Abstract
Objective:
To define the role of BRAF gene mutation in the progression of papillary thyroid carcinoma.
Summary Background Data:
BRAF gene mutation is frequently detected in papillary thyroid carcinoma. Its role in pathogenesis or progression is under investigation.
Methods:
Patients who underwent thyroidectomy and sentinel lymph node biopsy for papillary thyroid cancer were accrued. BRAF mutation was assessed in primary tumors and matched sentinel lymph nodes by a quantitative real-time PCR assay.
Results:
Tissue specimens from 103 consecutive patients were evaluated. BRAF mutation of the primary tumor was detected in 34 (33%) patients. In 26 of 34 (76%) patients with BRAF mutation, concomitant lymph node metastasis was detected. On the contrary, in 69 patients with BRAF mutation-negative primary tumors, only 12 (17%) patients had lymph node metastasis (χ2, P < 0.0001). BRAF mutation was detected in 20 of 26 (77%) lymph node metastases matched to BRAF mutation-positive primary tumors; it was not detected in lymph node metastases matched to BRAF mutation-negative primary tumors. Univariate analysis identified age, stage, tumor size, and BRAF mutation as prognostic factors for lymph node metastasis. In multivariate analysis, only BRAF mutation remained a significant prognostic factor for lymph node metastasis (odds ratio = 10.8, 95% confidence interval, 3.5–34.0, P < 0.0001).
Conclusions:
BRAF mutation may be a key genetic factor for the metastatic progression of papillary thyroid carcinoma. The study demonstrates that this gene mutation is a significant risk factor for locoregional lymph node metastasis and has potential utility as a surrogate marker.
Since BRAF mutation was first identified in papillary thyroid cancer, its role in pathogenesis or progression has been investigated. Lymphatic mapping and genetic analysis of the sentinel lymph node has allowed precise characterization of tumor progression. With these techniques, BRAF mutation is recognized as a risk factor for metastasis of papillary thyroid cancer.
BRAF gene mutation has been identified in several different cancers.1–4 Papillary thyroid carcinoma is among the most common epithelial tumors that bear this mutation, occurring in 36% to 69% of reported cases.2,5,6 Although the role of BRAF mutation in the pathogenesis or progression of papillary thyroid cancer is still unclear, recent in vitro data have indicated that it may contribute to its pathogenesis.7–11 A potential etiologic role for the BRAF mutation in cancer has been previously suggested by Pollock et al,12 who reported BRAF mutation in nevi, the prospective precursor lesions of malignant melanoma.
BRAF gene encodes a protein that has a critical role in cellular signal transduction pathways. Much has been learned about BRAF, since mutations of the BRAF gene were first identified as a result of the Cancer Genome Project.1 These mutations were discovered to be restricted to the kinase domain at multiple sites located on exons 11 and 15. Although multiple mutations of the BRAF gene have been detected, the most common is a thymine to adenine transversion at nucleotide position 1796, which converts valine to glutamate.2 This BRAF V600E mutation, previously designated as BRAF V599E,13 can result in enhancement of cellular signaling activity far greater than the activity from wild-type BRAF.14 Since B-raf is a component of the mitogen-activated protein kinase pathway, BRAF mutation can amplify signal transduction upon activation of tyrosine kinase receptors.8 This and other in vitro data provide evidence that the BRAF mutation lends more aggressive properties to malignant thyroid cancer cells.5,8,9 Two recent in vitro studies, by Mitsutake et al15 and Melillo et al,16 corroborate the aggressive, invasive phenotype in thyroid cancer cells with BRAF gene mutation.
We hypothesized that BRAF mutation promotes the metastatic progression of papillary thyroid cancer to the regional lymphatic drainage basin, the most common site of metastasis. To test this hypothesis, we investigated patients undergoing thyroidectomy, lymphatic mapping, and sentinel lymph node biopsy. The lymphatic mapping technique can accurately detect regional lymph node metastasis by identifying the lymph node(s) most likely to harbor tumor metastasis, ie, the sentinel lymph node. We, therefore, determined whether detection of BRAF gene mutation of papillary thyroid carcinoma was associated with lymph node metastasis.
METHODS
Tissue Samples
Operative specimens were obtained for 103 consecutive patients who underwent thyroidectomy, lymphatic mapping, and sentinel lymph node biopsy with histopathologic diagnosis of primary papillary thyroid carcinoma between the years 2000 to 2003 at John Wayne Cancer Institute (Santa Monica, CA) (Table 1). Specimens from different patients with recurrence (n = 5) or distant metastasis (n = 1) during the study period were also identified and assessed. All patients provided informed consent for intraoperative lymphatic mapping and sentinel lymph node biopsy or specimen use as dictated by Institutional Review Board approved clinical protocols.
TABLE 1. Patient Demographics

Sentinel Lymph Node Biopsy
We have previously reported the technique of lymphatic mapping and sentinel lymph node biopsy for thyroid cancer.17 In brief, after exposure of the thyroid gland through a small, transverse low-collar skin incision, the suspicious thyroid nodule was identified. One percent isosulfan blue dye (0.5–2.0 mL; Tyco International, Norwalk, CT) was injected directly into the thyroid nodule using a 25-gauge needle and syringe. A blue-stained lymphatic channel was followed to a blue-stained node (ie, sentinel lymph node). Suspicious lymph nodes (ie, enlarged or hardened) not stained blue were also excised for diagnostic purposes. Central neck dissection was performed when frozen sections of lymph nodes contained metastasis greater than 2.0 mm in size.
Pathologic Analysis
All excised sentinel lymph nodes and other lymph nodes were routinely evaluated by frozen and permanent section. Each lymph node was processed by routine histopathologic techniques and stained with hematoxylin and eosin; when malignant cells were not detected by hematoxylin and eosin, immunohistochemistry for cytokeratin was performed to identify epithelial cells (ie, tumor cells). Criteria to identify malignant keratin-positive cells included strong cytokeratin immunoreactivity, anatomic location in nodal sinuses, and cytologic atypia similar to the primary carcinoma.18 In addition, the size of the metastasis was measured using standard hematoxylin and eosin and immunohistochemistry, whereas the primary tumors were evaluated by hematoxylin and eosin alone. The nomenclature to designate the size of lymph node metastases was isolated tumor cells (clusters ≤0.2 mm), micrometastases (>0.2 mm to ≤2.0 mm), or macrometastases (>2.0 mm) as previously dictated.18 Each tumor's location, histologic type, size, multifocality, grade, lymphovascular invasion, number of foci, and capsular extension were recorded. Tumors were staged according to criteria of the American Joint Committee on Cancer.19
Specimen Processing and PCR Analysis
Formalin-fixed, paraffin-embedded sections of primary tumors and matched lymph nodes were reviewed by surgical pathologists (R.R.T. and R.E.G.) to reconfirm the diagnosis of papillary thyroid carcinoma. The primary tumors and sentinel lymph nodes were then evaluated for the BRAF gene mutation. In patients with multicentric lesions (n = 51; 49%), the largest tumor lesion was assessed. Multiple primary lesions were analyzed in a select group of patients (n = 8) with multicentric tumors to determine whether detection of BRAF mutation was variable among lesions. In patients with multiple sentinel lymph nodes (n = 31; 30%), the additional lymph nodes were analyzed for BRAF mutation. This number of multiple sentinel lymph nodes is consistent with the range reported from smaller series.20–22
The specimen blocks were sectioned (60 μm) for DNA extraction, which was performed using a modified assay (QIAamp DNA Mini Kit, Qiagen, Ventura, CA) as previously described.23,24 In brief, DNA sections were deparaffinized with xylene, washed with 100% ethanol, and treated overnight with a proteinase K digestion buffer solution at 56°C. The next day, DNA was isolated, purified, and resuspended in molecular grade water. Quantitative real-time PCR was performed using a peptide nucleic acid (PNA) oligonucleotide clamp approach as previously described25 and locked nucleic acid (LNA) oligonucleotide to specifically detect the BRAF V600E mutation on exon 15 and allow a high-throughput platform to accurately assess large numbers of specimens.26 The PNA clamp binds with high specificity to the wild-type BRAF sequence by complementary annealing and, thereby, prevents its amplification. The LNA anneals to and reports the amplification of BRAF V600E gene mutation. DNA from paraffin-embedded tissue sections (60 μm) with the BRAF mutation was serially diluted to assess the sensitivity of the qRT assay; ≤5 copies of BRAF mutation could be detected in 20 ng of DNA. Thus, the optimized qRT assay enabled detection of 1 to 5 copies of BRAF gene mutation in 20 ng of DNA extracted from paraffin-embedded tumors.
PCR was performed using the following primers and probe. BRAF: 5′-CCTCACAGTAAAAATAGGTG-3′ (forward); 5′-ATAGCCTCAATTCTTACCA-3′ (reverse); 5′-CTACAGAGAAATCTCGAT-BHQ-1-3′ (LNA); ATCGAGATTTCACTGTAG (PNA). The PCR assay was performed with the iCycler iQ RealTime PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Genomic DNA (20 ng) from paraffin-embedded tumors was amplified using real-time PCR (iCycler) in a 20 μL reaction containing each PCR primer, LNA, PNA, deoxynucleotide triphosphate, MgCl 2, PCR buffer, and AmpliTaq Gold Polymerase (Applied Biosystems, Branchburg, NJ). Each PCR reaction was subjected to 55 cycles at 94°C for 60 seconds, 72°C for 50 seconds, and 53°C for 50 seconds and 72°C for 60 seconds. Each sample was assayed in triplicate with appropriate positive and negative cell line and reagent controls.
BRAF Gene Sequencing
Representative BRAF mutation positive and negative tumors (n = 14) were sequenced to confirm the accuracy of the PCR assay. PCR amplification was performed using the following primers for BRAF: 5′-TGTTTTCCTTTACTTACTACACCTCA-3′ (forward); and 5′-AGCATCTCAGGGCCAAAAAT-3′ (reverse). The PCR products were purified with QIAquick PCR Purification kit (Qiagen) and, subsequently, direct-sequenced at 58°C using Dye Terminator Cycle Sequence Quick Start kit (Beckman Coulter; Fullerton, CA) according to the manufacturer's instructions. Dye-terminated products were assessed by capillary array electrophoresis on a CEQ8000XL Genetic Analysis System (Beckman Coulter).
Statistical Analysis
Patient characteristics and detection of BRAF mutation were summarized using mean and frequency. Univariate logistic regression models were constructed to examine the effect of prognostic factors including age, gender, TNM T-stage, tumor size, and BRAF mutation on the probability of having lymph node metastasis. Tumor grade was not assessed secondary to the predominance of well-differentiated tumors. A multivariate analysis using logistic regression was also performed to evaluate the prognostic significance of detecting BRAF mutation when clinical prognostic factors were adjusted. All analyses were performed using Splus (Insightful, Seattle, WA) and tests were 2-sided with a significance level of P < 0.05.
RESULTS
Papillary Thyroid Cancer and BRAF Gene Mutation
BRAF gene mutation was detected in the primary tumors of 34 of 103 (33%) patients; 69 tumors had no evidence of BRAF mutation. Genomic sequencing of 14 papillary thyroid tumors confirmed the accuracy of detecting BRAF mutation by the PCR assay (Fig. 1). In patients with assessment of multicentric lesions (n = 8), BRAF was consistently detected in the largest lesion; heterogeneity in BRAF mutation was observed in 3 patients.
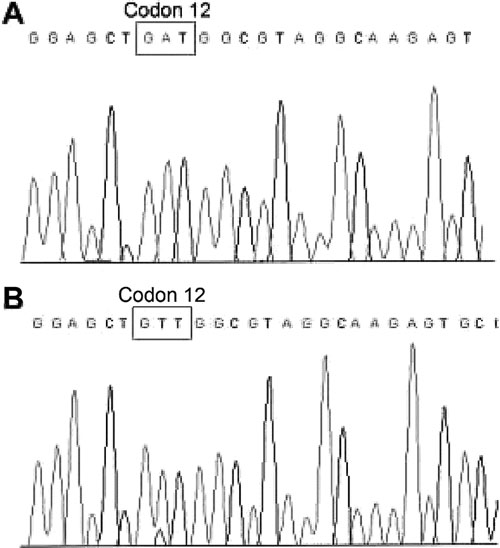
FIGURE 1. Representative sequences of (A) mutant and (B) wild-type BRAF in paraffin-embedded thyroid cancer specimens. The BRAF V600E mutation, as shown, is a single nucleotide mutation from thymine (T) to adenine (A).
At least one sentinel lymph node was identified in each of the 103 patients. Lymph node metastasis occurred in 38 of 103 (37%) patients and was uniformly detected in the sentinel lymph node. All 38 lymph node metastases were identified by hematoxylin and eosin staining without immunohistochemistry, and tumor foci were macrometastatic (n = 22; 58%) or micrometastatic (n = 16; 42%). The correlation of BRAF mutation of the primary tumor to lymph node metastasis was then assessed. Lymph node metastasis was identified in 26 of 34 (76%) patients with BRAF mutation-positive primary tumors versus 12 of 69 (17%) patients with BRAF mutation-negative primary tumors, thus demonstrating a significant association of BRAF mutation of the primary tumor with locoregional lymph node metastasis (χ2, P < 0.0001).
To determine whether BRAF mutation occurred and could be detected in metastases, sentinel lymph nodes and recurrent/metastatic lesions were analyzed by PCR. BRAF mutation was identified in the lymph node metastases from 20 of 26 (77%) patients with BRAF mutation-positive primary tumors. All BRAF mutation-positive lymph nodes were sentinel lymph node metastases, 5 of which had micrometastatic foci (>0.2 mm to ≤2.0 mm). In the 6 lymph node metastases in which BRAF mutation was not detected, tumor foci was uniformly ≤0.3 mm; and the primary tumor was unifocal (n = 3) or multicentric (n = 3). BRAF mutation was not detected in any of the histologically benign sentinel lymph nodes (n = 69 of 103; 63%). When recurrent/metastatic tumors were assessed, BRAF mutation was detected in all 6 lesions.
Univariate and Multivariate Analyses
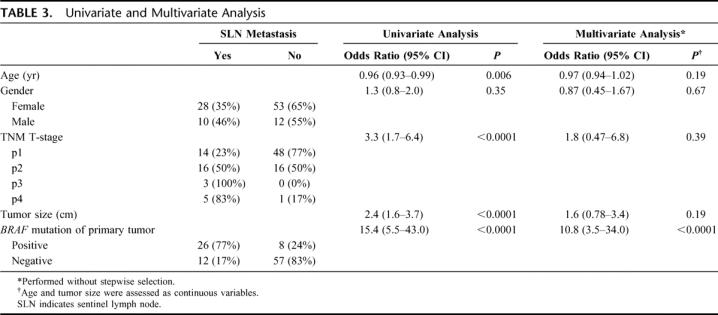
The association of clinicopathologic factors with B-RAF mutation of the primary papillary thyroid tumor was assessed. Age, TNM T-stage, tumor size, and BRAF mutation in lymph node metastases all had significant correlation with BRAF mutation of the primary tumor (Table 2). To determine the prognostic significance of clinicopathologic factors in determining lymph node metastasis, univariate and multivariate analyses were conducted. For univariate analysis, age, TNM T-stage, tumor size, and BRAF mutation were significant prognostic factors for lymph node metastasis (Table 3).
TABLE 2. Comparison of Clinicopathologic Factors and B-RAF Mutation

TABLE 3. Univariate and Multivariate Analysis
Clinicopathologic variables and lymph node metastasis were then assessed in relation to risk factor groups, which have been previously designed to classify patients with thyroid cancers as low- or high-risk patients with respect to poor clinical outcomes.27–34 According to the AMES classification (Age, distant Metastasis, tumor Extent, Size), patients with papillary thyroid cancer have high-risk disease by age (men >40 years, women >50 years), distant metastasis (most commonly to the lung), tumor extent (extrathyroid tumor spread), and tumor size (>3.0 cm).33,34 When the high-risk criteria of the AMES classification was assessed by univariate analysis, this group also had prognostic significance for lymph node metastasis (χ2, P = 0.018).
A multivariate analysis was performed by logistic regression method to predict lymph node metastasis. Clinicopathologic factors were measured with and without stepwise regression analysis to determine the best set of predictors. In both cases, BRAF mutation was the only significant prognostic factor for lymph node metastasis (odds ratio = 12.8; 95% confidence interval, 4.2–38.8, P < 0.0001; and odds ratio = 10.8; 95% confidence interval, 3.5–34.0, P < 0.0001; respectively) (Table 3); the high-risk group did not have prognostic significance. In summary, our results show that BRAF gene mutation of primary papillary thyroid carcinoma and concomitant lymph node metastasis correlated with younger age, advanced tumor stage, and larger tumor size by univariate analysis. None of the clinical factors, including the high-risk AMES group, had prognostic significance by multivariate analysis. BRAF gene mutation was the only significant prognostic factor for lymph node metastasis.
DISCUSSION
Cervical lymph node metastases have been reported in 30% to 90% of patients with papillary thyroid cancer.35 Our study narrows this range and provides an accurate identification of all potential sites of papillary thyroid cancer metastasis. We and others have reported the high sensitivity of lymphatic mapping in identifying sentinel lymph node(s) for papillary thyroid cancer.36–38 In this study, we detected a sentinel lymph node in all 103 study patients; the lymph node metastasis was uniformly detected in the sentinel lymph node. Because of the accurate detection of all lymph node metastases, we could determine whether the BRAF gene mutation correlated with lymph node metastasis.
The discovery of the BRAF gene mutation, which was first reported in 2002,1 has resulted in numerous studies theorizing its functional and pathologic role in cancer cells. These studies demonstrate the effects of the gene mutation on deregulated cell signaling.7,8,14–16 Here, we provide the initial evidence of progression of papillary thyroid carcinoma in relation to the BRAF gene mutation. Moreover, our analysis, in a cohort of 103 consecutive patients with papillary thyroid carcinoma, establishes the clear association of lymph node metastasis with BRAF mutation. This connection corroborates recent in vitro studies that assert a more aggressive behavior in thyroid cancer cells that harbor the BRAF mutation.15,16
We noted the detection of BRAF mutation in metastatic lymph nodes, a finding that supports recent reports.39,40 These metastatic lymph nodes that harbored the BRAF mutation were uniformly matched to primary tumors that also harbored the gene mutation. However, BRAF mutation was not detected in 6 patients with lymph node metastases. Several explanations could account for this disparity. In 3 patients with BRAF mutation-negative lymph nodes, the matched primary tumors were multicentric. A report by Shattuck et al41 has proposed different clonal origins of multicentric tumors. In this report, we noted heterogeneous detection of BRAF V600E mutation in multicentric tumors from a separate cohort of 3 patients; an assessment of BRAF mutation in relation to pathologic findings demonstrated no correlation to tumor multicentricity. It is a reasonable hypothesis, therefore, that a non-BRAF mutation tumor metastasized to the lymph node. Furthermore, Vasko et al39 and Oler et al40 have recently shown a disparity between the specific BRAF gene mutation in the primary tumor and the matched lymph node metastasis.
Six patients during the study period underwent surgical resection for recurrence of papillary thyroid carcinoma in the soft tissues of the neck (n = 5) or distant metastasis to lung (n = 1). None of these patients was part of the original study cohort. The BRAF mutation was detected in all 6 of these metastatic tissues, providing additional clinical evidence to support that BRAF gene mutation may confer metastatic capacity to papillary thyroid cancer. As such, we provide evidence that BRAF mutation can be detected in locoregional, recurrent, and distant metastatic papillary thyroid tumors. Therefore, the BRAF mutation has the potential to be a valuable surrogate marker for detection of regional/distant metastasis.
Our findings of aggressive tumor behavior were independent of poor tumor histology as the predominant pathologic tumor grade in our study cohort was well-differentiated tumors and none had evidence of vascular invasion. For reasons that remain unclear, BRAF mutation was associated with a relatively younger age at diagnosis of thyroid cancer (Table 2). This age discrepancy was evident by univariate analysis, which showed age, in addition to TNM T-stage, tumor size, and BRAF mutation as significant prognostic factors for lymph node metastasis. However, only BRAF mutation remained a significant prognostic factor by multivariate analysis. This clinical evidence corroborates in vitro data, demonstrating that BRAF mutation appears to function as a significant molecular alteration that promotes pathologic disease progression.
CONCLUSION
We have demonstrated that BRAF gene mutation is associated with disease progression. Moreover, a significant risk for lymph node metastasis was present when the primary tumor harbored the BRAF mutation. We conclude that BRAF V600E DNA mutation is associated with progression of disease to the regional lymphatic basins, and we propose that large cohort, long-term studies may show that patients with BRAF gene mutation, in addition to high-risk clinical features, will have worse disease outcomes. In our consecutive series of 103 patients, BRAF mutation was the only clinicopathologic factor to significantly increase the risk for lymph node metastasis. Accordingly, BRAF gene mutation may be an appropriate target for small molecule inhibitors in patients with high-risk disease progression.
Footnotes
Supported in part by P01 CA12582 and CA29605, NCI, NIH; Harold J. McAlister Foundation, Los Angeles, CA; and Martin H. Weil Research Laboratories, John Wayne Cancer Institute, Santa Monica, CA.
Reprints: Dave S. B. Hoon, PhD, Department of Molecular Oncology, John Wayne Cancer Institute, 2200 Santa Monica Blvd., Santa Monica, CA 90404. E-mail: hoon@jwci.org.
REFERENCES
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. [DOI] [PubMed] [Google Scholar]
- 2.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. [DOI] [PubMed] [Google Scholar]
- 3.Tuveson DA, Weber BL, Herlyn M. BRAF as a potential therapeutic target in melanoma and other malignancies. Cancer Cell. 2003;4:95–98. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun ES, Jones JB, Ashfaq R, et al. BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in distinct subsets of pancreatic cancer: potential therapeutic targets. Am J Pathol. 2003;163:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 6.Xu X, Quiros RM, Gattuso P, et al. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. 2003;63:4561–4567. [PubMed] [Google Scholar]
- 7.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of BRAF. Cell. 2004;116:855–867. [DOI] [PubMed] [Google Scholar]
- 8.Garnett MJ, Marais R. Guilty as charged: BRAF is a human oncogene. Cancer Cell. 2004;6:313–319. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. [DOI] [PubMed] [Google Scholar]
- 10.Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. [DOI] [PubMed] [Google Scholar]
- 11.Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. [DOI] [PubMed] [Google Scholar]
- 12.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Angelini S, Czene K, et al. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003;9:3362–3368. [PubMed] [Google Scholar]
- 14.Dibb NJ, Dilworth SM, Mol CD. Switching on kinases: oncogenic activation of BRAF and the PDGFR family. Nature. 2004;4:718–727. [DOI] [PubMed] [Google Scholar]
- 15.Mitsutake N, Knauf JA, Mitsutake S, et al. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. 2005;65:2465–2473. [DOI] [PubMed] [Google Scholar]
- 16.Melillo RM, Castellone MD, Guarino V, et al. The RET/PTC-RAS-B-RAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115:1068–1081. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Kelemen PR, Van Herle AJ, Giuliano AE. Sentinel lymphadenectomy in thyroid malignant neoplasms. Arch Surg. 1998;133:288–292. [DOI] [PubMed] [Google Scholar]
- 18.Turner RR. Histopathologic processing of the sentinel lymph node. Semin Breast Dis. 2002;5:35–40. [Google Scholar]
- 19.American Joint Committee on Cancer. AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag, 2002. [Google Scholar]
- 20.Catarci M, Zaraca F, Angeloni R, et al. Preoperative lymphoscintigraphy and sentinel lymph node biopsy in papillary thyroid cancer: a pilot study. J Surg Oncol. 2001;77:21–24. [DOI] [PubMed] [Google Scholar]
- 21.Chow TL, Lim BH, Kwok SP. Sentinel lymph node dissection in papillary thyroid carcinoma. ANZ J Surg. 2004;74:10–12. [DOI] [PubMed] [Google Scholar]
- 22.Tsugawa K, Ohnishi I, Nakamura M, et al. Intraoperative lymphatic mapping and sentinel lymph node biopsy in patients with papillary carcinoma of the thyroid gland. Biomed Pharmacother. 2002;55:100–103. [DOI] [PubMed] [Google Scholar]
- 23.Hoon DS, Spugnardi M, Kuo C, et al. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23:4014–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753–1757. [DOI] [PubMed] [Google Scholar]
- 25.Taback B, Bilchik AJ, Saha S, et al. Peptide nucleic acid clamp PCR: a novel K-ras mutation detection assay for colorectal cancer micrometastases in lymph nodes. Int J Cancer. 2004;111:409–414. [DOI] [PubMed] [Google Scholar]
- 26.Mouritzen P, Nielsen AT, Pfundheller HM, et al. Single nucleotide polymorphism genotyping using locked nucleic acid (LNA). Expert Rev Mol Diagn. 2003;3:27–38. [DOI] [PubMed] [Google Scholar]
- 27.McDermott WV Jr, Morgan WS, Hamlin E Jr, et al. Cancer of the thyroid. J Clin Endocrinol Metab. 1954;19:1336–1354. [DOI] [PubMed] [Google Scholar]
- 28.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma: a study of the EORTC thyroid cancer cooperative group. Eur J Cancer. 1979;15:1033–1041. [DOI] [PubMed] [Google Scholar]
- 29.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1,779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1058. [PubMed] [Google Scholar]
- 30.Shaha AR, Loree TR, Shah JP. Intermediate-risk group for differentiated carcinoma of thyroid. Surgery. 1994;116:1036–1041. [PubMed] [Google Scholar]
- 31.Eichhorn W, Tabler H, Lippold R, et al. Prognostic factors determining long-term survival in well-differentiated thyroid cancer: an analysis of four hundred eighty-four patients undergoing therapy and aftercare at the same institution. Thyroid. 2003;13:949–958. [DOI] [PubMed] [Google Scholar]
- 32.Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinoma: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720. [DOI] [PubMed] [Google Scholar]
- 33.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 34.Cady B. Our AMES is true: how an old concept still hits the mark; or, risk group assignment points the arrow to rational therapy selection in differentiated thyroid cancer. Am J Surg. 1997;174:462–468. [DOI] [PubMed] [Google Scholar]
- 35.Caron NR, Clark OH. Papillary thyroid cancer: surgical management of lymph node metastases. Curr Treat Options Oncol. 2005;6:311–322. [DOI] [PubMed] [Google Scholar]
- 36.Fukui Y, Yamakawa T, Taniki T, et al. Sentinel lymph node biopsy in patients with papillary thyroid carcinoma. Cancer. 2001;92:2868–2874. [DOI] [PubMed] [Google Scholar]
- 37.Arch-Ferrer J, Velazquez D, Fajardo R, et al. Accuracy of sentinel lymph node in papillary thyroid carcinoma. Surgery. 2001;130:907–913. [DOI] [PubMed] [Google Scholar]
- 38.Yao K, Turner R, Singer F, et al. Sentinel lymph node mapping in thyroid cancer. International Sentinel Node Congress, Yokohama, Japan, November 16–18, 2002.
- 39.Vasko V, Hu S, Wu G, et al. High prevalence and possible de novo formation of BRAF mutation in metastasized papillary thyroid cancer in lymph nodes. J Clin Endocrinol Metab. 2005;90:5265–5269. [DOI] [PubMed] [Google Scholar]
- 40.Oler G, Ebina KN, Michaluart P Jr, et al. Investigation of BRAF mutation in a series of papillary thyroid carcinoma and matched-lymph node metastasis reveals a new mutation in metastasis. Clin Endocrinol. 2005;62:509–511. [DOI] [PubMed] [Google Scholar]
- 41.Shattuck TM, Westra WH, Ladenson PW, et al. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. N Engl J Med. 2005;352:2406–2412. [DOI] [PubMed] [Google Scholar]


