they are comparable to those seen in many American series.
Finally, it is important to recognize that BMI is a “blunt” instrument of measurement in that, while it provides a reasonable estimate of excess adiposity relative to height, it does not provide information regarding adipose tissue distribution or its metabolic consequences. As such, while super-obesity is associated with significant excess body weight, it is not in and of itself necessarily predictive of the technical difficulty that one might encounter in the operating room or the severity of the patient's comorbidities.
Safely obtained significant and sustained excess weight loss is the key goal of surgical treatment of super-obesity. Our direct comparison of DS to RYGB demonstrates that the duodenal switch provides superior weight loss in the super-obese compared with gastric bypass. Further study and follow-up will be needed to confirm and extend the present findings, including a comparison of nutritional outcomes, resolution of comorbidities, and quality of life, such that an evidence-based rationale for procedure selection can be developed.
Discussions
Dr. Bruce M. Wolfe (Portland, Oregon): I commend Dr. Prachand and his colleagues for an excellent report and presentation. Clearly, the immediate goal of bariatric surgery is to accomplish weight loss, improve or resolve comorbidities, restore quality of life and extend longevity. The correlation of these outcomes with weight loss has been basically quite good. So it is appropriate, as you have done today, to focus on weight loss. It is also well established that there is variable weight loss following gastric bypass.
What is not so clear is just how much weight loss does an individual patient or patient population actually need in order to achieve the goals of comorbidity resolution and extended longevity? For example, the case you showed of a BMI of 64, does that patient really need to lose all the way down to a BMI of 25 or 30 in order to have a maximum benefit? More specifically, just how much additional benefit is achieved with a 69% excess weight loss at 3 years compared to 55%, as you report today? Most authors have reported a higher perioperative complication and mortality rate, although in your series that was not seen.
More concerning in my view is the potential for long-term metabolic or nutritional deficiencies. We know from intestinal bypass days as well as treating short bowel patients that as long as fat malabsorption is present there will necessarily also be malabsorption of calcium, magnesium and fat soluble volumes. Ren in New York and Fielding in Brisbane, Australia, have combined their series at 4 years and now report deficiencies of vitamins A, D and K, and hypersecretion of parathormone to maintain plasma calcium in more than 60% of patients, even though a rigorous replacement regime similar to what you recommend was reportedly adhered to. We have also seen renal failure due to oxalate deposition, another complication of malabsorption syndromes.
So my first question is, have you seen metabolic complications as the patients get farther out from your operation? And how are we going to resolve this difficult question of how much additional risk should a patient and their surgeon accept in order to achieve the level or degree of weight loss advantage that you report today?
Dr. Vivek N. Prachand (Chicago, Illinois): Thank you for your kind comments, Dr. Wolfe. We are in agreement with you regarding the concerns that you raise. Let me attempt, however, to address the second question first with regards to how much weight loss is enough and how do you weigh the potential risks and benefits of achieving greater weight loss. Certainly, from the standpoint of improvement of co-morbidities, only a relatively modest amount of weight loss is necessary to accomplish this, on the order to 10% to 20%. It is clear that both operations achieve this amount of weight loss very effectively.
Your question as to whether or not 64% versus 72% matters is a more difficult one to answer. Part of the rationale for performing bariatric surgery is the observation that individuals with higher BMI have an increased relative risk of premature mortality. Unfortunately, there is no direct evidence demonstrating the normalization of this risk following reduction of BMI after weight-loss surgery. There is some reasonable indirect evidence, however, suggesting that this might indeed be the case. Christou and colleagues recently compared mortality rates in 2 well-matched groups of severely obese patients, one of whom had bariatric surgery versus one managed medically which showed a 90% difference in mortality rate in favor of the bariatric surgery group, and data from Washington state suggested that there was a 33% reduction in mortality rate after one year after surgery. Neither study attempted to correlate the magnitude of weight loss with the change in mortality rate.
So, to get back to your question as to how much weight loss is enough, I don't think that we really know the scientific, evidence-based answer. There is another, perhaps more philosophical way, of framing the question. As surgeons, when we operate for GI bleeding or bowel obstruction, at the end of the operation, the bleeding should be controlled or the bowel obstruction relieved. In other words, we strive to eliminate the criteria for which the operation was performed in the first place. In a similar fashion, if we agree that it is appropriate to use BMI as a measure of the severity of obesity to help decide whether to perform bariatric surgery, it seems appropriate to expect that the patient should lose enough weight such that their BMI is no longer at a level that would potentially make that patient a candidate for bariatric surgery. Admittedly, this is more difficult to achieve in super-obese patients – hence, the potential role for the duodenal switch in this patient population.
Dr. Henry Buchwald (Minneapolis, Minnesota): I would like to thank Dr. Prachand for sending the manuscript ahead of time and I thank the authors for an excellent contribution to the medical literature. I have 3 brief comments and 2 questions.
Thank you for choosing to cite our meta-analysis of the effects of bariatric surgery. I would like to give some additional numbers from that meta-analysis. In this study, encompassing the entire world literature in bariatric surgery, published in JAMA in 2004, the percentage excess weight loss comparing gastric bypass and biliopancreatic diversion/duodenal switch was 62% versus 70%. The less than 30-day mortality was 0.5% versus 1.1%. But most interestingly, for resolution of diabetes the difference was 84% versus 99%, hyperlipidemia 97% versus 99%, hypertension 68% versus 83%, and obstructive sleep apnea 80% versus 92%. So in all categories of co-morbidities analyzed, the duodenal switch had an edge over the gastric bypass.
In our own series, and we just recently reviewed 105 consecutive cases of duodenal switch operations, we had 0% mortality, our complication rate was fairly minor, and there was no real difference in morbidity between patients with a BMI less than 50 and those with a BMI over 50. It has been predicted that there would be a convergence of operations towards the gastric bypass, which sits in the middle of the spectrum of difficulty for bariatric procedures. I think what we are really seeing in this country today, as well as worldwide, is a divergence from the center toward both poles, with more people seeking the lap band, the simplest operation, and the biliopancreatic diversion/duodenal switch, the most difficult operation.
With respect to my 2 questions: Putting aside insurance carriers, and other factors, would you recommend to all patients with a BMI over 50 that they should have a duodenal switch? Would you recommend this to all patients with a BMI over 40?
Dr. Vivek N. Prachand (Chicago, Illinois): Thank you, Dr. Buchwald, for examining our manuscript as well as your kind comments. With regards to whether we would recommend a duodenal switch for all patients with a BMI over 50, that is a difficult question to answer. I routinely tell my super-obese patients that the likelihood of achieving successful weight loss is greater with the duodenal switch compared to the gastric bypass, but that there is a greater potential for the development of nutritional complications if compliance to postoperative diet, vitamin supplementation, and follow-up is not adequate. Having said that, from my standpoint, given the similarity in morbidity and mortality rates that we have observed when comparing duodenal switch and gastric bypass, successful weight loss is the most important factor when making a recommendation regarding procedure selection. Nonetheless, we do see super-obese patients who are adamant about having a LapBand placed because of its perceived advantage with regards to safety despite the knowledge that the success rate is not going to be as high. In the end, each of these procedures is a tool that the patient needs to feel comfortable using, and as such, while we do give a specific recommendation based on the severity of the obesity and the nature of concomitant comorbidities, it is really up to the patient to make the final decision.
In regards to your second question, we have been rather hesitant to recommend duodenal switch for people with BMI of under 50: the weight loss achieved with gastric bypass in this patient group is generally very good, and there is probably a higher risk of excessive weight loss with the duodenal switch in this group. There is not a single “ideal” bariatric procedure that can be applied to all severely obese patients, and I do not claim that the duodenal switch is the be-all, end-all bariatric operation. As such, it is critical that we try to come up with a rational approach to procedure selection, and with this in mind, we feel that it is reasonable to start by directly comparing duodenal switch and gastric bypass, first for patients with a BMI of greater than 50, and then, after accumulating that knowledge, we can take a look and see whether it is something to be considered in the lower BMI range.
Dr. Clifford W. Deveney (Portland, Oregon): I have 2 questions I want to focus on the technical aspects of the duodenal switch particularly done laparoscopically.
I think that you can tweak this operation to produce greater or lesser weight loss. And one way you can do that is in constructing the sleeve gastrectomy. If you are very severe in the making of your gastric tube, you essentially are adding a fairly large restrictive component to the operation as well as the malabsorptive approach. So I would like you to comment on how you made your tube. I notice you use a dilator. Do you really have the dilator with your stapler or do you give it some leeway when you are making this tube.
The other factor is the length of the common channel in the elementary limb. And certainly in the duodenal switch I think these lengths are critical. Can you tell us how you measure these lengths when you do it laparoscopically?
Dr. Vivek N. Prachand (Chicago, Illinois): Dr. Deveney, we generally use a 60 French Maloney dilator oriented along the lesser curve, and we do not hug it very tightly. We routinely use buttressing material along the entire staple line.
With regards to the measurement of the common channel, while other groups have described a common channel length between 50 and 100 cm, we selected 100 centimeters as our routine common channel length, erring on the side of perhaps inadequate weight loss in an effort to reduce the development of nutritional deficiencies.
With regards to the measurement itself, we do the measurements under medium stretch using the tips of the instruments as a means to measure. Having said that, the fact is that if you were to take measurements of the small bowel 5 times consecutively in the same patient you will probably have at least a 10% variation each time you do the measurements. In the end, the measurement of limb lengths is in fact a proxy for measurement of the total surface area of that bowel, and given the microstructural contributions of the villi, brush border, and so forth, length is really a very rough an inexact proxy at that. As such, I believe that being consistent with the technique of measurement and careful follow-up of patients is probably more important than the specific technique used.
Footnotes
Reprints: Vivek N. Prachand, MD, University of Chicago, 5841 S. Maryland Ave., MC 5036, Chicago, IL 60637. E-mail: vprachan@surgery.bsd.uchicago.edu.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. [DOI] [PubMed] [Google Scholar]
- 3.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health Consensus Development Panel. Gastrointestinal surgery for severe obesity. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 5.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 6.Clegg AJ, Colquitt J, Sidhu MK, et al. The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation. Health Technol Assess. 2002;6:1–153. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen NT, Root J, Zainabadi K, et al. Accelerated growth of bariatric surgery with the introduction of minimally invasive surgery. Arch Surg. 2005;140:1198–202; discussion 1203. [DOI] [PubMed]
- 8.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157–1164. [DOI] [PubMed] [Google Scholar]
- 10.Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003;163:2146–2148. [DOI] [PubMed] [Google Scholar]
- 11.Mason EE, Doherty C, Maher JW, et al. Super obesity and gastric reduction procedures. Gastroenterol Clin North Am. 1987;6:495–502. [PubMed] [Google Scholar]
- 12.Kral JG. Morbidity of severe obesity. Surg Clin North Am. 2001;81:1039–1061. [DOI] [PubMed] [Google Scholar]
- 13.Fazylov RM, Savel RH, Horovitz JH, et al. Association of super-super-obesity and male gender with elevated mortality in patients undergoing the duodenal switch procedure. Obesity Surg. 2005;15:618–623. [DOI] [PubMed] [Google Scholar]
- 14.Schauer P, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artuso D, Wayne M, Kaul A, et al. Extremely high body mass index is not a contraindication to laparoscopic gastric bypass. Obes Surg. 2004;6:750–754. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 17.Brolin RE, Kenler HA, Gorman JG, et al. Long-limb gastric bypass in the superobese: a prospective randomized study. Ann Surg. 1992;215:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloosmston M, Zervos EE, Camps MA, et al. Outcome following bariatric surgery in super versus morbidly obese patients: Does weight matter? Obes Surg. 1997;7:414–419. [DOI] [PubMed] [Google Scholar]
- 19.MacLean LD, Rhode B, Nohn CW. Late outcome of isolated gastric bypass. Ann Surg. 2000;231:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murr MM, Balsiger BM, Kennedy FP, et al. Malabsorptive procedures for severe obesity: comparison of pancreaticobiliary bypass and very, very long limb Roux-en-Y gastric bypass. J Gastrointest Surg. 1999;3:607–612. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen NT, Ho HS, Palmer LS, et al. Laparoscopic Roux-en-Y gastric bypass for super/super obesity. Obes Surg. 1999;9:403–406. [DOI] [PubMed] [Google Scholar]
- 22.Farkas DT, Vemulapalli P, Haider A, et al. Laparoscopic Roux-en-Y gastric bypass is safe and effective in patients with a BMI ≥60. Obes Surg. 2005;15:486–493. [DOI] [PubMed] [Google Scholar]
- 23.Choban PS, Flancbaum LJ. The effect of Roux limb lengths on outcome after Roux-en-Y gastric bypass: a prospective randomized clinical trial. Obes Surg. 2002;12:540–545. [DOI] [PubMed] [Google Scholar]
- 24.Brolin RE, LaMarca LB, Kenler HA, et al. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195–205. [DOI] [PubMed] [Google Scholar]
- 25.Sugerman JH, Kellum JM, DeMaria EJ. Conversion of proximal to distal gastric bypass for failed gastric bypass for superobesity. J Gastrointest Surg. 1997;1:517–525. [DOI] [PubMed] [Google Scholar]
- 26.Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8:267–282. [DOI] [PubMed] [Google Scholar]
- 27.Marceau P, Biron S, Bourque RA, et al. Biliopancreatic diversion with a new type of gastrectomy. Obes Surg. 1993;3:29–35. [DOI] [PubMed] [Google Scholar]
- 28.Marceau P, Hould FS, Simard S, et al. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947–954. [DOI] [PubMed] [Google Scholar]
- 29.DeMeester TR, Fuchs KH, Ball CS, et al. Experimental and clinical results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg. 1987;206:414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scopinaro N, Gianetta E, Civalleri D, et al. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66:618–620. [DOI] [PubMed] [Google Scholar]
- 31.Dolan K, Hatzifotis M, Newbury L, et al. A clinical and nutritional comparison of biliopancreatic diversion with and without duodenal switch. Ann Surg. 2004;240:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlin AM, Rao DS, Meslemani AM, et al. Prevalence of vitamin D depletion among morbidly obese patients seeking gastric bypass surgery. Surg Obes Relat Dis. 2006;2:98–103. [DOI] [PubMed] [Google Scholar]
- 33.Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55. [DOI] [PubMed] [Google Scholar]
- 34.DePrisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61. [DOI] [PubMed] [Google Scholar]
- 35.Bloomberg RD, Fleishman A, Nalle JE, et al. Nutritional deficiencies following bariatric surgery: what have we learned. Obes Surg. 2005;15:145–154. [DOI] [PubMed] [Google Scholar]
- 36.Anthone GJ, Lord RV, DeMeester TR, et al. The duodenal switch operation for the treatment of morbid obesity. Ann Surg. 2003;238:618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hess DS, Hess DW, Oakley RS. The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg. 2005;15:408–416. [DOI] [PubMed] [Google Scholar]
- 38.National Institutes of Health Consensus Development Panel. Gastrointestinal surgery for severe obesity. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 39.Deveney CW, MacCabee D, Marlink K, et al. Roux-en-Y divided gastric bypass results in the same weight loss as duodenal switch for morbid obesity. Am J Surg. 2004;187:655–659. [DOI] [PubMed] [Google Scholar]
- 40.Current Procedure Terminology 2004. Chicago: AMA Press, 2004. [Google Scholar]
- 41.Parikh MS, Shen R, Weiner M, et al. Laparoscopic bariatric surgery in super-obese patients (BMI >50) is safe and effective: a review of 332 patients. Obes Surg. 2005;15:858–863. [DOI] [PubMed] [Google Scholar]
- 42.Biron S, Hould FS, Lebel S, et al. Twenty years of biliopancreatic diversion: what is the goal of the surgery? Obes Surg. 2004;14:160–164. [DOI] [PubMed] [Google Scholar]


