Abstract
Objective:
The aim of this study is to clarify the difference of risk of recurrence after hepatic resection between patients with hepatitis B- and hepatitis C-related hepatocellular carcinoma (HCC).
Summary and Background Data:
HCC is a highly recurrent carcinoma. However, consensus has not yet been reached about the relationship between hepatitis virus types and risk of recurrence in a long-term follow-up for HCC patients who underwent hepatic resection.
Patients and Methods:
From the beginning of January 1990 to the end of December 1999, of 469 HCC patients who underwent curative hepatic resection, 66 (14%) patients with positive hepatitis B virus surface antigen (HBs-Ag) and negative hepatitis C virus antibody (HCV-Ab) were regarded to have B-type hepatitis (HB)-related HCC (HB-HCC) and 351 (75%) with negative HBs-Ag and positive HCV-Ab were regarded to have C-type hepatitis (HC)-related HCC (HC-HCC). A clinical follow-up was performed to assess the existence of recurrence with the median follow-up periods of 11.0 and 10.1 years for HB- and HC-HCC patients, respectively.
Results:
The 3-, 5-, and 10-year disease-free survival (DFS) rates of HC-HCC (40%, 24%, and 12%, respectively) were significantly shorter than those of HB-HCC (57%, 54%, and 28%, respectively) (P = 0.0001). In multivariate Cox proportional hazard analysis, viral type, TNM stage, surgical margin, and Edmondson's grade were significantly associated with risk of recurrence. The risk of recurrence from the initial HCC increased to 1.93 times (95% confidence interval, 1.27–2.93) greater in HC-HCC patients than in HB-HCC patients.
Conclusion:
Hepatitis viral type is an independent factor for recurrence of HCC in a long-term clinical follow-up. This finding suggests that we may need a different strategy to control postoperative recurrence by the viral types in HCC patients.
The disease-free survivals of hepatitis C-related hepatocellular carcinoma (HC-HCC) were significantly shorter than those of hepatitis B-related HCC (HB-HCC) in a long-term follow-up after surgery. The risk of recurrence from the initial HCC increased to 1.93 times (95% confidence interval, 1.27–2.93) greater in HC-HCC patients than in HB-HCC patients. We may need different strategy to control postoperative recurrence by the viral types in HCC patients.
Since the measurement of first- and second-generation hepatitis C virus antibody (HCV-Ab) has become available,1,2 it has been elucidated that most cases of non-A and non-B type chronic hepatitis or liver cirrhosis coexisting with hepatocellular carcinoma (HCC) are C-type hepatitis (HC). The number of patients with HCC originating from HC (HC-HCC) has increased year by year, along with a decrease of patients with HCC originating from B-type hepatitis (HB) (HB-HCC), especially in Japan. According to a nationwide survey in Japan in 2003,3 the frequency of patients with HC-HCC (72%) was much higher than that of patients with HB-HCC (17%). The frequency of HC-HCC patients in Japan is almost the same as that of patients in Italy and Spain.4,5 However, in Asian countries around Japan, such as China, Taiwan, and Korea, where the prevalence of HCC is high, HB-HCC is still dominant.6–8 Numerous epidemiologic and molecular-biologic studies about the association between hepatitis B virus (HBV) infection and the development of HCC have been reported.9–11 HBV is thought to induce development of HCC through integration,12,13 transactivation,14 mutation of tumor suppressor genes, and so forth, in addition to carcinogenesis on the sequential process of chronic hepatitis to liver cirrhosis.15 As the occurrence of HB-HCC is partly brought about by the direct oncogenic effect of HBV, the fibrotic change of the liver at carcinogenesis may not be so severe. On the other hand, hepatitis C virus (HCV) is an RNA virus that does not integrate to the DNA of hepatocytes, and its relationship to the oncogenic mechanism of HCC is unclear. The carcinogenic potential of HC-HCC is known to increase in proportion to the progression from chronic hepatitis to liver cirrhosis, and actually, many of HC-HCCs occur from liver cirrhosis. Different mechanisms of HCC onset for these viruses may explain the differences in clinicopathologic features. Most chronic HBV infections are vertical transmissions during delivery, whereas HCV infections are mainly blood-borne such as from transfusions after reaching adulthood. Consequently, the mean age at occurrence of HCC is lower in HB-HCC than in HC-HCC. As mentioned above, characteristic differences of some etiologic or clinical factors have been pointed out between HB- and HC-HCC patients who underwent surgery.16,17 However, few data are available in the literature regarding the differences of recurrence between the two types of virus-originated HCC more than 10 years after hepatic resection. Herein, to clarify the difference of risk of recurrence after hepatic resection between patients with HB- and HC-HCC, we compared the long-term postoperative disease-free survival (DFS) of HCC between the two groups.
PATIENTS AND METHODS
Subjects
From the beginning of January 1990, when HCV-Ab could be generally measured in our hospital, to the end of December 1999, 469 patients underwent curative hepatic resection and were discharged. Curative resection was defined as complete macroscopic removal of the tumor without exposure of tumor cells on the cut surface. Of these patients, 66 (14%) were seropositive for hepatitis B virus surface antigen (HBs-Ag) and seronegative for HCV-Ab, 9 (2%) were seropositive for both HBs-Ag and HCV-Ab, 351 (75%) were seronegative for HBs-Ag and seropositive for HCV-Ab, and 43(9%) were seronegative for both HBs-Ag and HCV-Ab. The HCC patients with positive HBs-Ag and negative HCV-Ab were regarded to have HB-HCC, and those with negative HBs-Ag and positive HCV-Ab were regarded to have HC-HCC. The patients with HB-HCC and HC-HCC were enrolled in this study.
Assessment and Follow-up
Hepatic resection was offered by assessment of resectability based on both tumor progression and liver functional reserve. The degree of tumor progression was judged by radiographic findings from chest x-ray, ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and hepatic angiography. Liver function status was assessed by liver biochemistry status, indocyanine green retention test for 15 minutes (ICG-R15), and Child's classification18 as an overall estimate. No postoperative adjuvant therapies were given for all patients.
All patients were followed up for postoperative recurrence with assessment by tumor markers such as serum alpha-fetoprotein (AFP) level and serum protein induced by vitamin K absence II level, chest x-ray, and US or CT every 2 or 3 months after surgery until March 2005. At the end of March 2005, the number of patients who died of hepatic disease or other diseases without recurrence was 4 (6.1%) in HB-HCC patients and 26 (7.4%) in HC-HCC patients, respectively, and the number of patients who did not complete the follow-up about recurrence was 2 (3.0%) for HB-HCC patients and 5 (1.4%) for HC-HCC patients, respectively. When recurrence was discovered, the recurrent lesions were managed aggressively with a multimodal approach, including further surgery, transarterial chemoembolization, and percutaneous ethanol injection. The treatment method was decided by the pattern of recurrence and liver functional reserve at the time of recurrence.
Analysis
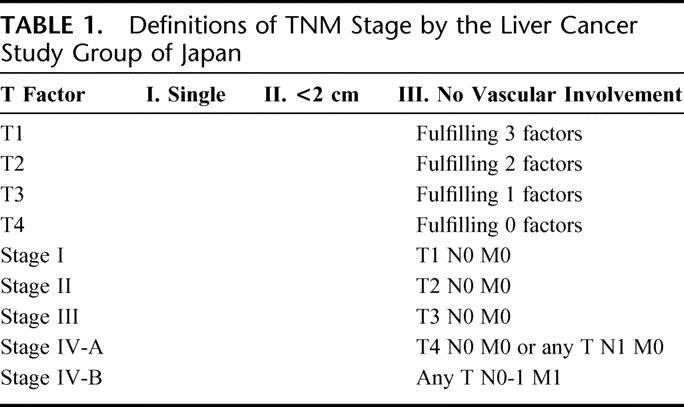
First, we compared the distributions of host factors including activity of hepatitis and liver functional reserve, tumor factors, surgical factors, and pathologic factors between the HB- and HC-HCC patients. Second, we compared the long-term survival and DFS curves after surgery between the HB- and HC-HCC patients. Moreover, to examine the differences of the DFS curves by the degree of tumor progression and liver function, HB-HCC patients and HC-HCC patients were separately stratified into these subgroups and the DFS curves of HB- and HC-HCC were compared on the same grade. TNM Stage by the Liver Cancer Study Group of Japan (LCSGJ),19 which is concordant with TNM classification by the International Hepato-Pancreato-Biliary Association and the International Union Against Cancer (Table 1) 20 was used for the grade of tumor progression, and Child's classification was used for the grade of liver functional reserve. Finally, to examine whether viral type is associated with the risk of recurrence for HCC, we calculated hazard ratio for recurrence in univariate and multivariate analysis.
TABLE 1. Definitions of TNM Stage by the Liver Cancer Study Group of Japan
Statistics
The statistical analysis was carried out with Student t[r] test for unpaired observations and the χ2 test for the frequency of various attributes between the groups. Survival and DFS curves were analyzed using the Kaplan-Meier method. Differences between curves were assessed according to the log-rank test. In univariate analysis, statistical comparisons between the subgroups of patients were made using the Mantel-Cox test. The Cox proportional hazards regression model was used for multivariate analysis. Differences with a P value less than 0.05 were considered significant. All P values were two-tailed. Statistical analysis was performed using a Power Macintosh G4 and Stat View 5.0 software (SAS Institute, Berkley, CA).
RESULTS
Comparison of Clinicopathologic Characters Between HB- and HC-HCC Patients
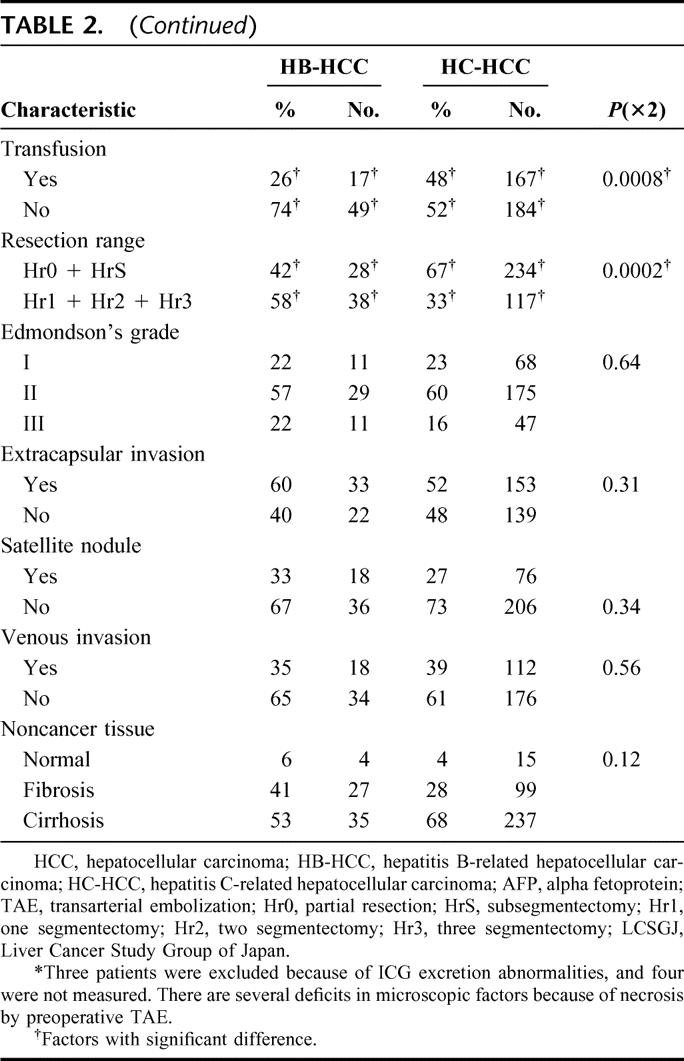
The distribution of selected characteristics by hepatitis virus among HCC patients was examined (Table 2). The percentages of older patients, patients with stronger hepatic inflammatory activity [the values of serum alanine aminotransferase (ALT)], and those with poorer liver functional reserve (the values of albumin and ICG-R15, and Child's classification) were significantly higher in the HC-HCC group than in the HB-HCC group. In tumor-related factors, the value of serum AFP was significantly smaller in the HC-HCC group than in the HB-HCC group. However, there were no statistical differences among the factors of the tumor size, the number of tumors, or TNM stage by LCSGJ. In surgery-related factors, although no significant differences were recognized for the operation time, blood loss during operation, and surgical margin, the patients who had smaller resection range and perioperative transfusion were more likely in the HC-HCC group than in the HB-HCC group. In pathologic factors, there were no significant differences in any factors such as the grade of differentiation of the tumor (Edmondson and Steiner's classification),21 extracapsular invasion, satellite nodules, vascular invasion, or histology of noncancerous lesions.
TABLE 2. Distribution of Selected Characteristics by Viral Hepatitis Among HCC Patients Who Underwent Hepatic Resection
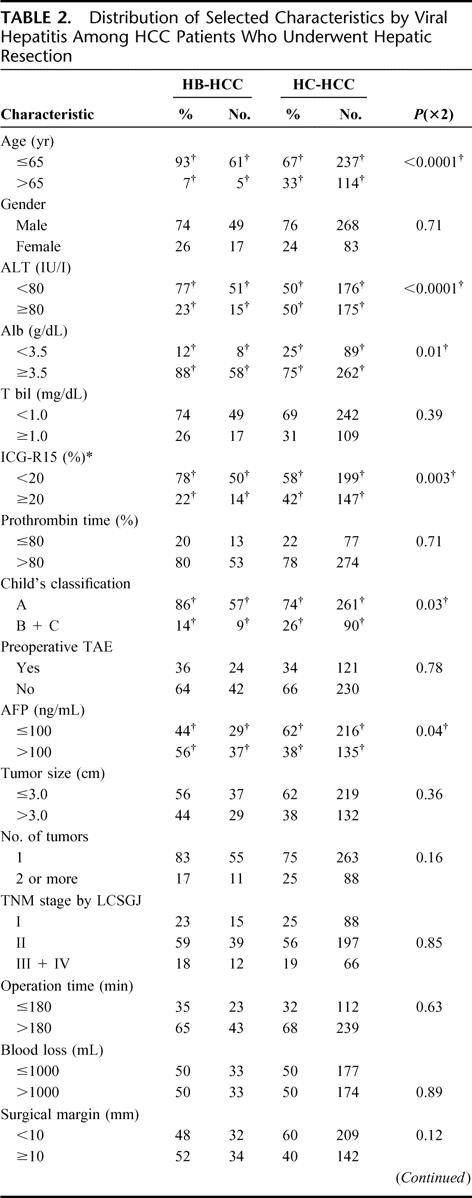
TABLE 2. (Continued)
Comparison of Survival and DFS After Surgery Between HB- and HC-HCC Patients
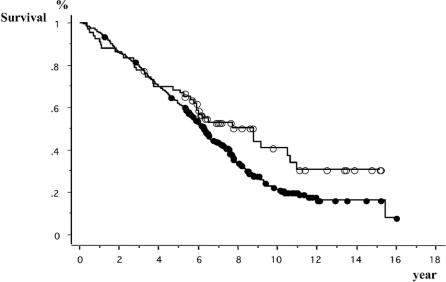
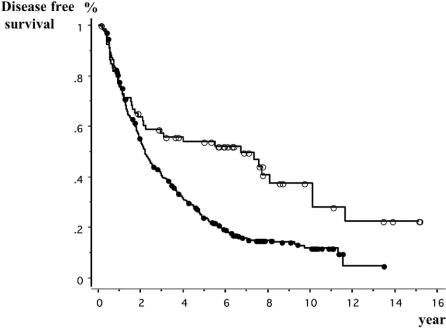
The median follow-up times of HB- and HC-HCC patients were 11.0 and 10.1 years, respectively. The number of recurrent patients was 39 (59%) in HB-HCC patients and 282 (80%) in HC-HCC patients, respectively. As for the forms of first recurrence, the number and percentage of patients with intrahepatic recurrence or distant metastasis was 35 (90%) and 4 (10%) in HB-HCC patients and 273 (97%) and 9 (3%) in HC-HCC patients. The 3-, 5-, and 10-year survival rates of HB-HCC group were 78%, 69%, and 41%, respectively, and those of HC-HCC group were 80%, 62%, and 22%, respectively, and no significant difference was recognized between the survival curves of the two groups (P = 0.068) (Fig. 1). Until 6 years after surgery, the survival curves of both groups were almost the same. Afterward, however, they began to separate and at 10 years after surgery, the survival rate of the HC-HCC group was lower than that of the HB-HCC group. On the other hand, the 3-, 5-, and 10-year DFS rates of the HC-HCC group, 40%, 24%, and 12%, respectively, were significantly lower than those of the HB-HCC group, 57%, 54%, and 28%, respectively (P = 0.0001) (Fig. 2). While the DFS curve of the HB-HCC group started to slope down slowly 2 years after surgery and became almost level subsequently, the curve of the HC-HCC group kept falling until 7 years after surgery.
FIGURE 1. Comparison of survivals between patients with hepatitis-B-related hepatocellular carcinoma (HCC) and patients with hepatitis-C-related HCC. Open circles, hepatitis B-related HCC patients (n = 66). Closed circles, hepatitis C-related HCC patients (n = 351). Log-rank test: P = 0.068.
FIGURE 2. Comparison of disease-free survivals between patients with hepatitis-B-related hepatocellular carcinoma (HCC) and patients with hepatitis-C-related HCC. Open circles, hepatitis B-related HCC patients (n = 66). Closed circles, hepatitis C-related HCC patients (n = 351). Log-rank test: P = 0.0001.
Comparison of DFS Curves Between the HB-HCC Group and the HC-HCC Group by TNM Stage and Child's Classification
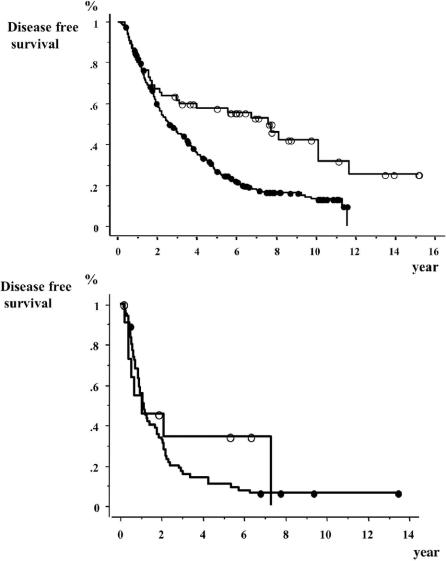
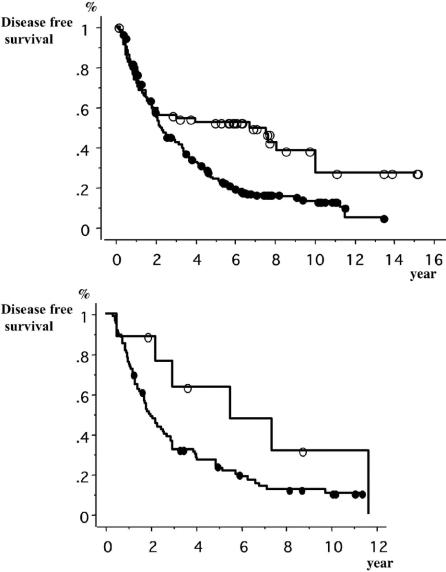
The DFS curves between the HB-HCC group and the HC-HCC group were compared in TNM Stage by LCSGJ (Fig. 3) and Child's classification (Fig. 4), respectively. In stages I and II, the DFS of the HC-HCC group was significantly lower than that of the HB-HCC group, and the DFS of the two groups were remarkably different 2 years after surgery and later. In contrast, there were no significant differences between the DFS of the two groups in stages III and IV. In the comparison of Child's classification, both in Child's A and in Child's B + C, the DFS of the HC-HCC group was lower than that of the HB-HCC group, especially 2 years after surgery and later.
FIGURE 3. Comparison of disease-free survivals between patients with hepatitis-B-related hepatocellular carcinoma (HCC) and patients with hepatitis-C-related HCC in TNM Stages by Liver Cancer Study Group of Japan. Top, stages I and II. Open circles, hepatitis-B-related HCC patients (n = 54). Closed circles, hepatitis-C-related HCC patients (n = 285). Log-rank test: P = 0.0002. Bottom, stages III and IV. Open circles, hepatitis-B-related HCC patients (n = 12). Closed circles, hepatitis-C-related HCC patients (n = 66). Log-rank test: P = 0.42.
FIGURE 4. Comparison of disease-free survivals between patients with hepatitis-B-related hepatocellular carcinoma (HCC), and hepatitis-C-related HCC using Child's classification. Top, Child's A. Open circles, hepatitis-B-related HCC patients (n = 57). Closed circles, hepatitis-C-related HCC patients (n = 261). Log-rank test: P = 0.001. Bottom, Child's B + C; Open circles, hepatitis-B-related HCC patients (n = 9). Closed circles, hepatitis-C-related HCC patients (n = 90). Log-rank test: P = 0.06.
Univariate and Multivariate Analysis for DFS of HCC Patients Who Underwent Hepatic Resection
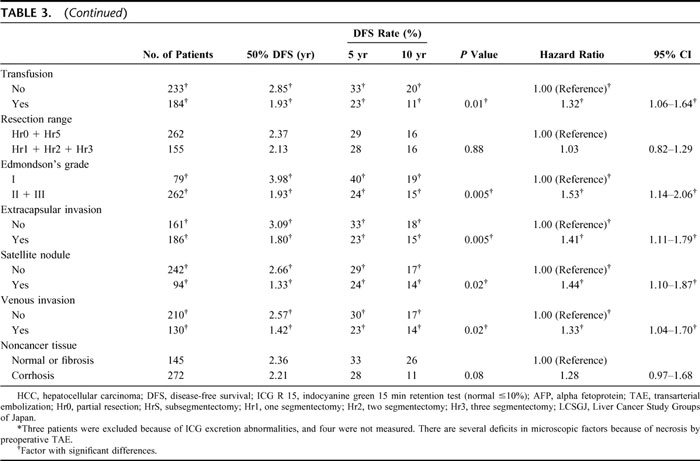
Univariate analysis and unadjusted hazard ratios for DFS were calculated on the HCC patients who underwent hepatic resection (Table 3). The risk of recurrence from HCC was 1.92 times greater in HC-HCC patients than in HB-HCC patients (P = 0.002, 95% confidential interval [CI], 1.37–2.70). In the other factors, ALT and ICG-R15 were selected from the clinical factors for DFS. From tumor factors: tumor size, number of tumors, and TNM stage by LCSGJ; from surgical factors: operation time, blood loss, perioperative transfusion, and surgical margin; and from pathologic factors: histologic grade (Edmondson's grade), extracapsular invasion, satellite nodules, and venous invasion were significantly strong predicators of risk for recurrence.
TABLE 3. Univariate Analysis and Unadjusted Hazard Ratios of Prognostic Factors for Disease-Free Survival on HCC Patients Who Underwent Hepatic Resection
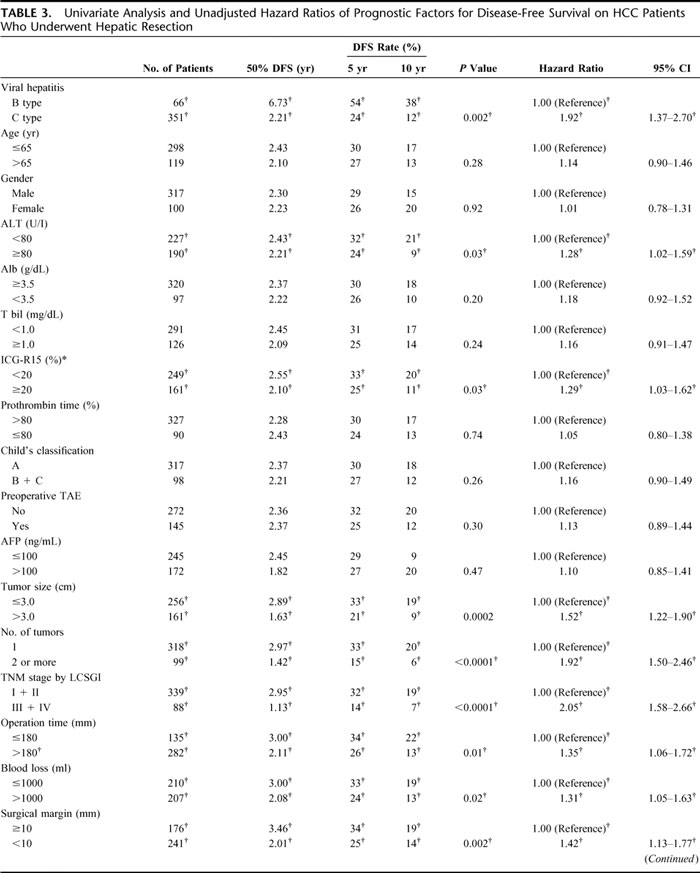
TABLE 3. (Continued)
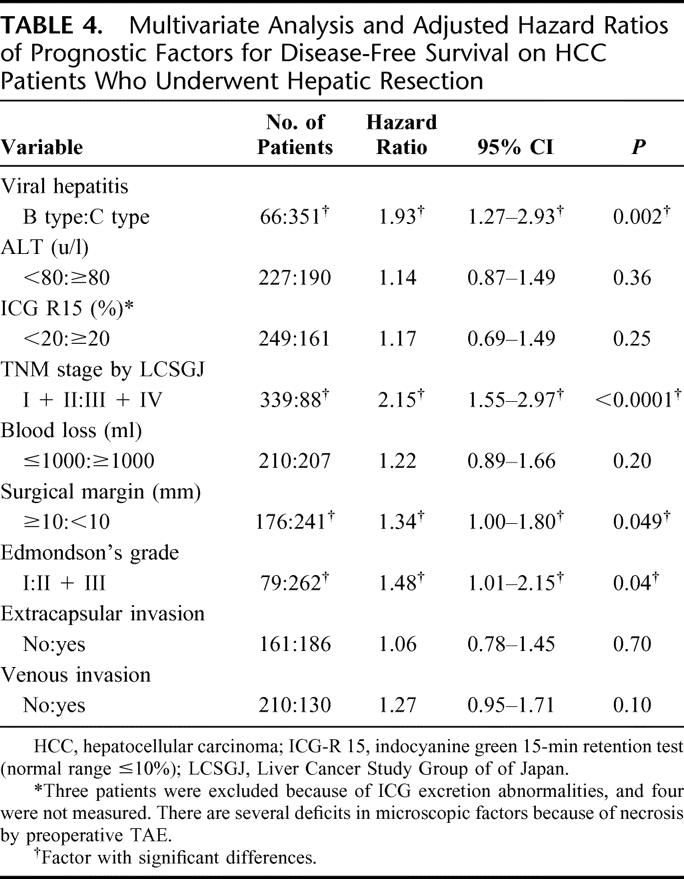
Multivariate analysis was performed for DFS using the selected variables of which the P values were less than 0.05 in univariate analysis (Table 4). Viral hepatitis was chosen for a serologic factor, and ALT was chosen from hepatic inflammatory activity factors, ICG-R15 from hepatic functional reserve factors, and from tumor factors TNM stage by LCSGJ, which includes the factors of both tumor size and tumor number. Blood loss and surgical margin were selected from operative factors, and Edmondson's grade, extracapsular invasion, and venous invasion from pathologic factors. As a result, viral hepatitis was one of the independent prognostic factors for DFS together with TNM stage, surgical margin, and Edmondson's grade (P = 0.002). The risk of recurrence from HCC increased to 1.93 times greater in HC-HCC patients than in HB-HCC patients after adjustment and the 95% CI became 1.27 to 2.93. When the patients whose first recurrence was distant metastasis were accounted for censored cases at the time of discovering the metastasis, the adjusted hazard ratio for the viral types was not changed (hazard ratio, 2.11; 95% CI, 1.36–3.28).
TABLE 4. Multivariate Analysis and Adjusted Hazard Ratios of Prognostic Factors for Disease-Free Survival on HCC Patients Who Underwent Hepatic Resection
DISCUSSION
The findings about the differences of various kinds of clinicopathologic characters such as age, hepatic inflammatory activity, liver functional reserve, and tumor and operative factors, etc., between HB-HCC and HC-HCC patients, which we indicated in this study, were consistent with those in previous reports for the most part17,22 (Table 2). On the other hand, the opinions about the differences of long-term clinical course after hepatic resection of the two groups were controversial. Takenaka et al17 reported the comparison of the outcome of a total of 126 patients with HB- or HC-HCC who underwent hepatic resection, and described that the survival and DFS at 5 years were similar in both groups. Similarly, some other reports described that long-term prognosis was not influenced by hepatitis virus types.23–26 On the other hand, Wu et al7 reported that the DFS rates between the two groups were not significantly different, but that the survival of HB-HCC patients was significantly lower than that of HC-HCC patients. They described that the poorer prognosis in HB-HCC patients was caused by a higher incidence of poor prognostic characters in the HB-HCC group. Haratake et al27 similarly reported that the prognosis of HB-HCC patients was worse than that of HC-HCC patients. These inconsistent findings possibly resulted from insufficient number of patients and follow-up periods for satisfactory analysis. We have shown the difference of hepatitis viral type had a dramatic impact on long-term DFS after surgery by a long follow-up period and a large number of patients (Fig. 2). If the follow-up period of our study was short and the comparative study was only about survivals, as the survivals of the two groups were almost the same until 7 years after surgery even in our study, our conclusions might be similar to previous reports,23–26 which stated hepatitis virus type was not influential in long-term prognosis.
In both the HB-HCC and HC-HCC groups, the recurrence curves were steep for the first 2 years. Afterward, though the curve of the HB-HCC group became very gentle, ie, the recurrence rate decreased dramatically, that of the HC-HCC group kept going down until 7 years after surgery, ie, the recurrence rate remained high during the later years (Fig. 2). The recurrences in the early period after surgery were considered to be mostly attributed to intrahepatic metastasis in both the HB-HCC and HC-HCC groups,28 whereas the recurrences occurring in the later follow-up years after surgery in the HC-HCC group can be presumed to have been caused by the higher frequency of metachronous carcinogenesis.29 We showed HC-HCC had the stronger hepatic inflammatory activity shown by the higher level of ALT, and the poorer liver functional reserve shown by the worse grade of Child's classification (Table 2). These findings are regarded to be the high-risk factors of developing HCC. However, even in the same liver function subgroup, which was separated based on Child's classification categorizing the degree of liver function, the potential of recurrence in the later follow-up years was stronger in the HC-HCC group than in the HB-HCC group regardless of the grade of liver functional reserve (Fig. 4). In addition, multivariate analysis showed that the difference of viral hepatitis itself was one of the independent prognostic factors for risk of recurrence (Table 4). These data may suggest that the high frequency of recurrence due to metachronous carcinogenesis in HC-HCC patients is caused not only by the high inflammatory activity or poor functional reserve but also by the other etiologic pathway induced by chronic HCV infection. Takano et al30 reported that the incidence of HCC in chronic hepatitis C patients was 2.7 times higher than that in chronic hepatitis B patients. Ikeda et al31 described that the incidence rates of HCC in HC patients and HB patients were 4.8% and 2.1% at the 5th year, 13.6% and 4.9% at the 10th year, respectively, and that the rates showed significant differences.
Even in earlier stages, such as TNM stages I and II, the recurrence rates of HC-HCC continued increasing until the later follow-up years, although the rates of HB-HCC decreased 2 years after surgery and later (Fig. 3, top). On the other hand, in more advanced stages, such as TNM stages III and IV, most recurrences happened in the early follow-up years in both HC-HCC and HB- HCC patients (Fig. 3, bottom). These findings may suggest that it is necessary to change the treatment strategy for HCC patients not only by the stage of progression or liver function but also the difference of viral hepatitis type. Namely, intrahepatic metastasis is considered to be the main form of recurrence in advanced stages of HCC. Therefore, for the more advanced stages of HCC, such as stages III or IV, regardless of the difference of hepatitis virus, we should pay attention to the appearance of intrahepatic metastasis in the early follow-up years after surgery, and an anticancer therapy such as chemotherapy may be recommended to reduce this type of recurrence. On the other hand, mainly for HC-HCC in less-advanced HCC, such as stages I or II, we should make a close follow-up to check intrahepatic recurrence by multicentricity even 2 years after surgery or later, and antiviral treatment such as interferon with or without ribavirin might be useful for preventing a second primary occurrence.32
Footnotes
Reprints: Yo Sasaki, MD, PhD, Department of Surgery, Osaka Medical Center for Cancer and Cardiovascular Diseases, 1-3-3, Nakamichi, Higashinari-ku, Osaka, 537-8511, Japan. E-mail sasaki-yo@mc.pref.osaka.jp.
REFERENCES
- 1.Choo QL, Kuo G, Weiner AJ, et al. Isolation of a cDNA clone derived from a blood born non-A, non-B virus hepatitis genome. Science. 1989;244:359–362. [DOI] [PubMed] [Google Scholar]
- 2.Kuo G, Choo QL, Alter HJ, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. [DOI] [PubMed] [Google Scholar]
- 3.Ikai I, Itai Y, Okita K, et al. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21–29. [DOI] [PubMed] [Google Scholar]
- 4.Colombo M, Kuo G, Choo QL, et al. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006–1008. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Barrera JM, Calvet X, et al. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004–1006. [DOI] [PubMed] [Google Scholar]
- 6.Tsai JF, Chang WY, Jeng JE, et al. Hepatitis B and C virus infection as risk factors for liver cirrhosis and cirrhotic hepatocellular carcinoma: a case-control study. Liver. 14:98–102. [DOI] [PubMed]
- 7.Wu CC, Ho WL, Chen JT, et al. Hepatitis viral status in patients undergoing liver resection for hepatocellular carcinoma. Br J Surg. 1999;86:1391–1396. [DOI] [PubMed] [Google Scholar]
- 8.Shim J, Kim BH, Kim NH, et al. Clinical features of HBsAg-negative but anti-HBc-positive hepatocellular carcinoma in a hepatitis B virus endemic area. J Gastroenterol Hepatol. 2005;20:746–751. [DOI] [PubMed] [Google Scholar]
- 9.Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- 10.Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. Lancet. 1981;2(suppl):1129–1133. [DOI] [PubMed] [Google Scholar]
- 11.Okuda K. Hepatocellular carcinoma: recent progress. Hepatology. 1992;15:948–963. [DOI] [PubMed] [Google Scholar]
- 12.Brechot C, Poucel C, Louise A, et al. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286:533–535. [DOI] [PubMed] [Google Scholar]
- 13.Shafritz DA, Kew MC. Identification of integrated hepatitis B virus DNA sequences in human hepatocellular carcinomas. Hepatology. 1981;1:1–8. [DOI] [PubMed] [Google Scholar]
- 14.Kim CM, Koike K, Saito I, et al. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. [DOI] [PubMed] [Google Scholar]
- 15.Sherlock S. Viruses and hepatocellular carcinoma. Gut. 1994;35:828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanabe G, Nuruki K, Baba Y, et al. A comparison of hepatocellular carcinoma associated with HBV or HCV infection. Hepatogastroenterology. 1999;46:2442–2446. [PubMed] [Google Scholar]
- 17.Takenaka K, Yamamoto K, Taketomi A, et al. A comparison of the surgical results in patients with hepatitis B versus hepatitis C-related hepatocellular carcinoma. Hepatology. 1995;22:20–24. [PubMed] [Google Scholar]
- 18.Pugh RNH, Murray-Lyon IM, Dawson JL. Transection of the esophagus for bleeding esophageal varices. Br J Surg. 1973;60:646–669. [DOI] [PubMed] [Google Scholar]
- 19.Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 2nd English ed. Tokyo: Kanehara, 2003. [Google Scholar]
- 20.Henderson JM, Sherman M, Tavill A, et al. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. J Hepatobiliary Pancreat Surg. 2003;5:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,000 necropsies. Cancer. 1954;7:462–502. [DOI] [PubMed] [Google Scholar]
- 22.Chen MF, Jeng LB, Lee WC. Surgical results in patients with hepatitis virus-related hepatocellular carcinoma in Taiwan. World J Surg. 2002;26:742–747. [DOI] [PubMed] [Google Scholar]
- 23.Chen MF, Jeng LB, Lee WC, et al. Surgical results in patients with dual hepatitis B- and C-related hepatocellular carcinoma compared with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Surgery. 1998;123:554–559. [DOI] [PubMed] [Google Scholar]
- 24.Miyagawa S, Kawwasaki S, Makuuchi M. Comparison of the characteristics of hepatocellular carcinoma between hepatitis B and hepatitis C viral infection: tumor multicentricity in cirrhotic liver with hepatitis C. Hepatology. 1996;24:307–310. [DOI] [PubMed] [Google Scholar]
- 25.Shiraishi M, Hiroyasu S, Nagahama M, et al. Characteristics of hepatocellular carcinoma in patients with negative virus markers: clinicopathologic study of resected tumors. World J Surg. 1999;23:301–305. [DOI] [PubMed] [Google Scholar]
- 26.Yamanaka N, Tanaka T, Tanaka W, et al. Correlation of hepatitis virus serologic status with clinicopathologic features in patients undergoing hepatectomy for hepatocellular carcinoma. Cancer. 1997;79:1509–1515. [DOI] [PubMed] [Google Scholar]
- 27.Haratake J, Takeda S, Kasai T, et al. Predictable factors for estimating prognosis of patients after resection of hepatocellular carcinoma. Cancer. 1993;72:1178–1183. [DOI] [PubMed] [Google Scholar]
- 28.Sakon M, Umeshita K, Nagano H, et al. Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg. 2000;135:1456–1459. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki Y, Imaoka S, Masutani S, et al. Influence of coexisting cirrhosis on long-term prognosis after surgery in patients with hepatocellular carcinoma. Surgery. 1992;112:515–521. [PubMed] [Google Scholar]
- 30.Takano S, Yokosuka O, Imazeki F, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650–655. [PubMed] [Google Scholar]
- 31.Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–938. [DOI] [PubMed] [Google Scholar]
- 32.Kubo S, Nishiguchi S, Hirohashi K, et al. Effect of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma: a randomized, controlled trial. Ann Intern Med. 2001;134:963–967. [DOI] [PubMed] [Google Scholar]