Abstract
Objective:
To examine the association between biochemical markers of brain injury (MBI) and the inflammatory response in relation to neurocognitive deficiency (NCD) after cardiopulmonary bypass (CPB).
Summary Background Data:
In cardiac surgery, NCD is a common but underdiagnosed complication with an unclear pathophysiology leading to significant morbidity. Despite extensive investigation, identification of a MBI for clinical use and clarifying the pathophysiology of NCD have not been achieved.
Methods:
Forty patients undergoing CABG and/or valve procedures using CPB were administered a validated neurocognitive battery preoperatively and postoperatively at day 4 and 3 months. S-100b, neuron specific enolase (NSE), and tau protein were assayed as MBIs preoperatively and postoperatively at 6 hours and day 4. C-reactive protein (CRP), interleukin (IL)-6, C3a, and total peroxide levels were also quantified from serum. Impact of cardiotomy suction and antifibrinolytics on markers of brain injury was assessed.
Results:
The incidence of early NCD was 40% (16 of 40). NSE and tau protein at the 6-hour time point were both significantly elevated in the presence of NCD (NCD group) compared with those without NCD (NORM group) (8.69 ± 0.82 vs. 5.98 ± 0.61; P = 0.018 and 68.8 vs. 29.2%; P = 0.015; respectively). S-100b increase was not different between the NCD and NORM groups. Cardiotomy suction significantly elevated S-100b levels, whereas NSE and tau were not significantly influenced. Aprotinin did not have an effect on NCD or levels of MBIs. Also, the NCD group had significantly elevated CRP and peroxide levels compared with the NORM group at postoperative day 4 while C3a was significantly elevated at 6 hours.
Conclusion:
NSE and tau are better associated with NCD and less influenced by cardiotomy suction compared with S-100β. Inflammatory and oxidative stress is associated with NCD post-CPB.
Neurocognitive decline (NCD) following cardiac surgery is a common and widely underdiagnosed complication due to unclear pathophysiology. Biochemical markers of brain injury studied revealed that neuron-specific enolase and tau are better predictors of NCD compared with S-100β following cardiopulmonary bypass. Also, the association between inflammatory response and NCD was explored.
Brain injury following cardiopulmonary bypass (CPB) remains a common and serious complication that is often misdiagnosed.1,2 The American College of Cardiology/American Heart Association guidelines for coronary artery bypass graft (CABG) surgery divide postoperative neurologic deficits into 2 categories.3 Type 1 deficits include major focal neurologic events, stupor and coma. Type 2 deficits describe more global cognitive deficits such as deterioration in intellectual function, memory and confusion without evidence of focal injury. Type 1 deficits are usually caused by identifiable sources of cerebral hypoxia due to intraoperative hypoperfusion or embolic phenomena. In contrast, the etiology of type 2 deficits is unclear and likely multifactorial; where factors such as hypoxia, time on CPB, age, type of procedure, preoperative creatinine levels, and perioperative inflammatory response have been implicated in its pathophysiology.4 While physical examination and neuroimaging modalities have proven valuable for the detection and treatment of acute focal brain damage postoperatively, mild and diffuse injuries such as neurocognitive decline (NCD) seen in type 2 deficits would benefit from improvements in the early diagnosis and identification of these patients. Neuropsychologic tests, the current gold standard for detecting NCD, are complex and difficult to use routinely in the postoperative setting. Identifying biochemical surrogate markers for brain dysfunction would greatly assist in the diagnosis and timely treatments of patients with this complication. Recent studies have focused on proteins that are expressed predominantly in cerebral cells and released into the cerebrospinal fluid and blood following an acute insult to the brain such as CPB.5–12 One of the most studied potential protein marker of brain injury is S-100β, an astroglial protein that leaks from damaged cells and across the blood-brain barrier. Unfortunately, S-100β has fallen out of favor as a marker of subclinical and cognitive dysfunction post-CPB as its levels will be artificially elevated by retransfused mediastinal blood that is drained by the cardiotomy suction.7,13–16 Neuron specific enolase (NSE), one of the 5 isozymes of the glycolytic enzyme, enolase, is another potential serum marker of brain injury.17 A third marker that has recently been tested in other areas of medicine but has not been studied in cardiac surgery prior to our recent report41 is the microtubule-associated tau protein that is released following axonal injury.9,10
Our main objective for this study was to compare serum markers of brain injury with respect to the strength of their association to post-CPB NCD, cardiotomy suction, and aprotinin. We also studied the association of post-CPB early NCD to markers of acute inflammation.
PATIENTS AND METHODS
Patient Enrollment
We carried out a single-institution, prospective cohort study that was approved by the Beth Israel Deaconess Medical Center Institutional Review Board/Committee on Clinical Investigations. Inclusion criteria included consenting patients scheduled for elective or urgent primary CABG, valvular surgery (aortic or mitral), or a combination of the 2 using CPB. Exclusion criteria included: subjects undergoing aortic arch/root procedures and those with calcified aorta, recent stroke, severe preoperative neurologic deficits, known high-grade carotid stenosis, advanced hepatic disease (cirrhosis), and chronic renal failure (serum creatinine >2.0 mg/dL). Subjects who were unable to complete the baseline neuropsychologic battery due to severe cognitive impairment, psychiatric disease, substance abuse, blindness, or poor English were also excluded.
Anesthetic and Surgical Technique
Procedures were completed by 3 surgeons following the same conventional operative approach at our institution, including induction of general anesthesia, invasive monitoring, midline sternotomy, and systemic heparinization. CPB was initiated via cannulation of the right atrium and ascending aorta with a nonpulsatile system, membrane oxygenator, and a 40-μm arterial filter. Crystalloid pump prime was used. For all patients, mild hypothermic CPB (minimum temperature 32–34°C) with intermittent cold-blood hyperkalemic (25 mmol/L) cardioplegia was used. Continuous patient core temperature measurement was carried out via pulmonary artery catheter. Serum glucose levels were monitored and attempted to keep <130 mg/dL by intermittent intravenous insulin injections or insulin infusion. During CPB, pump blood flow was maintained at 2 to 2.4 L/min/m2 body surface area. Arterial partial oxygen pressure was kept between 150 and 250 mm Hg and alpha-stat pH monitoring was used. Mean blood pressure was maintained at between 50 and 90 mmHg using conventional vasoactive medications. Two of the surgeons exclusively used standard cardiotomy suction for retransfusion of shed mediastinal blood while the third surgeon used the Cell Saver (Hemonetics Corp., Braintree, MA) autologous blood recovery system exclusively. Aminocaproic acid (Amicar) (100 mg/kg load, 5 g pump prime and 30 mg/kg/hr infusion) or half-Hammersmith dose of aprotinin (Bayer Corporation, Westhaven, CT) at 1,000,000 KIU was administered to all patients at the surgeon's discretion.
Blood Sampling and Biochemical Assays
Blood samples were collected from the central venous line preoperatively after induction of anesthesia and before skin incision, 6 hours postoperatively in the cardiovascular intensive care unit, peripherally and on the morning of the fourth postoperative day prior to discharge. Samples were collected into sterile vacuum tubes, immediately centrifuged at 15,000g for 15 minutes, and serum/plasma samples frozen at −80°C until the time of assay at the conclusion of the study.
Serum samples were assayed in duplicate using commercially available high sensitivity immunoassays. Samples were assayed for markers of CNS injury S-100β (SYNX Pharma Inc., Toronto, Ontario, Canada), NSE (ALPCO Diagnostics, Windham, NH), and tau protein (Biosource Inc., Carlsbad, CA). The coefficient of variation for S-100β, NSE, and tau was 5.8%, 4.1%, and 5.2%, respectively.
Inflammatory stress was assessed via the early-phase reactant C reactive protein (CRP) (DSLabs Inc., Webster, TX), interleukin (IL)-6 (R&D Systems, Minneapolis, MN), and activated complement mediator C3a (Quidel Corporation, San Diego, CA). Oxidative stress was studied by quantifying total peroxide levels in serum (OxyStat, ALPCO Diagnostics). All assays were performed as per manufacturer's specifications.
Neurocognitive Assessment
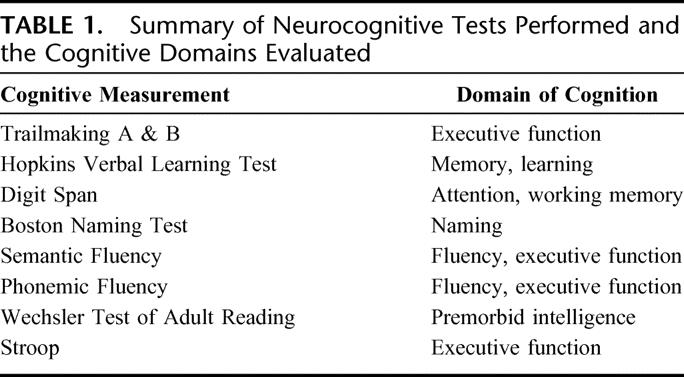
Patients underwent neurocognitive testing with a battery of evaluations preoperatively (1–10 days prior to surgery), then postoperatively prior to discharge at postoperative day 4 to 5 and at 3 months. Prior to baseline neurocognitive assessment, all patients underwent a depression assessment using the Geriatric Depression Scale (positive if score ≥9 of 15). All evaluations were carried out by a trained and dedicated psychometrician who was blinded to serologic testing. The battery was chosen to be consistent with the Statement of Consensus on Neurobehavioral Outcomes after Cardiac Surgery18 and consisted of 8 validated assessments covering memory, executive function, attention, language, and global cognition as summarized in Table 1.
TABLE 1. Summary of Neurocognitive Tests Performed and the Cognitive Domains Evaluated
Data Analysis
Clinical data were expressed as mean ± SD. Following the Consensus Statement on Neurobehavioral Changes Following Cardiac Surgery guidelines, we defined cognitive decline via dichotomous variable, as a 1 SD decline from baseline on 25% of the tasks (2 of 8 measures).18 The χ2 test or Fisher exact test was used to compare proportions, and the Student t test was used to compare continuous variables. All serum test values were described as mean ± standard error of the mean except for tau protein. As in previous recent studies, tau results were used as a dichotomous variable reflecting presence or absence of the protein in serum.10,41 Since the exact pharmacokinetics of tau are not yet known, drawing correlations and conclusions based on the quantitative levels would not have been justified. Repeated measures analysis of variance was used to determine significant rate of change at the time points studied for laboratory data. Multivariate linear regression was used for continuous outcomes, whereas logistic regression was used to analyze for brain injury markers as predictors of NCD while controlling for known possible confounders (age, preoperative creatinine, time on CPB, use of cardiotomy suction, and aprotinin use). Statistical significance was set at P < 0.025 given the large number of comparisons. Software packages used are GraphPad Prism 4 (GraphPad; Prism 4, San Diego, CA) and SPSS (SPSS 11.5 for Windows; SPSS Inc., Chicago, IL).
RESULTS
Patient Characteristics
From 68 patients who were eligible for the study and approached for enrollment/consent during the study period, 24 patients refused enrollment. One patient was approached but subsequently did not consent due to delirium. Forty-three patients provided informed written consent and were enrolled. One patient was subsequently excluded from the analysis because of refusal to complete neuropsychologic assessment prior to discharge (voluntary withdrawal). Another patient had a hemolyzed blood sample that was of insufficient quality for analysis and was excluded. A third patient was excluded as the procedure was converted intraoperatively into an aortic root replacement operation. Thus, 40 patients were included in the analysis.
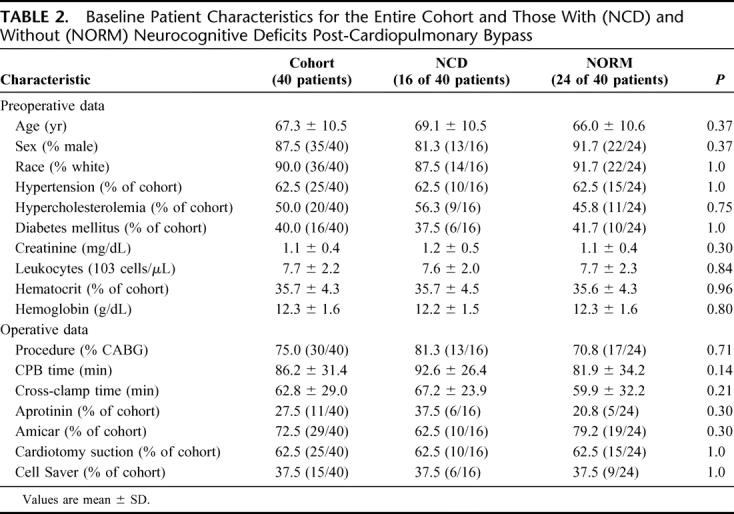
Early NCD rate at postoperative day 4 was 40% (16 of 40). Almost all patients returned to baseline cognitive function at the 3-month time point as the NCD rate was 2.5% (1 of 40). Baseline patient characteristics and key perioperative data were similar between patients who developed NCD (NCD group) at postoperative day 4 compared with those who did not develop NCD (NORM group) and are summarized in Table 2. Patients underwent similar intraoperative course with respect to CPB technique, temperature, anesthesia, and perioperative monitoring. No focal neurologic deficits or stroke occurred in enrolled patients. All patients tested negative for depression preoperatively.
TABLE 2. Baseline Patient Characteristics for the Entire Cohort and Those With (NCD) and Without (NORM) Neurocognitive Deficits Post-Cardiopulmonary Bypass
Serum Markers and Brain Injury
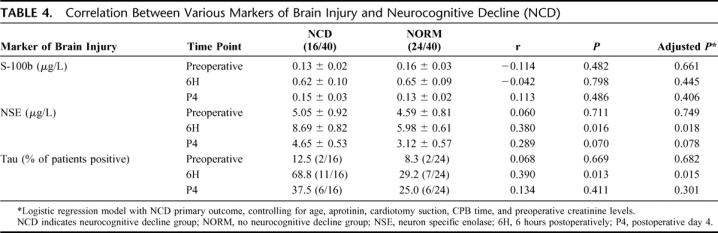
All 3 markers of brain injury studied were effectively increased following CPB when measured at the 6-hour time point. For S-100b and NSE, this rise was transient and returned to baseline when assessed at the postoperative day 4 time point (Table 3). Proportion of patients with positive tau levels remained elevated at the postoperative day 4 time point compared with baseline (30% vs. 10%; P < 0.001) but had started to decrease from the 6-hour level (42.5%). When all 3 markers of brain injury were compared with respect to their relationship to clinical NCD, we found that NSE and tau were both significantly elevated in the NCD group compared with the NORM group at the 6-hour time point. S-100b levels, however, were not significantly associated with NCD (Table 4).
TABLE 3. Brain Injury Markers at Various Time Points
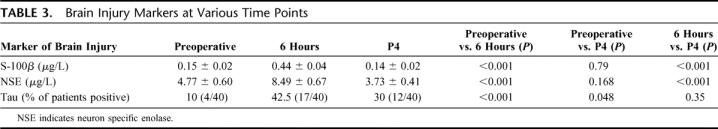
TABLE 4. Correlation Between Various Markers of Brain Injury and Neurocognitive Decline (NCD)
Markers of inflammatory (CRP, IL-6, and C3a) and oxidative response (peroxide) assessed, also showed the characteristic pattern of rise following CPB. CRP levels continued to rise until postoperative day 4, while IL-6, C3a, and peroxide levels rose sharply at the 6-hour time point and then declined at postoperative day 4, although did not return to their preoperative baseline levels (Table 5). While not statistically significant, there was a trend for higher CRP levels in the NCD group compared with the NORM group at 6 hours (Table 6). This trend persisted and reached statistical significance at the postoperative day 4 time point (adjusted P = 0.018). Complement activation (C3a) was significantly higher at 6-hour in the NCD group compared with the NORM group (adjusted P = 0.021), whereas peroxide levels were significantly higher at postoperative day 4 in the NCD group compared with the NORM group. IL-6 levels were not significantly different between the NCD and NORM groups.
TABLE 5. Markers of Inflammatory and Oxidative Stress at Various Time Points
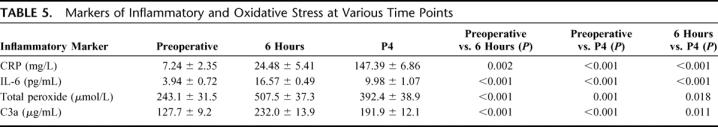
TABLE 6. Correlation Between Inflammatory and Oxidative Stress to Neurocognitive Decline (NCD)
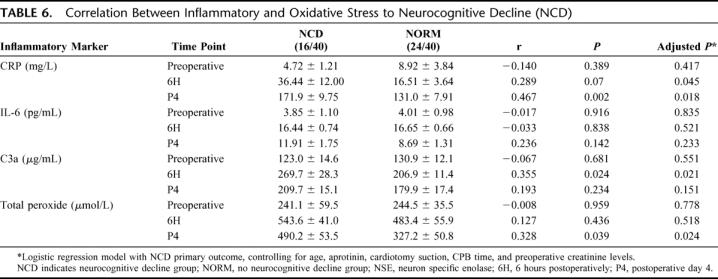
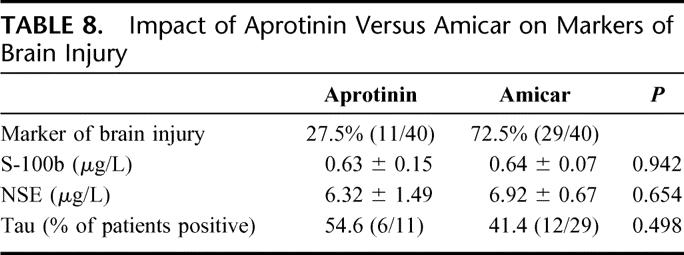
Cardiotomy suction was used in 62.5% (25 of 40) of patients in this cohort. The other 37.5% (15 of 40) underwent CPB with the use of Cell Saver. Comparing serum markers of brain injury studied, patients who underwent CPB with the assistance of cardiotomy suction had significantly elevated S-100b levels at 6 hours compared with Cell Saver (P = 0.013). In contrast, NSE levels and tau presence were not different whether cardiotomy suction or Cell Saver was used in this cohort (Table 7). Half-Hammersmith dose aprotinin was used in 27.5% (11 of 40), whereas Amicar was used in the remaining 72.5% of patients (29 of 40). None of the markers of brain injury studied was influenced by whether patients received aprotinin or Amicar (Table 8).
TABLE 7. Impact of Cardiotomy Suction Versus Cell Saver on Markers of Brain Injury
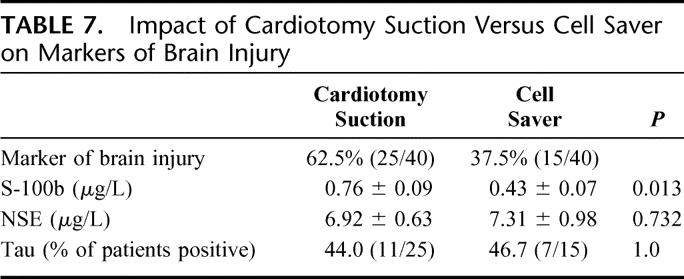
TABLE 8. Impact of Aprotinin Versus Amicar on Markers of Brain Injury
DISCUSSION
As one of the most scrutinized areas of medicine, cardiac surgical outcomes need to be constantly monitored and improved in a systematic and reliable manner to better serve our patients. A major area where the field has not made great progress in improving patient outcomes is brain injury following CPB. While stroke (type 1 deficit) is the most serious and carries an incidence of 1.5% to 5.2%, neurocognitive dysfunction (type 2 neurologic deficit) is arguably the most common complication following cardiac surgery, with a short-term incidence of 33% to 83% as well as a long-term incidence of 20% to 60%.19–21 The main reasons why we have been unsuccessful at improving these incredibly high rates are 2-fold. First, subtle changes in brain function that are manifested by memory and intellectual deficits have not been easy to detect and diagnose in the routine clinical setting postoperatively. Long and impractical questionnaires have been used successfully for research purposes but not clinically. Similarly, expensive and elaborate radiologic tests such as diffusion-weighted magnetic resonance imaging or CT scans22 are usually reserved for patients with clinical signs of focal injury and are of limited benefit for detection of nonfocal or subtle brain injury. While serum markers of injury are readily available for other organs, markers of brain injury (such as S-100β) have been largely unreliable and of limited clinical use post-CPB. The identification of a reliable serum marker of brain injury that can be used at the bedside would greatly improve early detection rates. Newman et al reported that cognitive function at discharge was a significant independent predictor of long-term decline at 5 years postoperatively.21 Second, our lack of clear understanding of the mechanisms leading to the pathophysiology of this complication has been a challenge to prevention and treatment. While the etiology of postoperative NCD is likely multifactorial, we, like others, hypothesize that the perioperative inflammatory response plays an important role in the development of post-CPB brain injury.6,23–30 This study was able to further strengthen this claim by identifying a strong association between the acute perioperative inflammatory response and NCD.
Our data confirm that early NCD remains a common complication following routine cardiac surgery involving CPB and carried an incidence of 40% (16 of 40) at discharge in this study. We also show, however, that this is a transient phenomenon as the rate of NCD 3 months following surgery is significantly lower at 3% (1 of 40). Certainly, this transient sharp decline in cognitive function immediately coincides with the sharp rise in inflammatory stress that patients are subjected to perioperatively and may be due to the its influence on the brain. It is also possible that this significant improvement in cognitive function a few months after the surgery could be influenced by a slight learning effect from repeating the cognitive battery. We speculate, however, that patients’ cognitive and brain function is influenced by the significant rise in the inflammatory and oxidative stress these patients go through during the perioperative and recovery period.
The primary aim of this study was to compare 3 neuronal proteins with respect to the strength of their relationship to clinical symptoms of subtle brain injury, namely NCD. We also explored the impact of intraoperative factors such as cardiotomy suction and use of different antifibrinolytics that may affect the interpretation of these markers. In another aim of this study, the relationship between the magnitude and persistence of post-CPB inflammatory response and the development of NCD clinically was strengthened. Clearly, the small sample size and multiple comparisons made in this study are limitations. It is possible that, given a larger sample size, factors known to influence the development of NCD postoperatively (such as age, CPB time, and preoperative creatinine levels) would have reached significance when comparing the NCD and NORM groups. However, attempts were made to control for these possible confounders via multivariate regression modeling, even though preoperative and operative characteristics between the groups were not statistically different. Moreover, despite the small sample size, we were able to show significant associations between NCD and markers of brain injury (NSE and tau) and inflammation (CRP, C3a, and peroxide), which attests to the strength of these relationships.
Markers of Brain Injury and NCD
The ideal biochemical serum marker of brain injury would be highly specific for the brain, highly sensitive for brain injury, and would be released only after irreversible destruction of brain tissue and have rapid appearance in serum across the blood-brain barrier.31,32 Importantly, its levels should not be influenced by the CPB apparatus or commonly used drugs such as heparin, protamine, or propofol. Moreover, unlike markers of other organ dysfunction, a marker of brain injury cannot necessarily be correlated with the magnitude of injury as injury to certain regions of the brain (eg, motor region) will have greater clinical repercussions compared with other areas (eg, frontal lobe). All these requirements make identifying a marker more challenging. After brain injury, several substances, including S-100β, NSE, brain-specific creatine phosphokinase, and glutamate, have been shown to be released from brain tissue. Other biochemical markers such as GOT, malate transaminase, 1,6-diphosphate aldolase, and alpha-hydroxybutyric acid dehydrogenase have been studied in the setting of head trauma, but none was found to be adequate for clinical use.31 S-100β, and to a lesser extent NSE, have been studied in the cardiac surgical setting with inconclusive results.7,8,12–14,16,17,31,33,34 Following early enthusiasm about the ability of S-100β to predict severity of neuronal damage in cardiac surgical patients, it has been recently discredited due to its lack of specificity. In the setting of CPB, high levels of S-100β are found in blood drained from the cardiotomy suction due to its presence in mediastinal fat cells. Jonsson et al found that S-100β levels are decreased significantly by replacing cardiotomy suction with Cell Saver.5 This implies that blood retransfused from the mediastinum artificially increases serum S-100β levels early following CPB without true brain injury. Previous studies that allowed for this confounder have yielded conflicting results when correlating S-100β levels with NCD.12,15,16
Our data confirm the significant increase in S-100β levels in patients where cardiotomy suction was used compared with Cell Saver.23 Also, even after adjusting for other factors such as age, sex, CPB time, cardiotomy suction, aprotinin use, and preoperative creatinine levels, S-100β failed to show an association to the development of early NCD in our cohort. NSE levels, on the other hand, were not influenced by the use of cardiotomy suction compared with cell saver. Increased levels of NSE were also significantly associated with early NCD. Similarly, tau protein presence was similar in both the cardiotomy suction and Cell Saver groups. Also, tau presence in serum was significantly higher for patients in the NCD group. Thus, this study shows that NSE and tau are both potentially better markers of brain injury compared with S-100β. Both NSE and tau, however, require further study before they can be used clinically. NSE release has also been found to occur from hemolyzed erythrocytes.35 Tau protein is limited so far by our ability to reliably detect it quantitatively in serum due to our lack of understanding of its pharmacokinetics and other technical issues.36 As the accuracy of imnunoassays improves for tau and the impact of hemolysis on NSE levels following CPB becomes clearer, combining these tests may improve their sensitivity and specificity for early detection of brain injury in cardiac surgical patients.
Significant recent attention has been generated by Mangano et al about the association of aprotinin and end-organ damage.37 They found that aprotinin (more than 2 million KIU throughout procedure) is associated with increased cerebrovascular risk (stroke, encephalopathy, or coma) compared with other antifibrinolytic agents. Our data show that there was not a significant difference between aprotinin and Amicar on the incidence of the more subtle outcome of NCD or the levels of brain injury markers studied.
Inflammation and Early NCD
The inflammatory response elicited following CPB has been implicated in end-organ damage.27,30,38,39 From this study, the strong association between the acute phase reactant CRP, activated complement mediator C3a, and peroxide levels in relation to early NCD clearly support this hypothesis. The significant rise in complement activation in the NCD group compared with the NORM group certainly points to the increased inflammatory activation present in that group. While this seems to subside at the postoperative day 4 time point, values were still higher in the patients with NCD. Interestingly, CRP and peroxide levels on the fourth postoperative day showed a stronger association to NCD than the earlier time point of 6 hours postoperatively (although a strong trend was present for CRP). This may be explained by the fact that CRP levels continued to rise until the postoperative day 4 time point; hence, it is at this point that we can detect the difference among the groups. Alternatively, CRP and peroxide increase may have been triggered in response to a brain injury, rather than being the cause of the injury. However, this question cannot be answered from data obtained in this study. Also, it is unclear why the IL-6 response failed to show an association to early NCD while other mediators were successful. While this study may have been underpowered to detect this difference, this may also be explained by to variations of this cytokine within the inflammatory response in relation to blood sampling time.
There has recently been an increase in our understanding of the inflammatory response following CPB and its role in the development of brain injury. This can be caused by mechanisms leading to humoral and cellular activation. First, it is caused by contact activation of the immune system following interaction between the patient's blood and the foreign surfaces of the CPB circuit. Second, ischemia-reperfusion injury of organs, such as the heart, lungs, and kidneys, after release of the aortic cross-clamp causes immune activation.19,24,40,41 Attempts to decrease this inflammatory response, via the use of heparin-bonded circuits, corticosteroids, or other pharmacologic agents during CPB have been shown to decrease brain injury and NCD.23 With the exception of a few centers, widespread use of such techniques has not yet been established.
An interesting question that arises from the associations found in this study between NCD and the inflammatory/oxidative stress is: why is it that some patients developed higher or prolonged stress levels possibly leading to NCD while others did not? The answer to this question is currently unknown. However, we think that answering this question requires studying the genetic predisposition (ie, certain alleles or variations in gene expression) that these patients may have to developing pronounced or prolonged inflammatory and oxidative responses following periods of stress such as CPB. Alternatively, it may be that some patients have a more fragile or sensitive blood-brain barrier that allows for the increased inflammatory effects on central nervous system neurons. These are all very challenging and interesting questions that we may need to probe to uncover the pathophysiology on NCD.
CONCLUSION
NSE and tau may be better serum markers of brain injury, compared with S-100β, as they appear to have a stronger association to NCD following CPB wile not being influenced by perioperative factors such a retransfusion of cardiotomy suction blood. As we learn more about the pharmacokinetics of these markers and better assays are developed to quantify them, it may be of benefit to combine results from both these markers to improve the accuracy of their detection of brain injury. Our results also showed the strong relationship between CRP, C3a, and peroxide levels in relation to early NCD and that aprotinin, compared with Amicar, did not influence brain injury.
Discussions
Dr. Irving L. Kron (Charlottesville, Virginia): I appreciate the opportunity to review this paper. And I am going to make a bold statement. We basically have the heart figured out. So the next frontier is the brain. The authors are to be congratulated for attempting to study in a prospective fashion one of the most difficult issues in cardiac surgery.
There has been a great deal of unwelcome publicity about an increased incidence of subtle neurocognitive defects after cardiopulmonary bypass. The authors have attempted to scientifically correlate these defects with specific markers of injury. They found that these markers were elevated in patients that had postoperative deficits, and there is evidence of inflammation as part of this as noted in the elevation of c-reactive protein, an area that we are particularly interested in at the University of Virginia.
Unfortunately, this is a small group of patients, as the authors have stated. Therefore, there is a large possibility of a type 2 error, and I would suggest that probably did occur. If you look at bypass time, cross-clamp time, aprotinin usage, the numbers were higher in those who had neurocognitive defects but I suspect the sample size is too small to bring these out. I would like to have the authors comment on this later on.
In addition, several previous studies have noted neurocognitive defects with procedures such as pump bypass, percutaneous coronary angioplasty and general anesthesia. Have the authors previously been studying any patients in these subsets and tried to correlate this with markers?
The final question is whether these deficits actually translate into more objective findings such as increased length of stay or more discharges to skilled nursing facilities.
The bottom line is that this is a really great beginning. This is, as far as I know, one of the first correlations of these defects to chemical markers, and this will be the source for many future studies.
Dr. Basel Ramlawi (Boston, Massachusetts): You are absolutely right; sample size is a limitation in this study. And given 100 patients or so, this could have been certainly true in terms of baseline markers in such as time on cardiopulmonary bypass and age, and all of these could have reached significance. However, in defense of the conclusions, the results that were pointed out using the neurologic markers of brain injury or the inflammatory markers did reach significance even at this low sample size. And therefore, that hopefully shows and points to the strength of this relationship and association between brain injury and the markers or oxidative and inflammatory stress. But you are absolutely right, it is a small sample size. And it was intended to be a pilot study. And that is what it is. In terms of long-term care and effects on patients postoperatively, none of these patients had any major neurologic deficits or any discharge to long-term rehab due to cognitive deficit. All patients had fairly good outcomes. And this was a subtle finding that was only observed based on the extensive test and battery test that was done to them. This is an active area of investigation. There doesn't seem to be a lot of consistency with the data out there with regards to which test to use and which is the better neurologic marker to use for predicting neurocognitive decline or brain injury, in general, following cardiac surgery. S100 beta has been studied and neuron-specific enolase has also been studied, but there is no comparison between the two. And certainly Tau protein has not been studied. So you are absolutely right in that there does remain a lot of work to be done in this area.
Dr. Frank C. Spencer (New York, New York): I rise to emphasize the importance of this line of inquiry. Because as you indicated, we badly need a serum marker or some that may predict a brain injury. Right now, you say you wait and see if they wake up. But the neurocognitive changes don't appear for months. And you have no way of knowing what you might do differently. So it is a very important line of work. These are preliminary, they are probably mostly signs of inflammation. Your studies only go for 4 days. And 40% is far too high to be clinically significant. It is probably a sign of edema or something with bypass.
My one question is, do you have any data to indicate how long these studies last? Because they went home in 4 days. Bring them back in a month and measure them again, or 2 months. There is the overall course to find a reliable test that would show the future but also give you a clue. There are many questions that are unanswerable about bypass, I mean, what level of perfusion, what level transfusion, different times with it? But now it is primarily a guessing game. If you had a measure you could tell – it would be great contribution.
Dr. Basel Ramlawi (Boston, Massachusetts): Generally, intraoperative factors will affect patient outcomes, and neurocognitive and brain injuries are prime among those. We did do a 3-month analysis. And just as in many other studies, the decline rate decreases significantly compared the neurocognitive decline rate immediately postoperatively. So you are absolutely right in that a lot of these may not be translated into long term and may be just due to perioperative inflammatory factors, as we have showed, or other factors such as edema.
Dr. Richard M. Engelman (Springfield, Massachusetts): I would ask the pathogenesis, and you just already mentioned perhaps edema, but I wonder if just edema would cause axonal injury and that you would be able to identify it. Secondly, do you foresee this injury to be a temporary or permanent process? Finally was there a distinction made in your series between valve patients having cardiotomies and CABG patients that did not require a cardiotomy?
Dr. Basel Ramlawi (Boston, Massachusetts): Certainly edema and several other etiological factors contribute to this. In fact, one of the other things we are doing with this is look at genetic predisposition and genetic variability in these patients with regards to predicting the effect of brain injury on these patients. That is upcoming and you should have the data for that fairly shortly. With regards to whether it is temporary, it certainly is. Several studies have been done, as in our case. This is likely a temporary phenomenon where you have a significant increase in neurocognitive decline, which subsequently improves. In a paper I mentioned it has been shown that this potentially comes back at a much later date. But it is certainly an area of controversy. Certainly, as has been shown by Dr. Baumgartner's group at Johns Hopkins, having a good control group in these patients is important.
Footnotes
Supported by Grant No. HL04095-06 from the NIH as well as a CIHR/Heart and Stroke Foundation of Canada Postdoctoral Research Fellowship (to B.R.); Grant No. 5 P60 AG08812-14 from the Older Americans Independence Center (to E.R.M., S.E.L.); K12 Mentored Clinical Scientist Award No. 5 K12 AG00294-18 (to J.L.R.); and Grant No. HL-46716 from the NIH (to F.W.S.).
Reprints: Frank W. Sellke, MD, Division of Cardiothoracic Surgery, BIDMC, LMOB 2A, 110 Francis Street, Boston, MA, 02215. E-mail: fsellke@caregroup.harvard.edu.
REFERENCES
- 1.Ahonen J, Salmenpera M. Brain injury after adult cardiac surgery. Acta Anaesthesiol Scand. 2004;48:4–19. [DOI] [PubMed] [Google Scholar]
- 2.Arrowsmith JE, Grocott HP, Reves JG, et al. Central nervous system complications of cardiac surgery. Br J Anaesth. 2000;84:378–393. [DOI] [PubMed] [Google Scholar]
- 3.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: executive summary and recommendations: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1991 guidelines for coronary artery bypass graft surgery). Circulation. 1999;100:1464–1480. [DOI] [PubMed] [Google Scholar]
- 4.Murkin JM. Etiology and incidence of brain dysfunction after cardiac surgery. J Cardiothorac Vasc Anesth. 1999;3(4 suppl 1):12–17; discussion 36–37. [PubMed]
- 5.Jonsson H, Johnsson P, Backstrom M, et al. Controversial significance of early S-100B levels after cardiac surgery. BMC Neurol. 2004;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalman J, Juhasz A, Bogats G, et al. Elevated levels of inflammatory biomarkers in the cerebrospinal fluid after coronary artery bypass surgery are predictors of cognitive decline. Neurochem Int. 2006;48:177–180. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd CT, Ascione R, Underwood MJ, et al. Serum S-100 protein release and neuropsychologic outcome during coronary revascularization on the beating heart: a prospective randomized study. J Thorac Cardiovasc Surg. 2000;119:148–154. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen LS, Christiansen M, Hansen PB, et al. Do blood levels of neuron-specific enolase and S-100 protein reflect cognitive dysfunction after coronary artery bypass? Acta Anaesthesiol Scand. 1999;43:495–500. [DOI] [PubMed] [Google Scholar]
- 9.Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001;310:173–186. [DOI] [PubMed] [Google Scholar]
- 10.Shaw GJ, Jauch EC, Zemlan FP. Serum cleaved tau protein levels and clinical outcome in adult patients with closed head injury. Ann Emerg Med. 2002;39:254–257. [DOI] [PubMed] [Google Scholar]
- 11.Vaage J, Anderson R. Biochemical markers of neurologic injury in cardiac surgery: the rise and fall of S-100beta. J Thorac Cardiovasc Surg. 2001;122:853–855. [DOI] [PubMed] [Google Scholar]
- 12.Westaby S, Saatvedt K, White S, et al. Is there a relationship between serum S-100beta protein and neuropsychologic dysfunction after cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2000;119:132–137. [DOI] [PubMed] [Google Scholar]
- 13.Hall RI. Serum S-100beta protein and postoperative neurological dysfunction: ready for prime time? Can J Anaesth. 2004;51:645–648. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson H, Johnsson P, Alling C, et al. S-100beta after coronary artery surgery: release pattern, source of contamination, and relation to neuropsychological outcome. Ann Thorac Surg. 1999;68:2202–2208. [DOI] [PubMed] [Google Scholar]
- 15.Ueno T, Iguro Y, Yamamoto H, et al. Serial measurement of serum S-100B protein as a marker of cerebral damage after cardiac surgery. Ann Thorac Surg. 2003;75:1892–1897; discussion 1897–1898. [DOI] [PubMed]
- 16.Wang KJ, Wu HH, Fang SY, et al. Serum S-100 beta protein during coronary artery bypass graft surgery with or without cardiopulmonary bypass. Ann Thorac Surg. 2005;80:1371–1374. [DOI] [PubMed] [Google Scholar]
- 17.Nygaard O, Langbakk B, Romner B. Neuron-specific enolase concentrations in serum and cerebrospinal fluid in patients with no previous history of neurological disorder. Scand J Clin Lab Invest. 1998;58:183–186. [DOI] [PubMed] [Google Scholar]
- 18.Murkin JM, Newman SP, Stump DA, et al. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–1295. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Taha R, Gauvin D, et al. Postoperative cognitive dysfunction after cardiac surgery. Chest. 2005;128:3664–3670. [DOI] [PubMed] [Google Scholar]
- 20.Royter V, Bornstein N, Russell D. Coronary artery bypass grafting (CABG) and cognitive decline: a review. J Neurol Sci. 2005;229–230:65-67. [DOI] [PubMed]
- 21.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. [DOI] [PubMed] [Google Scholar]
- 22.Stolz E, Gerriets T, Kluge A, et al. Diffusion-weighted magnetic resonance imaging and neurobiochemical markers after aortic valve replacement: implications for future neuroprotective trials? Stroke. 2004;35:888–892. [DOI] [PubMed] [Google Scholar]
- 23.Aldea GS, Soltow LO, Chandler WL, et al. Limitation of thrombin generation, platelet activation, and inflammation by elimination of cardiotomy suction in patients undergoing coronary artery bypass grafting treated with heparin-bonded circuits. J Thorac Cardiovasc Surg. 2002;123:742–755. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Abraham R, Weinbroum AA, Dekel B, et al. Chemokines and the inflammatory response following cardiopulmonary bypass: a new target for therapeutic intervention? A review. Paediatr Anaesth. 2003;13:655–661. [DOI] [PubMed] [Google Scholar]
- 25.Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55:552–559. [DOI] [PubMed] [Google Scholar]
- 26.Chew ST, Newman MF, White WD, et al. Preliminary report on the association of apolipoprotein E polymorphisms, with postoperative peak serum creatinine concentrations in cardiac surgical patients. Anesthesiology. 2000;93:325–331. [DOI] [PubMed] [Google Scholar]
- 27.Clermont G, Vergely C, Jazayeri S, et al. Systemic free radical activation is a major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology. 2002;96:80–87. [DOI] [PubMed] [Google Scholar]
- 28.Holmes JH, Connolly NC, Paull DL, et al. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res. 2002;51:579–586. [DOI] [PubMed] [Google Scholar]
- 29.Miller BE, Levy JH. The inflammatory response to cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:355–366. [DOI] [PubMed] [Google Scholar]
- 30.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition: the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. [DOI] [PubMed] [Google Scholar]
- 31.Ingebrigtsen T, Romner B. Serial S-100 protein serum measurements related to early magnetic resonance imaging after minor head injury: case report. J Neurosurg. 1996;85:945–948. [DOI] [PubMed] [Google Scholar]
- 32.Bakay RA, Ward AA Jr. Enzymatic changes in serum and cerebrospinal fluid in neurological injury. J Neurosurg. 1983;58:27–37. [DOI] [PubMed] [Google Scholar]
- 33.van Engelen BG, Lamers KJ, Gabreels FJ, et al. Age-related changes of neuron-specific enolase, S-100 protein, and myelin basic protein concentrations in cerebrospinal fluid. Clin Chem. 1992;38:813–816. [PubMed] [Google Scholar]
- 34.Herrmann M, Curio N, Jost S, et al. Protein S-100B and neuron specific enolase as early neurobiochemical markers of the severity of traumatic brain injury. Restor Neurol Neurosci. 1999;14:109–114. [PubMed] [Google Scholar]
- 35.Johnsson P, Blomquist S, Luhrs C, et al. Neuron-specific enolase increases in plasma during and immediately after extracorporeal circulation. Ann Thorac Surg. 2000;69:750–754. [DOI] [PubMed] [Google Scholar]
- 36.Teunissen CE, Dijkstra C, Polman C. Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol. 2005;4:32–41. [DOI] [PubMed] [Google Scholar]
- 37.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. [DOI] [PubMed] [Google Scholar]
- 38.Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–244. [DOI] [PubMed] [Google Scholar]
- 39.Luyten CR, van Overveld FJ, De Backer LA, et al. Antioxidant defence during cardiopulmonary bypass surgery. Eur J Cardiothorac Surg. 2005;27:611–616. [DOI] [PubMed] [Google Scholar]
- 40.Ohata T, Sawa Y, Kadoba K, et al. Normothermia has beneficial effects in cardiopulmonary bypass attenuating inflammatory reactions. ASAIO J. 1995;41:M288–M291. [DOI] [PubMed] [Google Scholar]
- 41.Baufreton C, Allain P, Chevailler A, et al. Brain injury and neuropsychological outcome after coronary artery surgery are affected by complement activation. Ann Thorac Surg. 2005;79:1597–1605. [DOI] [PubMed] [Google Scholar]


