Abstract
Objective:
This study tests the hypothesis that methylprednisolone may influence eNOS activity in renal arterial and venous vascular beds and impede subclinical renal injury.
Summary Background Data:
Acute renal failure is a major complication of cardiovascular surgery. Renal damage arises in part from excessive vasoconstriction mediated by an imbalance of vasoconstrictive ET-1 and vasodilatory NO produced by eNOS. While methylprednisolone may reduce subclinical renal injury as measured by urinary N-acetyl-beta-D-glucosaminidase (β-NAG), its effects upon eNOS activity in renal arterial and venous vascular beds, reflected by urinary nitrate levels, is unclear.
Methods:
A porcine model of normotensive, euvolemic infrarenal aortic ischaemia-reperfusion was used. Forty-two pigs underwent a 60-minute laparotomy followed by 150 minutes of infrarenal ischemia and 180 minutes of reperfusion. Animals were randomized to receive methylprednisolone 30 mg/kg or placebo after induction of general anesthesia. Urinary β-NAG levels were assessed as an index of subclinical renal injury, whereas urinary nitrate was assessed as an indicator of eNOS activity in renal arterial and venous vascular beds.
Results:
Methylprednisolone treatment did not influence mean arterial, central venous, or pulmonary artery wedge pressures but suppressed plasma IL-6 levels. After the ischemia-induced rise from preanaesthetic baseline levels, urinary β-NAG levels declined to significantly lower values in the MP group, indicative of MP renal protection (P < 0.05). Conversely, urinary nitrate levels indicative of vascular e-NOS activity remained significantly and persistently higher in MP-treated animals (P < 0.05).
Conclusion:
This study, in a porcine model of renal ischaemia-reperfusion injury, shows the benefits of methylprednisolone pretreatment in enhancing urinary nitrate levels indicative of vascular eNOS activity and the reduction of urinary β-NAG levels, which represent subclinical renal injury.
During cardiovascular surgery, renal injury arises partly because of an imbalance between vasoconstrictive ET-1 and vasodilatory NO. This study provides evidence of a potentially renal protective effect of methylprednisolone, by alteration of vascular eNOS activity, in a porcine model of aortic ischemia reperfusion.
Reactive vasoconstriction of the acutely ischemic kidney is harmful, first due at least in part to heightened endothelin-1 (ET-1) activity.1 Thus, bosentan, a dual endothelin (ET) receptor antagonist, reduces ischemia induced renal injury and protective effects of ischemic preconditioning against prolonged renal ischemia depend on endothelial nitric oxide synthase (eNOS). Second, ischemia reduces vasodilatory e-NOS, thus compounding ischemia-mediated renal injury through adversely altering the intrarenal ET-1/NO balance. Third, ischemia, by an NF kappa B-dependent mechanism, increases renal proinflammatory mediators, which may be renotoxic, indirectly by amplifying hypoxia-induced arterial vasoconstriction (through inhibition of vasodilatory e-NOS and promoting ET-1) and by direct renotoxic effects. A local renal proinflammatory response from localized hypoxia, superimposed upon a systemic proinflammatory response, may predispose to renal dysfunction. Measures to reduce the systemic proinflammatory response as well as heighten arterial eNOS might be expected to be renoprotective. Methylprednisolone (MP) reduces both plasma proinflammatory and urinary anti-inflammatory cytokines, suggesting reduced intrarenal proinflammatory activity. Moreover, steroids enhance arterial e-NOS activity, reducing vascular vasoconstriction in murine ischemic myocardium. Furthermore, dexamethasone lowers tumor necrosis factor (TNF)-mediated pulmonary ET-1. In renal damage, arising from vasoconstrictive effects of hypoxia and proinflammatory mediators, it is unknown if steroids favorably counter renal arterial eNOS/ET-1 imbalance as observed in myocardium and lung. This considers only vasoconstrictive effects of proinflammatory mediators on arterial eNOS. In contrast, intravenous IL-1 causes venodilation with increased venous eNOS expression. Thus, proinflammatory mediators increase venous e-NOS and impair arterial eNOS/ET-1 balance. Urinary nitrate reflects total eNOS activity of both arterial and venous vascular beds. We hypothesize that a proinflammatory response arising from infrarenal ischemia-reperfusion in a normotensive, normoxic subject increases overall urinary nitrate excretion, reflecting increased venous eNOS activity. We also hypothesized that MP may be renoprotective through further increasing urinary nitrate levels. The underlying reasoning is that higher urinary nitrate excretion would follow a MP-induced additional arterial contribution to urinary nitrate. Moreover, reducing vasoconstrictive renal damage in the MP group would enhance nitrate excretion and reduce subclinical renal injury as measured by urinary N-acetyl-beta-D-glucosaminidase (NAG)/creatinine (Cr) ratios.2–4 NAG is a proximal tubular lysosomal enzyme, neither filtered at the glomerulus nor absorbed nor secreted by tubules. Thus, urinary NAG arises from tubular cell damage.5,6
METHODS
General
The project was undertaken, after approval by the local animal ethical committee, in accordance with the United Kingdom Animal (Scientific Procedures) Act, 1986.
Forty-two White Landrace pigs (28–35 kg) underwent prolonged infrarenal aortic ischemia followed by reperfusion. The power calculation to determine group size was based on a pilot study in similar animals as follows. Assays for plasma interleukin-6 (IL-6) and urinary N-acetyl-beta-D-glucosaminidase/creatinine (NAG/Cr) in control animals were assessed for variability at the same endpoint. As there was evidence of skew the results were logarithmically transformed. The pooled standard deviations (log 10 scale) for 2 groups of 8 animals was S = 0.466. The number n per group required to have 90% power to detect a difference, d, on a log 10 scale as statistically significant (P < 0.05) is given by the formula; n > 2 (za + ab)2 (s/d)2 where az = 1.96 (5% significance level); zb = 1.282 (90% power). It was considered that if the geometric mean between groups differed by as much as a factor of 3 then this would be worth detecting. On the log 10 scale, this is equivalent to a difference d = log 10 (3) = 0.477. The numbers required were therefore: n > 2 (1.96 + 1.282)2 (0.466/0.477)2 = 20.1: n = 21 was therefore chosen. Animals were randomized prior to commencement of the study into two groups using commercial “Instat” software, Graph Pad.
After baseline intraoperative samples of blood and urine had been collected, the control received a saline placebo while the steroid group received MP 30 mg/kg (Solu-Medrone, Pharmacia), a dose comparable to many clinical studies investigating its effect.7–12
The total perioperative time course was 390 minutes, consisting of 60 minutes of preoperative set-up time followed by 150 minutes of ischemia and 180 minutes of reperfusion.
Anesthesia and Operative Setup
On the eve of surgery, animals were fasted for food but given unrestricted access to water overnight. On the morning of transfer to our operative unit (a distance of 8 miles), animals were sedated with intramuscular azaperone 40 mg/ml (Stresnil 1 mL per 20 kg, Boehringer-Ingelheim). Intravenous anesthesia was used throughout and directed in accordance with the requirements of a named governmental office veterinary surgeon. Anesthesia was induced, using a bolus of intravenous pentobarbitone sodium BP 40 mg/kg (Sagatal 60 mg/mL, Rhone Merieux, Essex), as per the manufacturer's instructions. This was given via a marginal ear vein cannulated with a 20-G Adsyte Pro Venflon (Becton Dickinson, St. Augustin, Del Gadelix, Madrid, Spain). The animal was placed on a prewarmed operating table and an immediate tracheostomy performed using a 7.5-mm endotracheal tube (Portex). The tube was connected to standard anesthetic circuitry and the lungs ventilated with an oxygen/air mixture by means of the Manley Ventilator (Blease Anesthetic Equipment Ltd.). The tidal volume was adjusted for body weight and set at 16 mL/kg. Oxygen/airflow rates were set at approximately 6 L/4 L, respectively, throughout to maintain an FiO2 of 0.7 throughout. These flow rates were constantly adjusted to maintain end-tidal carbon dioxide (EtCO2) and arterial partial pressure carbon dioxide (pCO2) levels within a normal range.
Following induction of anesthesia, tracheostomy and stabilization on the ventilator a left subclavian cut-down was performed. An 18-G Adsyte Pro Venflon was inserted and secured with silk. A bolus of 100 IU/kg intravenous heparin sodium (CP Pharmaceuticals Ltd, Ash Road North, Wrexham Industrial) was given prior to any further cannulae placement or instrumentation.
Normothermic Hartmann's 1.0-L bags (Baxter Health Care Ltd., Thetford, Norfolk, England), to each of which 1000 IU heparin was added, were prewarmed to 37°C overnight in a waterbath (Grant 5540 Waterbath, Grant Instruments Ltd., Barrington, Cambridge) prior to commencement of surgery.
Fluids were then instilled continuously via this subclavian route continuously throughout the perioperative time course at a rate of 30 mL/kg per hour via a roller pump (Gilson Minipuls 2). Maintenance of anesthesia continued via the previously cannulated marginal ear vein with pentobarbitone 13 mg/kg per hour (Sagatal 60 mg/mL, Rhone Merieux, Essex) infused by means of an Ohmeda 9000 digital volumetric syringe pump (Ohmeda, Station Road, Streeton, West Yorkshire, England), a 60-mL calibrated syringe and 1-m extension set.
A standard midline laparotomy was performed and a urinary cystostomy fashioned using a 2/o Prolene purse-string suture. A sterile urinary catheter (Silicon, 12Fr Portex, Portex Ltd., Hythe, Kent) was placed in the middle of the purse-string after a cystotomy was fashioned with diathermy. The purse-string was tightened around the catheter and the retaining balloon inflated with 5 mL sterile water. The catheter was then connected to a disposable hourly urometry bag (Bard, CR Bard International Ltd, Crawley, West Sussex).
During the period of infrarenal ischemia, the aorta was occluded using a standard angled arterial cross-clamp. The distal aorta was palpated to confirm total aortic occlusion. To allow reperfusion the cross-clamp was removed.
For arterial pressure monitoring and blood sampling, the right carotid sheath was dissected and both common carotid artery and internal jugular vein were identified and dissected free. Both vessels were ligated distal to point of cannulae insertion.
A 14-G Insyte arterial cannula was placed in the common carotid artery.
The pulmonary artery flotation catheter (Swan-Ganz, Baxter 831HF75, Baxter Healthcare Corp., Edwards Critical Care Division, Irvine, CA) was flushed with 5% dextrose, calibrated, and zero referenced. The catheter was inserted through a simple venotomy of the right internal jugular vein. Placement of the catheter was similar to clinical practice. The catheter was slowly advanced, with the balloon inflated, carefully observing right atrial, ventricular and eventually pulmonary artery tracing. The balloon was wedged and the pressure recorded before deflation.
Monitoring
A Hewlett Packard monitor, model number HP 78353B (HP Medical) was used for invasive monitoring of systemic blood pressure (systolic, diastolic, and mean), heart rate recording, Swan-Ganz catheter measurements of central venous pressure (CVP), systolic, diastolic, and mean pulmonary artery pressures (PAP), and pulmonary artery wedge pressure (PAWP) as well as esophageal temperature measurement.
An Ohmeda 4700 (Oxicap, Louisville, CO) was used for the measurement of the following noninvasive parameters: Oxygen saturation (%), heart rate (HR), end-tidal carbon dioxide measurement (EtCO2), and inspired oxygen concentration (FiO2).
A heparinized sample was obtained every 30 minutes throughout the perioperative time course for arterial blood gas estimation. Samples were tested on an automatic, self-calibrating, blood gas analyzer (AVL Compact 2, Roche Diagnostics Ltd, East Sussex, UK).
Sample Collection
Prior to transfer, a preoperative baseline urine sample was obtained. Intraoperative blood and urine samples were as follows; a baseline sample (t = 20 minutes) prior to methylprednisolone or placebo administration, a preischemia sample (t = 60 minutes), an end of ischemia sample (t = 210 minutes), 1 hour into reperfusion (t = 270 minutes), 2 hours into reperfusion (t = 330 minutes), and end of reperfusion samples (t = 390 minutes), respectively.
Whole blood was sampled from the carotid arterial line at the time points previously indicated. At each sample time the following were obtained.
A 4-mL clotted sample was collected for estimation of serum urea and creatinine. Ten milliliters of blood was collected in an EDTA coated bottle for later cytokine analysis. This was immediately transferred to 6 Eppendorf containers and centrifuged at 15,000 rpm for 2 minutes. The separated plasma was then placed in 4 Sardstedt containers and immediately placed in the freezer at −5°C. At the end of the experiment, these stored plasma samples were transferred to a −80°C freezer for cytokine estimation later.
In addition to the above, at the same time points and at 30 minute intervals, heparinized samples were collected for arterial blood gas estimation.
Urine was collected at the same sample times. Urine was collected and sent for estimation of urinary N-acetyl-β-D-glucosaminidase (β-NAG) and creatinine to the Department of Clinical Chemistry, Belfast City Hospital, Belfast.
Urine, for estimation of urinary cytokines and urinary nitrate/nitrite, was also transferred to 6 Eppendorf tubes and spun at 15,000 rpm for 2 minutes to remove any debris. The urine was then transferred to 5 Sarstedt containers and immediately frozen to −5°C. At the end of the experiment, the urine was transferred to a −80°C freezer for subsequent analysis.
Assay Measurement
Interleukin-6 Assay
An R&D Systems (Abingdon, Oxon, UK) swine specific interleukin-6 (IL-6) immunoassay was used for determination of IL-6 in swine plasma.
Urinary Nitrite/Nitrate Estimation (NO2−/NO3−)
Urinary nitrite/nitrate (NO2−/NO3−) concentrations were measured by a commercially available R&D systems kit (R&D Systems Europe Ltd., Abingdon, Oxon, UK).
Urinary N-Acetyl-β-D-Glucosaminidase (β-NAG)
NAG was analyzed by a spectrophotometric method (PPR Diagnostics Ltd., London, UK) by a method outlined by Price.2,3
RESULTS
Forty-two pigs were studied with no intraoperative failures with respect to anesthesia, instrumentation, cannulation, or sample collection. Nonparametric data were analyzed using Instat software (Graph Pad). Analysis between groups was undertaken using the Mann-Whitney U test and differences within groups using Friedman's repeated measures ANOVA with Dunn's multiple comparison post test.
In both groups, cross-clamping of the infrarenal aorta led to a significant rise in mean arterial pressure with a subsequent fall on removal of the cross-clamp. There was no significant difference between groups in mean arterial pressure throughout the perioperative time period as shown in Figure 1(i).
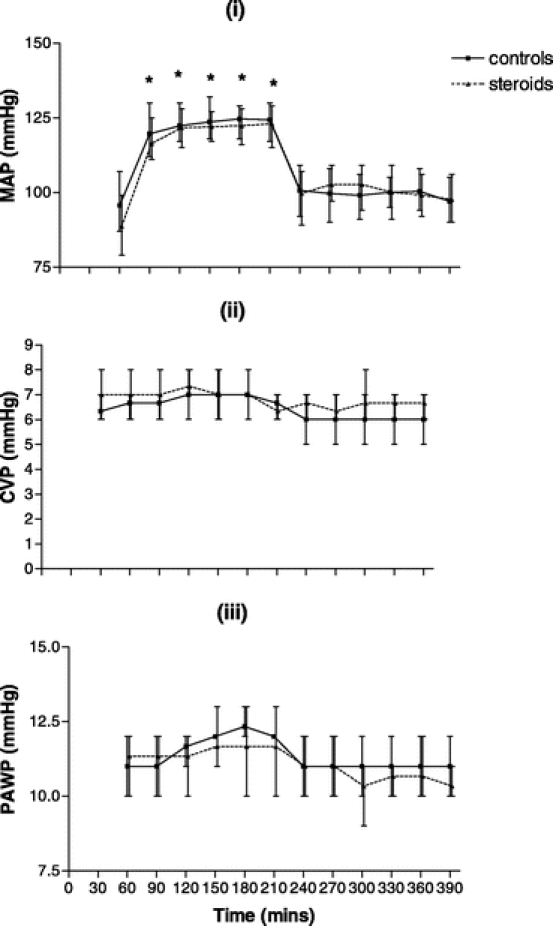
FIGURE 1. (i), Mean arterial pressure (MAP) throughout the perioperative time period in the control group (n = 21) and the steroid group (n = 21). (ii), Central venous pressure (CVP) throughout the perioperative time period in the control group and the steroid group. (iii), Pulmonary artery wedge pressure (PAWP) throughout the perioperative time period in the control group and the steroid group. X-Y plots show the median and interquartile ranges. *P < 0.05 within group compared with baseline.
There was no significant differences between or within group either in central venous or pulmonary artery wedge pressures throughout the perioperative time period as shown in Figures 1(ii) and 1(iii), respectively.
There was a significant rise in plasma interleukin-6 (IL-6) in controls at all time points as compared with the baseline. In the steroid group, there was a significant rise from the baseline only at the 210 minutes time point. There was significantly higher IL-6 level in controls compared with that in the steroid group at the end of reperfusion at times 330 and 390 minutes as shown in Figure 2.
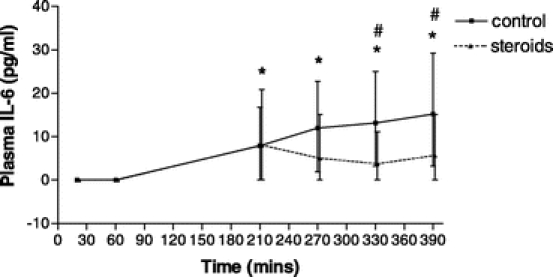
FIGURE 2. Plasma interleukin-6 (IL-6) throughout the perioperative time period in the controls (n = 21) and the steroid group (n = 21). X-Y plot shows the median and interquartile range. #P < 0.05 between groups. *P < 0.05 within group compared with baseline.
There was a significantly higher total urinary output in the steroid group compared with controls as shown in Figure 3.
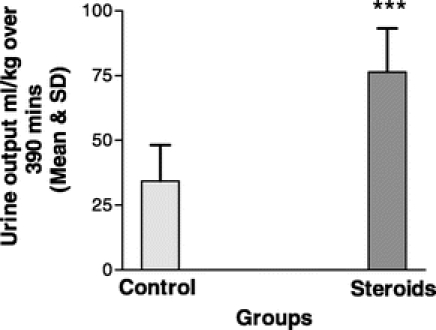
FIGURE 3. Total urinary output for the perioperative time period in the controls (n = 21) and the steroid group (n = 21). Bar graph shows the mean and standard deviation. ***P < 0.001 between groups.
Urinary N-acetyl-β-D-glucosaminidase/urinary creatinine ratios (β-NAG/Cr) rose significantly from the baseline in both groups in response to transfer, early anesthesia and laparotomy alone. While in the steroid group β-NAG/Cr ratios fell toward baseline values during reperfusion, higher levels were sustained in controls. There was a significantly higher β-NAG/Cr ratio in controls compared with that in the steroid group at times 210, 270, 330, and 390 minutes as shown in Figure 4(i).
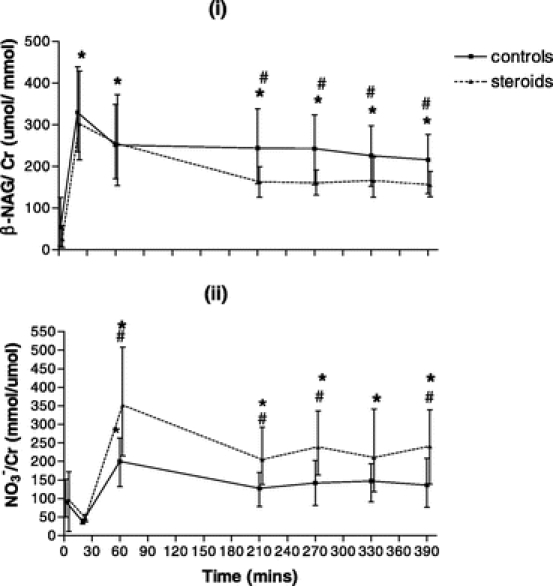
FIGURE 4. (i), Urinary N-acetyl-β-D-glucosaminidase/urinary creatinine ratios (NAG/Cr) throughout the perioperative time period in the controls (n = 21) and the steroid group (n = 21). (ii), Urinary nitrate/urinary creatinine ratios (NO3−/Cr) throughout the perioperative time period in the controls (n = 21) and the steroid group (n = 21). X-Y plot shows the median and interquartile range. #P < 0.05 between groups. *P < 0.05 within group compared with baseline.
Urinary nitrate/urinary creatinine ratios (NO3−/Cr) rose significantly in both groups from the baseline with transfer, early anesthesia, and laparotomy alone. While levels fell again in the controls, they remained significantly elevated throughout the ischemia-reperfusion period in the steroid group. There was a significantly higher NO3−/Cr ratio at times 60, 210, 270, and 390 minutes as shown in Figure 4(ii).
DISCUSSION
Renal dysfunction at cardiovascular surgery may arise directly from renal ischemia-reperfusion and indirectly from ischemia-reperfusion of other organs. Accordingly, transient ischemic episodes during surgery, whether related to systemic hypotension, hypovolemia, or hypoxia, contribute to subclinical renal injury detectable despite normal serum urea or creatinine by increased urinary NAG/creatinine ratios.5,6,13 Since we focused on indirect renal effects of remote organ ischemia reperfusion, animals remained normovolemic and normoxic without evidence of renal arterial hypotension as confirmed in pilot work, where intraoperative Doppler probes showed preserved renal artery blood flow throughout infrarenal clamping. Moreover, lack of between-group differences in CVP, PAWP, and MAP excluded hemodynamic factors as directly contributing to β-NAG differences.
At cardiovascular surgery, it has been suggested that filtered proinflammatory mediators, particularly IL-1β and TNF-α, indirectly contribute to subclinical renal injury.13 Recently, in a murine model of ischemia-reperfusion induced renal injury, a more pivotal role for IL-6 in proinflammatory cytokine-mediated renal injury was identified, where IL-6 knockout mice compared with wild type, had reduced renal tissue expression of the other key proinflammatory mediators TNF-α and IL-1β. Renal injury, dysfunction, and inflammation in IL-6 knockout animals were reduced.14 Since IL-6 influences ischemia-reperfusion associated renal injury,14 we monitored IL-6 levels.
We found an initial peak in β-NAG after laparotomy, remaining elevated in relation to the baseline during ischemia-reperfusion in controls but not the steroid group. Here, despite a similar early rise, a significant fall from the 20 minutes peak was observed, following which β-NAG values were significantly lower than controls.
The early β-NAG peak in both groups suggests subclinical renal injury after 20 minutes arising from laparotomy alone, even before reperfusion injury commenced. It is unsurprising that later reperfusion injury in placebo animals failed to increase urinary β-NAG when these results are compared with cardiac surgery where an initial postcardiopulmonary bypass peak13 in β-NAG was followed by a later peak at 24 hours. One limitation of this present model is its duration of only 390 minutes, precluding determination of MP modulation of a delayed peak as occurred at cardiac surgery. However, the greater fall from the initial β-NAG peak in the steroid animals compared with controls suggests MP-related renal protection as found at cardiac surgery.13
The second finding was that, in contrast to the significantly lower urinary β-NAG in the steroid group, urinary nitrate was significantly higher in this group.
To explain the immediate increase in urinary nitrate in controls, we note that a pro-inflammatory insult stimulates venous eNOS to produce NO.15 This accounts at least partly for the mild rise at T = 60 in controls. As the inflammatory response intensifies, however, venous eNOS activity in controls might be expected to increase leading to a further rise in urinary nitrate excretion. This did not take place because NO levels in the control group leveled out at t = 60 and remained at this level thereafter. An explanation for this, at least in part, arises from the observation that an inflammatory response reduces arterial eNOS and increases arterial ET-1 and that this is associated with impaired renal function (as indicated by the peak in β-NAG at T = 20) in such a manner that the ability to excrete nitrate is limited. Hence a further increase in urinary nitrate after t = 60 was not observed despite later IL-6 rises. Although others have shown that MP reduces IL-6,7 it is worth noting that Kang et al16 showed that NO impairs IL-6 production. If MP heightens NO production, as evidenced by increased urinary nitrate, and NO impairs IL-6 production16 (a pivotal cytokine in ischemia-reperfusion mediated renal injury14), it is perhaps unsurprising that in the steroid group IL-6 remained lower than controls while urinary nitrate excretion increased accompanied by reduced proximal tubular injury as demonstrated by lower β-NAG. There are several possible explanations for the immediate urinary nitrate increase remaining greater in the steroid group than controls throughout the experiment.
1. The reduction in β-NAG in the steroid group along with elevated urinary output suggests renal protection contributing at least in part to the enhanced ability to excrete urinary nitrate in the steroid group.
2. We know that MP increases arterial eNOS.17 This means that, in addition to the nitrate arising from venous eNOS in the steroid animals, a further contribution of nitrate emerges from arterial eNOS. This effect is amplified by improved renal excretion of NO in the steroid group as a result of enhanced preservation of renal function.
3. Since exogenously administered steroids lead to arterial eNOS activation,17,18 and in our model to increased urinary nitrate, the possibility of stress-induced endogenous cortisols beneficially activating arterial eNOS should be considered.
Enhancement of renal function in the steroid group is consistent with findings in vivo.19 This present study, however, shows that increased urinary nitrate excretion is associated with renal protection.
The findings are consistent with the proposition that MP preserves and even enhances urinary nitrate excretion during an ongoing inflammatory response because:
Arterial e-NOS activity is directly increased, thereby protecting against renotoxic effects of hypoxia-mediated ET-1/NO imbalance.
Direct renal protection resulting from this preservation of ET-1/NO balance allows continued nitrate excretion, which would otherwise be impaired if potential renotoxic effects of hypoxia or inflammatory related renal damage were to progress unopposed.
Since Kang et al have noted that increased NO production leads to impaired IL-6, the MP-related increase in NO production may contribute to the observed inhibitory effects of MP on IL-6, a pivotal cytokine in ischemia-reperfusion mediated renal injury, 14 therefore leading to preservation of renal function.
Further work is needed to obtain tissue samples to measure eNOS expression in renal tissue as well as tissue expression of IL-6 and other proinflammatory mediators. This, together with measurement of plasma arterial and venous NO markers, would help determine whether changes in NO directly contribute to the observed renoprotection or constitute an indirect association.
CONCLUSION
This study shows the benefits of methylprednisolone pretreatment by enhancement of urinary nitrate levels indicative of vascular eNOS activity and reduction of urinary β-NAG levels, which represents subclinical renal injury, in a porcine model of renal ischemia-reperfusion injury.
ACKNOWLEDGMENTS
The authors thank Mrs. E. Fleming for her help with analytical aspects of this work.
Footnotes
Supported by the British Heart Foundation, Northern Ireland Kidney Research Fund, and the AAB Barros D'Sa/Thompson Vascular Education and Research Fund.
Reprints: Robert C. Baker, MD, Department of Surgery, Queen's University Belfast, Grosvenor Road, Belfast, BT12 6BA, Northern Ireland, United Kingdom. E-mail: robincbaker@hotmail.com.
REFERENCES
- 1.Jerkic M, Miloradovic Z, Jovovic D, et al. Relative roles of endothelin-1 and angiotensin II in experimental post-ischaemic acute renal failure. Nephrol Dial Transplant. 2004;19:83–94. [DOI] [PubMed] [Google Scholar]
- 2.Price RG. The role of NAG (N-acetyl-beta-D-glucosaminidase) in the diagnosis of kidney disease including the monitoring of nephrotoxicity. Clin Nephrol. 1992;38(suppl 1):14–19. [PubMed] [Google Scholar]
- 3.Price RG. Measurement of N-acetyl-beta-glucosaminidase and its isoenzymes in urine methods and clinical applications. Eur J Clin Chem Clin Biochem. 1992;30:693–705. [PubMed] [Google Scholar]
- 4.Kunin CM, Chesney RW, Craig WA, et al. Enzymuria as a marker of renal injury and disease: studies of N-acetyl-beta-glucosaminidase in the general population and in patients with renal disease. Pediatrics. 1978;62:751–760. [PubMed] [Google Scholar]
- 5.Jorres A, Kordonouri O, Schiessler A, et al. Urinary excretion of thromboxane and markers for renal injury in patients undergoing cardiopulmonary bypass. Artif Organs. 1994;18:565–569. [DOI] [PubMed] [Google Scholar]
- 6.Westhuyzen J, McGiffin DC, McCarthy J, et al. Tubular nephrotoxicity after cardiac surgery utilising cardiopulmonary bypass. Clin Chim Acta. 1994;228:123–132. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura T, Inada K, Nara N, et al. Influence of methylprednisolone on cytokine balance during cardiac surgery. Crit Care Med. 1999;27:545–548. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura T, Inada K, Okada H, et al. Methylprednisolone inhibits increase of interleukin 8 and 6 during open heart surgery. Can J Anaesth. 1995;42:399–403. [DOI] [PubMed] [Google Scholar]
- 9.Toft P, Christiansen K, Tonnesen E, et al. Effect of methylprednisolone on the oxidative burst activity, adhesion molecules and clinical outcome following open heart surgery. Scand Cardiovasc J. 1997;31:283–288. [DOI] [PubMed] [Google Scholar]
- 10.Jansen NJ, van Oeveren W, van Vliet M, et al. The role of different types of corticosteroids on the inflammatory mediators in cardiopulmonary bypass. Eur J Cardiothorac Surg. 1991;5:211–217. [DOI] [PubMed] [Google Scholar]
- 11.Inaba H, Kochi A, Yorozu S. Suppression by methylprednisolone of augmented plasma endotoxin-like activity and interleukin-6 during cardiopulmonary bypass. Br J Anaesth. 1994;72:348–350. [DOI] [PubMed] [Google Scholar]
- 12.Chaney MA, Nikolov MP, Blakeman B, et al. Pulmonary effects of methylprednisolone in patients undergoing coronary artery bypass grafting and early tracheal extubation. Anesth Analg. 1998;87:27–33. [DOI] [PubMed] [Google Scholar]
- 13.Gormley SM, McBride WT, Armstrong MA, et al. Plasma and urinary cytokine homeostasis and renal dysfunction during cardiac surgery. Anesthesiology. 2000;93:1210–1216 ; discussion 1215A. [DOI] [PubMed]
- 14.Patel NS, Chatterjee PK, Di Paola R, et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. 2005;312:1170–1178. [DOI] [PubMed] [Google Scholar]
- 15.Bhagat K, Hingorani AD, Palacios M, et al. Cytokine-induced venodilatation in humans in vivo: eNOS masquerading as iNOS. Cardiovasc Res. 1999;41:754–764. [DOI] [PubMed] [Google Scholar]
- 16.Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073. [DOI] [PubMed] [Google Scholar]
- 17.Hafezi-Moghadam A, Simoncini T, Yang E, et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8:473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dschietzig T, Richter C, Pfannenschmidt G, et al. Dexamethasone inhibits stimulation of pulmonary endothelins by proinflammatory cytokines: possible involvement of a nuclear factor kappa B dependent mechanism. Intensive Care Med. 2001;27:751–756. [DOI] [PubMed] [Google Scholar]
- 19.McBride WT, Allen S, Gormley SM, et al. Methylprednisolone favourably alters plasma and urinary cytokine homeostasis and subclinical renal injury at cardiac surgery. Cytokine. 2004;27:81–89. [DOI] [PubMed] [Google Scholar]


