Abstract
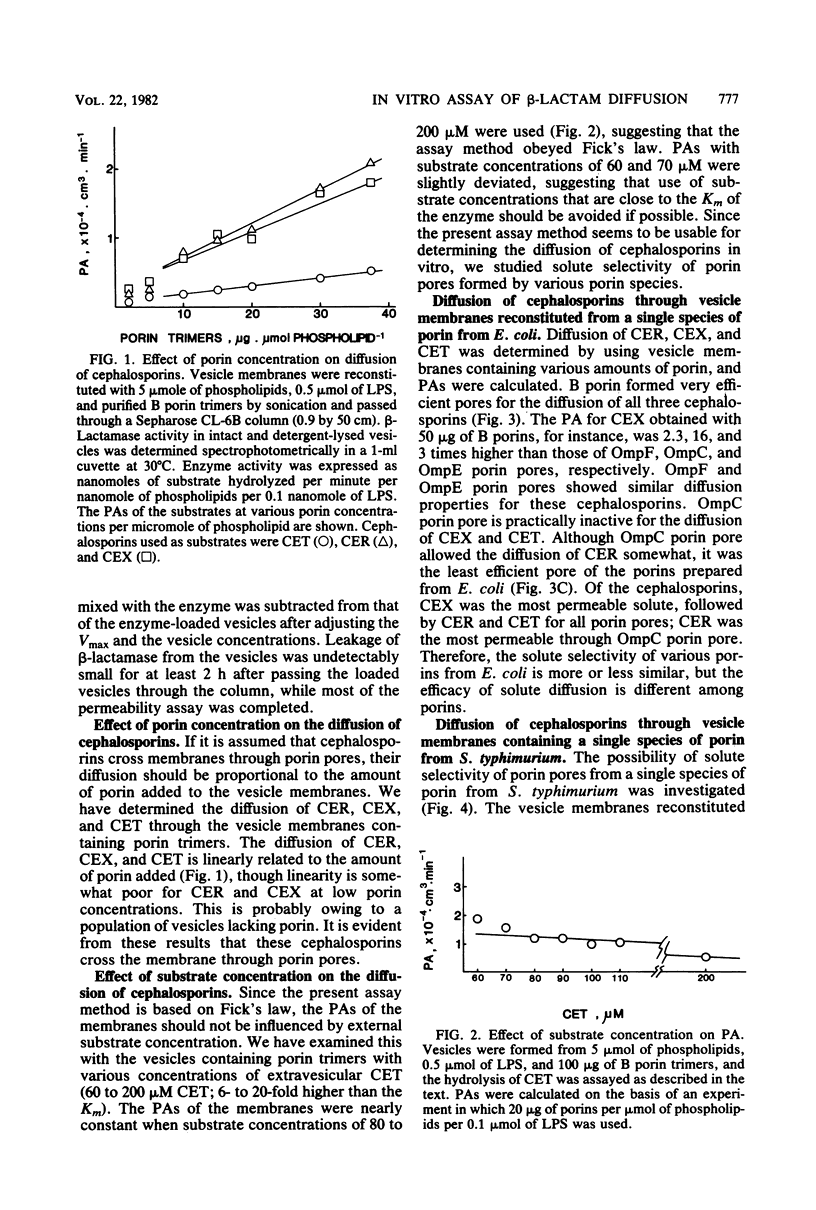
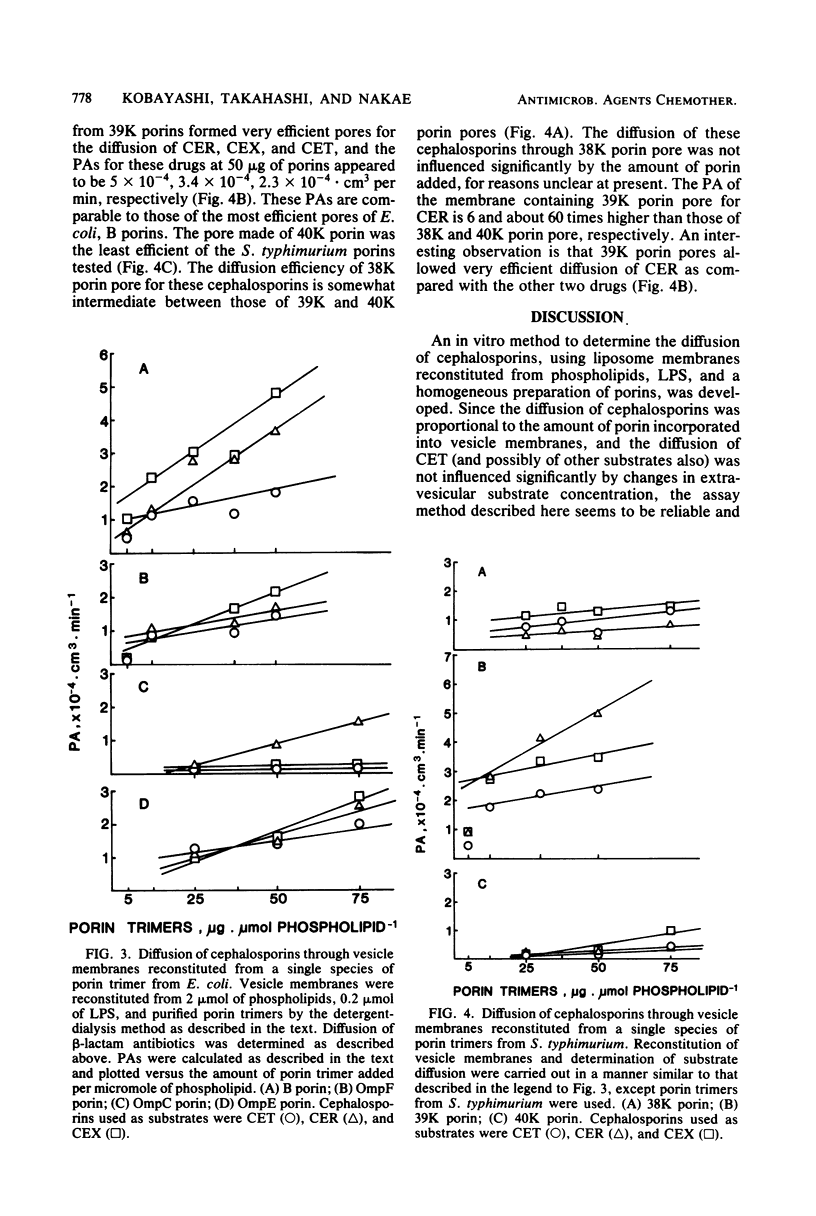
A method to determine the diffusion of cephalosporins through porin pores in vitro was developed, using liposomes reconstituted from phospholipids, lipopolysaccharides, and purified porin trimers. With this method, the roles of several species of porin pores from Escherichia coli and Salmonella typhimurium in the diffusion of cephalexin, cephaloridine, and cephalothin were examined. Results clearly showed that porins from E. coli B and 39,000-molecular-weight porins from S. typhimurium formed the most efficient pores. Thus, these were considered to represent a single functional group. OmpF and OmpE porins of E. coli K-12 and 38,000-molecular-weight porins of S. typhimurium formed moderately efficient pores. OmpC porins of E. coli K-12 and 40,000-molecular-weight porins of S. typhimurium were the least efficient pore formers. The present method can be used to distinguish the role of individual porin pores in the diffusion of cephalosporins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Ishii J., Nakae T. Determination of ion permeability through the channels made of porins from the outer membrane of Salmonella typhimurium in lipid bilayer membranes. J Membr Biol. 1980 Aug 21;56(1):19–29. doi: 10.1007/BF01869348. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Läuger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1979 Mar 8;551(2):238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii J., Nakae T. Subunit constituent of the porin trimers that form the permeability channels in the outer membrane of Salmonella typhimurium. J Bacteriol. 1980 Apr;142(1):27–31. doi: 10.1128/jb.142.1.27-31.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Mimms L. T., Zampighi G., Nozaki Y., Tanford C., Reynolds J. A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Ishii J., Tokunaga M. Subunit structure of functional porin oligomers that form permeability channels in the other membrane of Escherichia coli. J Biol Chem. 1979 Mar 10;254(5):1457–1461. [PubMed] [Google Scholar]
- Nakae T., Ishii J. Transmembrane permeability channels in vesicles reconstituted from single species of porins from Salmonella typhimurium. J Bacteriol. 1978 Mar;133(3):1412–1418. doi: 10.1128/jb.133.3.1412-1418.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Lounatmaa K., Sarvas M., Mäkelä P. H., Nakae T. Bacteriophage-resistant mutants of Salmonella typhimurium deficient in two major outer membrane proteins. J Bacteriol. 1976 Aug;127(2):941–955. doi: 10.1128/jb.127.2.941-955.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Yura T. Regulatory mutations conferring constitutive synthesis of major outer membrane proteins (OmpC and OmpF) in Escherichia coli. J Bacteriol. 1981 Jan;145(1):88–96. doi: 10.1128/jb.145.1.88-96.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Matsuba K., Yamagishi S. A method for measuring the outer membrane-permeability of beta-lactam antibiotics in gram-negative bacteria. J Antibiot (Tokyo) 1977 Dec;30(12):1134–1136. doi: 10.7164/antibiotics.30.1134. [DOI] [PubMed] [Google Scholar]
- Sawai T., Mitsuhashi S., Yamagishi S. Drug resistance of enteric bacteria. XIV. Comparison of beta-lactamases in gram-negative rod bacteria resistant to alpha-aminobenzylpenicillin. Jpn J Microbiol. 1968 Dec;12(4):423–434. doi: 10.1111/j.1348-0421.1968.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga H., Tokunaga M., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 1. Chemical analysis. Eur J Biochem. 1979 Apr;95(3):433–439. doi: 10.1111/j.1432-1033.1979.tb12982.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Nakae T. The outer membrane permeability of Gram-negative bacteria: determination of permeability rate in reconstituted vesicle membranes. FEBS Lett. 1979 Oct 1;106(1):85–88. doi: 10.1016/0014-5793(79)80700-0. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Localization of phoE, the structural gene for outer membrane protein e in Escherichia coli K-12. J Bacteriol. 1981 Jul;147(1):118–123. doi: 10.1128/jb.147.1.118-123.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]



