Abstract
Objective:
The objective of this study was to clarify the incidence and risk factors for developing incisional surgical site infection (SSI) in both elective colon and rectal surgery.
Summary Background Data:
SSI is a frequent complication after elective colorectal resection. The National Nosocomial Infection Surveillance system surveys all colorectal surgeries together, without differentiating the type of colorectal surgery performed. However, rectal surgery may have a higher risk for SSI, and identifying risk factors that are more specific to each procedure would be more predictive.
Methods:
We conducted prospective SSI surveillance of all elective colorectal resections performed by a single surgeon in a single institution from November 2000 to July 2004. The data for colon and rectal surgeries were collected separately. The outcome of interest was incisional SSI. Univariate and multivariate analyses were performed to determine the predictive significance of variables in each type of surgery.
Results:
A total of 556 colorectal resections, consisting of 339 colon and 217 rectal surgeries, were admitted to the program. The incisional SSI rates in colon and rectal surgeries were 9.4% and 18.0%, respectively (P = 0.0033). Risk factors for developing incisional SSI in colon surgery were ostomy closure (OR = 7.3) and lack of oral antibiotics (OR = 3.3), while in rectal surgery, risk factors were preoperative steroids (OR = 3.7), preoperative radiation (OR = 2.8), and ostomy creation (OR = 4.9).
Conclusions:
Colon and rectal surgeries differ with regard to incidence and risk factors for developing incisional SSI. SSI surveillance for such surgeries should be performed separately, as this should lead to more efficient identification of risk factors and a reduction in SSI.
Prospective surveillance of incisional surgical site infection (SSI) following elective colorectal surgery was conducted. The incidence of SSI was higher in rectal surgery than in colon surgery, and the risk factors also differed between these surgeries. Colon and rectal surgery should be surveyed separately to achieve more efficient SSI surveillance.
Surgical site infection (SSI) is the most frequent nosocomial infection among surgical patients, accounting for 38% of all such infections.1,2 It increases medical costs, prolongs hospital stay, and occasionally leads to mortality.1,3,4 Nationwide SSI surveillance has been conducted in more than 300 hospitals in the United States since the establishment of the National Nosocomial Infection Surveillance (NNIS) system in 1970 and, as a result, an enormous amount of data has been accumulated.5
In colorectal surgery, in particular, SSI is a frequent cause of morbidity with an incidence of up to 30% in previous studies.6–10 The NNIS system categorizes all colorectal surgeries into the same “COLO” group, and the SSI rates within this group are stratified according to the NNIS risk index, which consists of the following three factors: an American Society of Anesthesiologists (ASA) score of 3, 4, or 5; a wound classification of contaminated or dirty-infected; and duration of operation lasting more than 3 hours.5 However, previous studies have suggested that SSI rates might not be the same in colon and rectal surgery.11,12 Surgery for rectal cancer is often associated with ostomy formation, preoperative radiation and total mesorectal excision (TME) with anastomosis close to the anal verge, all of which could lead to surgery that lasts longer and has greater bacterial contamination.13–15,16 Therefore, rectal surgery might have a higher risk for developing SSI. Furthermore, the NNIS risk index has been criticized as being unsuitable for risk evaluation in elective colorectal surgery because most patients undergoing such a procedure have an ASA score of 1 or 2 and a wound classification of clean-contaminated.10,16,17 The risk factors for colon and rectal procedures developing SSI may differ, and risk factors that are more specific to each procedure would be more predictive.
In an attempt to clarify the difference between colon and rectal surgery with regard to SSI, we started a prospective SSI surveillance program in a department specializing in colorectal surgery at a university hospital in Japan. The aim of this study was: 1) to determine the actual incidence of incisional SSI in elective colon and rectal surgery, and 2) to identify the risk factors for developing incisional SSIs in these two procedures.
METHODS
Surveillance Methods and Surgical Protocols
From November 2000 to July 2004, all patients undergoing elective resection of the colon and rectum via laparotomy were admitted to our SSI surveillance program at the Department of Colorectal Surgery, University of Tokyo, Japan, using the NNIS System.1,18 All operations were performed or supervised by 1 surgeon (T.W.). Attending physicians and nurses observed all wounds at least once a day until the patients were discharged. Postdischarge surveillance of SSIs was carried out in the outpatient clinic, and all patients were followed up for at least 30 days postoperatively. The diagnosis of SSI was made after discussion among attending physicians, nurses and a member of the infection control staff (T.K.), based on the definitions stated in the guidelines issued by the Center for Disease Control and Prevention.1 The latter person (T.K.) prospectively collected the surveillance data, including patients’ names, age, gender, height, weight, diagnosis, history of diabetes, preoperative steroid use, preoperative albumin level, preoperative hemoglobin level, ASA score as determined by the anesthesiologist, use of nonabsorbable antibiotic bowel preparations, procedures performed, additional operative procedures including ostomy creation and ostomy closure, use of laparoscopy, date of operation, duration of operation, surgical wound classification, preoperative irradiation, and perioperative transfusion. Outcome variables included development and date of incisional SSI (superficial or deep).
The only modification to the NNIS system in our surveillance program was that the surgical procedures were subclassified into colon surgery and rectal surgery, and the data were collected separately. Colon surgery was defined as a procedure manipulating only above the peritoneal reflection, while rectal surgery was defined as manipulating below the peritoneal reflection and resecting the extraperitoneal portion of the rectum.
All elective procedures had preoperative bowel preparation with oral laxative and glycerin enema. All cases after June 2003 received oral antibiotic preparation on the day before the operation, using metronidazole and kanamycin. With regard to the regimen of systemic prophylactic antibiotics, the use of second-generation cephalosporins was strictly ensured for all cases, and administration was started 30 minutes before incision, repeated every 3 hours during surgery, and then stopped within 24 hours after the operation. Routine preoperative shaving was performed using electric clippers. Abdominal incisions were closed primarily in all cases using polydioxanone monofilament absorbable sutures for the fascia and nonabsorbable sutures for the skin, in principle. Subcutaneous drains were not used.
Dependent Variables
The outcome of interest was a diagnosis of incisional SSI (superficial or deep), as defined by the NNIS system.1 The criteria for superficial incisional SSI were an infection that occurred at the incision site within 30 days after surgery involving only the skin and subcutaneous tissue, and at least one of the following: purulent drainage from the incision; an organism isolated from a culture of fluid from the incision; incisional pain, tenderness, localized swelling, redness, or heat, and opening of the wound. The criteria for deep incisional SSI were an infection that occurred within 30 days after surgery involving the muscle and fascial layers but not organ space, and at least one of the following: purulent drainage from the deep incision; an incision that spontaneously dehisced or was deliberately opened by a surgeon in the presence of the signs and symptoms of infection described previously. Superficial and deep incisional SSIs were combined into the same diagnosis of incisional SSI because in some cases it was difficult to determine whether a diagnosis of deep or superficial SSIs was appropriate, which might have led to misclassification.
Independent Variables
Patient age was evaluated as a continuous and categorical variable (≤60 years, 61–70 years, ≥71 years). Body mass index (BMI) in kg/m 2, calculated from height and weight, was evaluated as a categorical variable (<25, ≥25). Preoperative serum albumin level was evaluated as a categorical variable (≤3.0 and ≥3.1). Anemia was defined as a preoperative hemoglobin level less than 11.0. ASA score was evaluated as a categorical variable (≤2 and ≥3). Duration of operation was evaluated as a categorical variable (<3 hours, 3–4 hours, 4–5 hours, >5 hours). Surgical wound class was evaluated as a categorical variable consisting of clean-contaminated (class 2) and contaminated-dirty (class 3 or 4). Perioperative transfusion of cellular or plasma products was evaluated as a single categorical variable. Other variables were all categorical variables and are presented in the results.
Statistical Analysis
Statistical analysis was performed using JMP software (SAS Institute Inc., Cary, NC). The univariate relation between each independent variable and incisional SSI was evaluated using a logistic model for continuous variables and Pearson's χ2 test for categorical variables. Independent variables with a P value ≤0.2 in the univariate analysis were entered into the multivariate logistic regression model, using a Wald statistic backward stepwise selection. P values <0.05 were considered to be statistically significant.
RESULTS
A total of 556 elective colorectal resections, consisting of 339 colonic surgeries (61%) and 217 (39%) rectal surgeries, were admitted to our 46-month surveillance program, with all cases being eligible for the study. In patients who underwent colonic surgery, the mean age was 63 years (range, 20–94 years), and 219 (65%) were male. The most common procedure performed was left-sided colectomy 155 (46%), followed by right-sided colectomy 94 (28%) and other colectomies 90 (27%). A total of 32 (9.4%) incisional SSIs were identified out of 339 colonic surgeries. Patients who underwent rectal surgery, on the other hand, had a mean age of 59 years (range, 15–89 years) and included 150 (69%) males. The procedures performed included low anterior resection 132 (61%), abdominoperineal resection 51 (24%), and total colectomy or proctocolectomy 34 (16%). A total of 39 (18.0%) incisional SSIs were diagnosed among 217 rectal surgeries. Thus, the incidence of incisional SSI in rectal surgery was statistically higher than in colonic surgery (P = 0.0033, Pearson's χ2 test).
Univariate Analysis
None of the variables relating to patient characteristics was statistically associated with the development of incisional SSI (Table 1). However, preoperative steroid use, diabetes, and anemia showed a tendency to develop incisional SSIs in those patients who underwent rectal surgery, as their P values were less than 0.2.
TABLE 1. Patient Characteristics and Incisional SSI
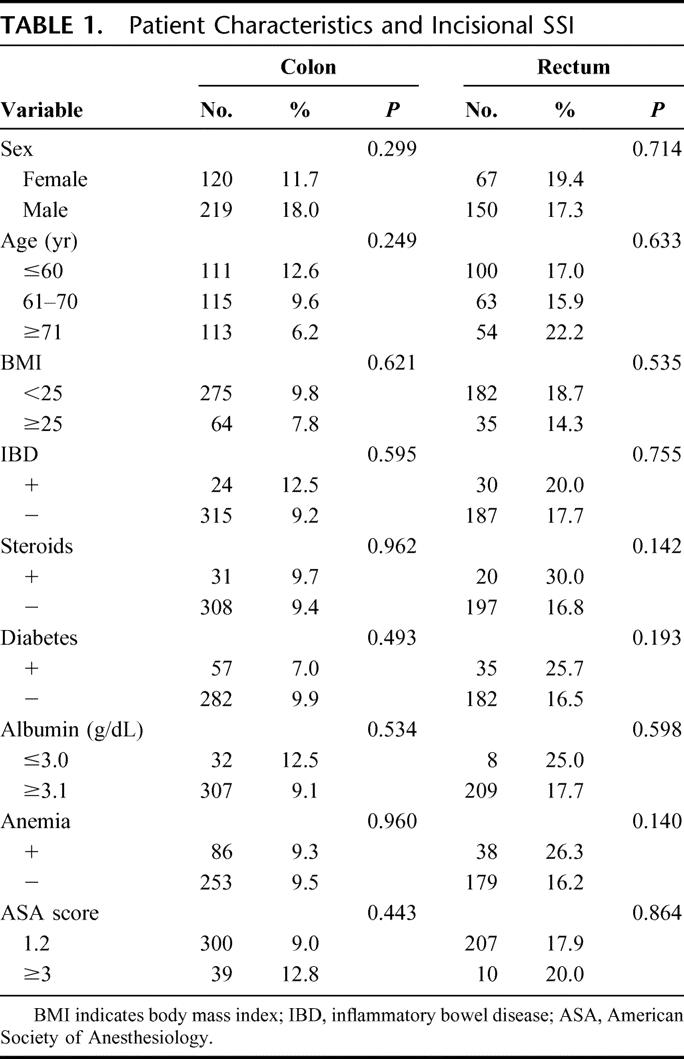
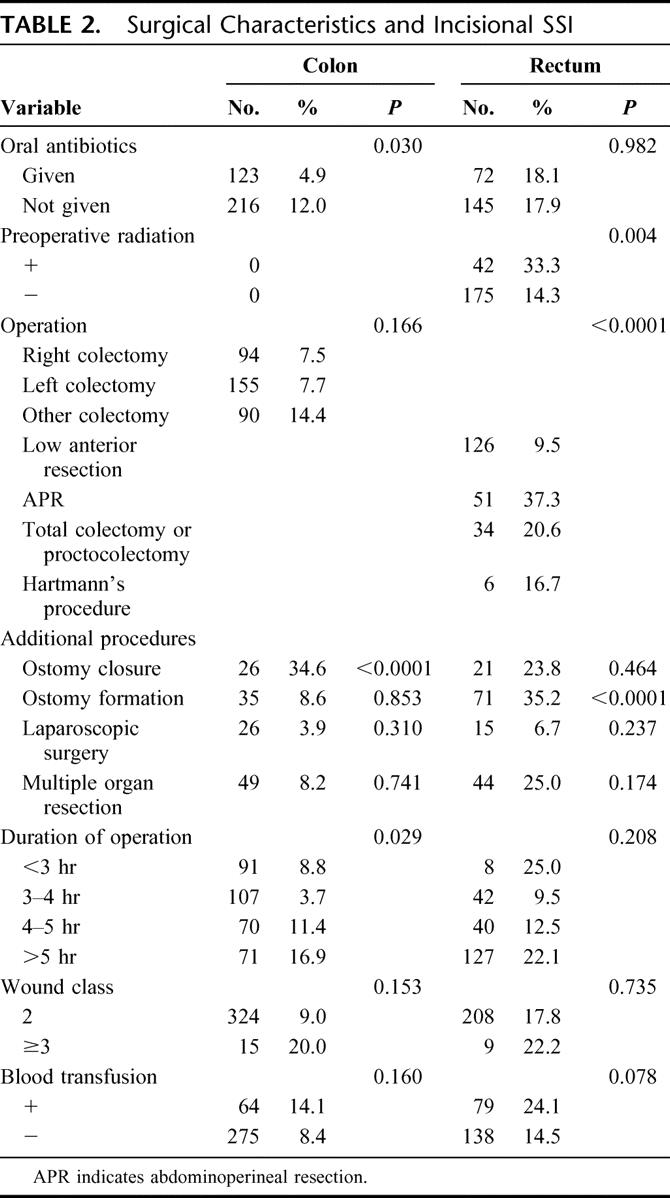
Table 2 shows the association between surgical characteristics and incisional SSI. In colonic surgery, ostomy closure, longer duration of operation, and lack of preoperative oral antibiotics were statistically associated with a higher incidence of incisional SSI. Contaminated or dirty wound, operative procedure, and blood transfusion also exhibited a tendency to develop incisional SSI, as their P values were less than 0.2. In rectal surgery, on the other hand, ostomy creation, preoperative radiation, and operative procedure were statistically associated with the development of incisional SSI. Blood transfusion and simultaneous multiple organ resection also showed a trend toward developing incisional SSI.
TABLE 2. Surgical Characteristics and Incisional SSI
Multivariate Analysis
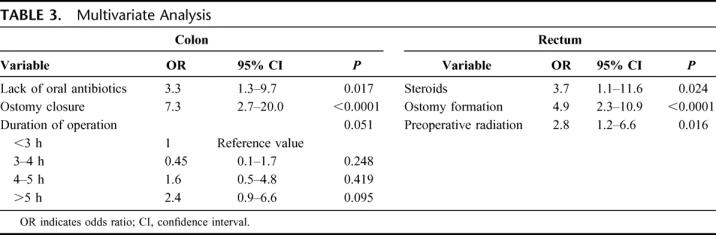
Following univariate analysis, those variables with a P value less than 0.2 were selected for multivariate analysis using a stepwise logistic regression model. Table 3 summarizes the results of multivariate analysis. Lack of oral antibiotics and ostomy closure were independently predictive of developing incisional SSIs after colonic surgery. Longer duration of operation also showed a trend toward developing incisional SSI (P = 0.051). In contrast, independent risk factors for incisional SSI after rectal surgery were preoperative use of steroids, ostomy creation, and preoperative radiation.
TABLE 3. Multivariate Analysis
DISCUSSION
SSI surveillance has been shown to reduce SSI.1,4,19 A successful surveillance program includes stratification of operative procedures according to SSI rates and risk factors that enable analysis of procedure-specific risk factors, subsequent clinical intervention, and periodical feedback of actual SSI rates to surgeons.1,20 Our study demonstrated that colonic and rectal surgeries differ with regard to the incidence of incisional SSI. We found that the incisional SSI rate in rectal surgery was 18.0%, which was nearly twice as high as the 9.4% found in colonic surgery. Furthermore, the risk factors for developing incisional SSI were different. Independent risk factors in colonic surgery were colostomy closure and lack of oral antibiotics. In contrast, risk factors in rectal surgery were creation of colostomy, preoperative radiation, and preoperative use of steroids. In the NNIS system, colonic and rectal surgeries are categorized into the same “COLO” group.5 However, our results suggest that colonic and rectal surgery should be surveyed separately to achieve more efficient SSI surveillance, as defined in the Japanese Nosocomial Infection Surveillance System.21
Antimicrobial bowel preparation with nonabsorbable oral antibiotics for elective colorectal surgery is strongly recommended in the NNIS guidelines for prevention of SSI.1 However, there has been great controversy regarding the efficacy of this. Some studies have reported that oral antibiotics are of no added value when appropriate parenteral antibiotics are administered,11,22,23 and more than 50% of surgeons in the United States feel that prophylactic oral antibiotics are doubtful or even unnecessary.24 Our results have revealed that the use of oral antibiotics was associated with a lower incisional SSI rate in colonic surgery. On the other hand, the effect on rectal surgery was not remarkable. Although microbial flora is heavier in the distal bowel,25 a proper mechanical preparation would eliminate the differential bacterial counts in the colon and rectum due to stool presence. Therefore, oral antibiotics are expected to be equally effective in reducing the number of microorganisms in the colon and rectum. A prospective randomized trial is needed to clarify whether the efficacy of oral antibiotics truly differs in colon and rectal surgery.
The use of diversion colostomy in anterior resection has been controversial.16 Previous studies have demonstrated that diversion colostomy enhances the risk for developing incisional SSI without reduction of clinical anastomotic leakage.16,26–28 The present study provides supportive evidence that creation of colostomy is an independent risk factor for incisional SSI in rectal surgery. Furthermore, closure of diversion colostomy can again lead to wound infection. The present study indicates that colostomy closure is an independent risk factor for incisional SSI among colonic surgeries. Previous reports also stated that closure of colostomy with primary wound closure has a high wound infection rate of up to 41%.29,30 Thus, both our results and previous reports suggest that the use of diverting colostomy in anterior resection should be carefully examined and could be justified only in high-risk patients or procedures such as TME or sphincter-saving resection with low level anastomosis.
The present study has revealed that preoperative radiation is an independent risk factor for incisional SSI in rectal surgery. However, it remains questionable whether preoperative radiation itself is actually a cause of incisional SSIs. A large randomized control study comparing TME, with and without preoperative radiation, demonstrated that there was no difference regarding the wound infection rate between these 2 groups.31 In our institution, indication for preoperative radiation has been limited to rectal cancer in the lower rectum, and all such patients underwent TME following radiation, consisting of abdominoperineal resection or low anterior resection with anastomosis close to the anal verge. Previous studies have reported that TME, including both low anterior resection and abdominoperineal resection, was associated with high incisional SSI rates of up to 32%, even without preoperative radiation.10,32 Thus, preoperative radiation in our study might be an indicator of a more difficult procedure, which could have led to preoperative radiation being a risk factor for incisional SSI.
The present study was performed within a single institution. All operations were performed or supervised by 1 surgeon (T.W.), and all diagnoses of SSI, data collection, and analysis were prospectively conducted by 1 member of the infection control staff (T.K.). This enabled us to minimize interhospital or interobserver variations, such as differences in antibiotics regimen, methods of bowel preparation, operating room environment, aseptic and surgical technique, diagnosis of SSI, and surveillance coverage after discharge.
There are several important limitations to be noted with this study. First, this was a retrospective analysis of prospectively collected data, and not a randomized controlled study to examine the significance of specific risk factors for incisional SSI. Statistical correlations between the risk factors and incisional SSI do not determine any “cause-and-effect” relationship between them. Thus, it is important to carefully examine the results of the analysis to avoid misinterpretation. Second, there are other known risk factors that were not evaluated but that could predispose a patient to SSI, including history of smoking,33 weight loss,34 intraoperative hypotension or hypothermia,10,35 postoperative glucose control,36 and arterial hypoxemia.37 In the present study, however, all variables to be recorded were determined from the start of our surveillance program, and all the data were collected prospectively. The addition of retrospective research by reviewing medical records was discouraged because it could have degraded the quality of the data by the accumulation of incorrect or missing data. Despite these limitations, we think that this study reflects actual incisional SSI rates, as well as risk factors in this patient population and data setting.
CONCLUSION
The incidence of incisional SSI was higher in rectal surgery than in colonic surgery, and the risk factors for incisional SSI were also different between these surgeries. We think that SSI surveillance for these surgeries should be performed separately, which should lead to more efficient identification of the risk factors and a further reduction in SSI.
ACKNOWLEDGMENTS
The authors thank all the members of the surgical staff at the Department of Colorectal Surgery in University of Tokyo for help with data recording, Dr. Naoyuki Umetani for support in statistical analysis, and both Dr. Keita Morikane at National Institute of Infectious Diseases and Dr. Kazuhiko Fukatsu at National Defense Medical College Research Institute for supervision of our SSI surveillance program.
Footnotes
Supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports, Culture, and Technology of Japan and from the Ministry of Health, Labour and Welfare, and in part by the Public Trust of Surgery Research Fund.
Reprints: Tsuyoshi Konishi, MD, Department of Surgical Oncology, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan. E-mail: KONISHIT-SUR@h.u-tokyo.ac.jp.
REFERENCES
- 1.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999: Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278; quiz 279–280. [DOI] [PubMed]
- 2.Weiss CA 3rd, Statz CL, Dahms RA, et al. Six years of surgical wound infection surveillance at a tertiary care center: review of the microbiologic and epidemiological aspects of 20,007 wounds. Arch Surg. 1999;134:1041–1048. [DOI] [PubMed] [Google Scholar]
- 3.Olson MM, Lee JT Jr. Continuous, 10-year wound infection surveillance: results, advantages, and unanswered questions. Arch Surg. 1990;125:794–803. [DOI] [PubMed] [Google Scholar]
- 4.Cruse PJ, Foord R. The epidemiology of wound infection: a 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27–40. [DOI] [PubMed] [Google Scholar]
- 5.National Nosocomial Infections Surveillance (NNIS) System Report. Data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. [DOI] [PubMed] [Google Scholar]
- 6.Bullard KM, Trudel JL, Baxter NN, et al. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48:438–443. [DOI] [PubMed] [Google Scholar]
- 7.Clarke JS, Condon RE, Bartlett JG, et al. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg. 1977;186:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppa GF, Eng K, Gouge TH, et al. Parenteral and oral antibiotics in elective colon and rectal surgery: a prospective, randomized trial. Am J Surg. 1983;145:62–65. [DOI] [PubMed] [Google Scholar]
- 9.Stone HH, Hooper CA, Kolb LD, et al. Antibiotic prophylaxis in gastric, biliary and colonic surgery. Ann Surg. 1976;184:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann Surg. 2004;239:599–605; discussion 605–607. [DOI] [PMC free article] [PubMed]
- 11.Nichols RL, Choe EU, Weldon CB. Mechanical and antibacterial bowel preparation in colon and rectal surgery. Chemotherapy. 2005;51(suppl 1):115–121. [DOI] [PubMed] [Google Scholar]
- 12.Coppa GF, Eng K. Factors involved in antibiotic selection in elective colon and rectal surgery. Surgery. 1988;104:853–858. [PubMed] [Google Scholar]
- 13.Enker WE, Merchant N, Cohen AM, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg. 1999;230:544–552; discussion 552–554. [DOI] [PMC free article] [PubMed]
- 14.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutton CD, Williams N, Marshall LJ, et al. A technique for wound closure that minimizes sepsis after stoma closure. Aust NZ J Surg. 2002;72:766–767. [DOI] [PubMed] [Google Scholar]
- 16.Tang R, Chen HH, Wang YL, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg. 2001;234:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenbroucke-Grauls C, Schultsz C. Surveillance in infection control: are we making progress? Curr Opin Infect Dis. 2002;15:415–419. [DOI] [PubMed] [Google Scholar]
- 18.Konishi T, Watanabe T, Morikane K, et al. Prospective surveillance effectively reduced surgical site infections in elective colorectal surgery at a university hospital in Japan. Infect Control Hosp Epidemiol. 2006;27:526–528. [DOI] [PubMed] [Google Scholar]
- 19.Condon RE, Schulte WJ, Malangoni MA, et al. Effectiveness of a surgical wound surveillance program. Arch Surg. 1983;118:303–307. [DOI] [PubMed] [Google Scholar]
- 20.Consensus Paper on the Surveillance of Surgical Wound Infections. The Society for Hospital Epidemiology of America, the Association for Practitioners in Infection Control, the Centers for Disease Control, the Surgical Infection Society. Infect Control Hosp Epidemiol. 1992;13:599–605. [PubMed] [Google Scholar]
- 21.Morikane K, Konishi T, Nishioka M, et al. Initiation of nationwide nosocomial infection surveillance in Japan. Infect Control Hosp Epidemiol. 2000;21:156. [Google Scholar]
- 22.Song F, Glenny AM. Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials. Health Technol Assess. 1998;2:1–110. [PubMed] [Google Scholar]
- 23.Espin-Basany E, Sanchez-Garcia JL, Lopez-Cano M, et al. Prospective, randomised study on antibiotic prophylaxis in colorectal surgery: is it really necessary to use oral antibiotics? Int J Colorectal Dis. 2005;20:542–546. [DOI] [PubMed] [Google Scholar]
- 24.Zmora O, Wexner SD, Hajjar L, et al. Trends in preparation for colorectal surgery: survey of the members of the American Society of Colon and Rectal Surgeons. Am Surg. 2003;69:150–154. [PubMed] [Google Scholar]
- 25.Nichols RL, Miller B, Smith J, et al. Anaerobic infections following abdominal surgery. Clin Med. 1974;81:23–26. [Google Scholar]
- 26.Mealy K, Burke P, Hyland J. Anterior resection without a defunctioning colostomy: questions of safety. Br J Surg. 1992;79:305–307. [DOI] [PubMed] [Google Scholar]
- 27.Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355–358. [DOI] [PubMed] [Google Scholar]
- 28.Dehni N, Schlegel RD, Cunningham C, et al. Influence of a defunctioning stoma on leakage rates after low colorectal anastomosis and colonic J pouch-anal anastomosis. Br J Surg. 1998;85:1114–1117. [DOI] [PubMed] [Google Scholar]
- 29.Yakimets WW. Complications of closure of loop colostomy. Can J Surg. 1975;18:366–370. [PubMed] [Google Scholar]
- 30.Hackam DJ, Rotstein OD. Stoma closure and wound infection: an evaluation of risk factors. Can J Surg. 1995;38:144–148. [PubMed] [Google Scholar]
- 31.Marijnen CA, Kapiteijn E, van de Velde CJ, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20:817–825. [DOI] [PubMed] [Google Scholar]
- 32.Nagawa H, Muto T, Sunouchi K, et al. Randomized, controlled trial of lateral node dissection vs. nerve-preserving resection in patients with rectal cancer after preoperative radiotherapy. Dis Colon Rectum. 2001;44:1274–1280. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen LT, Horby J, Friis E, et al. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol. 2002;28:815–820. [DOI] [PubMed] [Google Scholar]
- 34.Malone DL, Genuit T, Tracy JK, et al. Surgical site infections: reanalysis of risk factors. J Surg Res. 2002;103:89–95. [DOI] [PubMed] [Google Scholar]
- 35.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization: Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–1215. [DOI] [PubMed] [Google Scholar]
- 36.Chong T, Sawyer R. Update on the epidemiology and prevention of surgical site infections. Curr Infect Dis Rep. 2002;4:484–490. [DOI] [PubMed] [Google Scholar]
- 37.Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic: the effect of inspired oxygen on infection. Arch Surg. 1984;119:199–204. [DOI] [PubMed] [Google Scholar]


