Abstract
Objective:
This study tested the validity of the hypothesis that eradication of esophageal varices by repeated injection sclerotherapy would reduce recurrent variceal bleeding and death from bleeding varices in a high-risk cohort of alcoholic patients with cirrhosis.
Summary Background Data:
Although banding of esophageal varices is now regarded as the most effective method of endoscopic intervention, injection sclerotherapy is still widely used to control acute esophageal variceal bleeding as well as to eradicate varices to prevent recurrent bleeding. This large single-center prospective study provides data on the natural history of alcoholic cirrhotic patients with bleeding varices who underwent injection sclerotherapy.
Methods:
Between 1984 and 2001, 287 alcoholic cirrhotic patients (225 men, 62 women; mean age, 51.9 years; range, 24–87 years; Child-Pugh grades A, 39; B, 116; C, 132) underwent a total of 2565upper gastrointestinal endoscopic sessions, which included 353 emergency and 1015 elective variceal injection treatments. Variceal rebleeding, eradication, recurrence, and survival were recorded.
Results:
Before eradication of varices was achieved, 104 (36.2%) of the 287 patients had a total of 170 further bleeding episodes after the first endoscopic intervention during the index hospital admission. Rebleeding was markedly reduced after eradication of varices. In 147 (80.7%) of 182 patients who survived more than 3 months, varices were eradicated after a mean of 5 injection sessions and remained eradicated in 69 patients (mean follow-up, 34.6 months; range, 1–174 months). Varices recurred in 78 patients and rebled in 45 of these patients. Median follow-up was 32.3 months (mean, 42.1 months; range, 3–198.9 months). Cumulative overall survival by life-table analysis was 67%, 42%, and 26% at 1, 3, and 5 years, respectively. A total of 201 (70%) patients died during follow-up. Liver failure was the most common cause of death.
Conclusion:
Repeated sclerotherapy eradicates esophageal varices in most alcoholic cirrhotic patients with a reduction in rebleeding. Despite control of variceal bleeding, survival at 5 years was only 26% because of death due to liver failure in most patients.
A total of 287 alcoholic cirrhotic patients with bleeding esophageal varices were treated with endoscopic injection sclerotherapy. Varices were eradicated in 81% of patients surviving more than 3 months but recurred in half. Cumulative overall survival by life-table analysis was 67%, 42%, and 26% at 1, 3, and 5 years, respectively.
Cirrhosis is the third leading cause of death in urban males in the United States, and alcohol abuse is the leading etiology.1–3 Major bleeding from esophageal varices is the commonest cause of death in alcoholic cirrhotics who have portal hypertension with a reported mortality of up to 50% for the initial bleed and 30% for subsequent bleeds.4–8 Endoscopic therapy is the emergency treatment of choice if actively bleeding esophageal varices are present.9–12 Even though the initial bleed may be controlled effectively by endoscopic therapy, the risk of subsequent rebleeding is substantial.13–18 There is general consensus that patients surviving a bleeding episode should be treated to prevent rebleeding.19–21 A considerable body of evidence supports the use of repeated endoscopic treatment to obliterate esophageal varices to prevent further variceal bleeding.22–28 Although banding of esophageal varices is now regarded as the most effective endoscopic method of intervention,29,30 injection sclerotherapy is still widely used to control esophageal variceal bleeding as well as to eradicate varices to prevent rebleeding.31 The major limitations of variceal injection sclerotherapy are recurrent variceal bleeds from residual varices prior to eradication, the cumulative risk of complications in patients having repeated injections,32–34 and the fiscal and logistic implications35 because of the need for continued and prolonged surveillance endoscopy.
Despite the widespread use of endotherapy, data on long-term recurrence, rebleeding and survival after variceal eradication, and the perceived need and optimal frequency of endoscopic surveillance in alcoholic cirrhotic patients with bleeding esophageal varices are limited.36–39 Our unit has previously published our early results of sclerotherapy in bleeding esophageal varices, initially using rigid endoscopy,28,40,41 and subsequently with fiberoptic endoscopy.42 The present study evaluates the overall long-term clinical results of flexible injection sclerotherapy for a large cohort of consecutively treated alcoholic cirrhotic patients with endoscopically proven bleeding esophageal varices followed up prospectively in a single surgical unit. The data presented is based on a protocol using a standardized injection technique with eradication of esophageal varices the predetermined endpoint of repeated sclerotherapy.43
PATIENTS AND METHODS
Consecutive adult alcoholic cirrhotic patients with endoscopically proven esophageal variceal bleeding admitted to a specialist surgical gastroenterology unit with a particular interest in portal hypertension in Groote Schuur Hospital, Cape Town between January 1984 and December 2001 were assessed. All patients included in the study were either referred at the time of their first variceal bleed or after a proven variceal bleed and received their first emergency endoscopic sclerotherapy injection in our unit. All injection treatments, both emergency and subsequent elective injections, were analyzed to assess the role of sclerotherapy in long-term management. All patients were studied prospectively and the data entered on a computer program by a dedicated research assistant. The diagnosis of cirrhosis was established by findings on liver function tests, ultrasound and portal Doppler assessment, liver biopsy and, in selected patients, hepatic vein wedge pressure measurements. Cirrhosis was considered to be alcohol related if patients gave a history of sustained heavy alcohol consumption over several years with corroborative histologic evidence and exclusion of other causes.
During the 210-month study period, 378 consecutive adult alcoholic cirrhotic patients were treated for esophageal variceal bleeding. Seventy-two patients who had received either variceal band ligation or who had undergone esophageal transection and gastric devascularization as part of a randomized trial after initial control of acute variceal bleeding by sclerotherapy or underwent liver transplantation were excluded from the study group and the analysis. Nineteen patients who had cirrhosis and an alcohol history and in addition had positive viral markers were also excluded from the study. Data were analyzed in April 2004 after a minimum 26-month follow-up period.
Technique of Sclerotherapy
A standard injection sclerotherapy technique was used.43 After intravenous sedation with midazolam, diagnostic endoscopy and injection sclerotherapy were performed using either a fiberoptic endoscope (model GIF 1T20 or K10; Olympus Corp, Lake Success, NY) during the first decade of the study or video-endoscopy during the last decade. The sclerosant, 5% ethanolamine oleate, was injected using a combined intravariceal and paravariceal technique.43 A maximum total volume of 25 mL of sclerosant was injected at any one endoscopy session for control of acute variceal bleeding or when large varices (grade 4 or 5) were encountered during elective sclerotherapy. An intravariceal technique alone with smaller total volumes of sclerosant was used for elective injection sclerotherapy when varices were grade 3 or less in size.43 The initial and the second sclerotherapy session a week later were performed during the index admission to hospital. Subsequent sclerotherapy was undertaken at 1-week intervals on an outpatient basis until the varices were eradicated. After variceal obliteration, surveillance endoscopy was performed at 3 and 6 months and then annually to identify patients in whom varices had recurred. Repeat injection sclerotherapy was performed whenever residual or recurrent varices were identified during surveillance endoscopy. No alcoholic cirrhotic patient was routinely given beta–blockers during follow-up.
Rebleeding
Recurrent bleeding was defined as any episode of upper gastrointestinal bleeding that occurred after the first sclerotherapy session or subsequently between scheduled treatment sessions. All such bleeding episodes were investigated by means of emergency endoscopy, undertaken promptly after admission to hospital. Rebleeding was treated according to endoscopic findings. Additional sclerotherapy was undertaken if bleeding was due to patent residual or recurrent varices. Other sources of bleeding, such as peptic ulcers, gastric varices, erosive gastritis, or portal hypertensive gastropathy were included in the definition of rebleeding. Eradication of varices was defined as the absence of esophageal varices on repeat endoscopic examination during follow-up visits.
Survival Analysis
Actuarial survival was calculated using the Kaplan-Meier method. Differences in survival in the 3 Child-Pugh grades were examined using the log-rank test. A P value less than 0.05 was considered significant. SAS System Package version 8.2 software (SAS Systems International, Cary, NC) was used for statistical analysis.
RESULTS
The 287 patients evaluated in this study included 225 men and 62 women (mean age, 51.97 years; range, 24–87 years); 39 patients were Child-Pugh grade A, 116 were grade B, and 132 were grade C when assessed on first admission.
Recurrent Bleeding Before Variceal Eradication
Rebleeding after the index injection procedure occurred in 104 of the 287 (36.2%) patients before eradication either during their index admission or after discharge from hospital. These 104 patients had a total of 170 bleeds during 164 subsequent admissions before the varices were eradicated. Ninety-one patients bled from varices and 13 patients bled from nonvariceal sources. The 91 patients had 124 variceal bleeds which were successfully treated with acute emergency injection sclerotherapy on 113 occasions. The remaining 13 bleeding episodes were from gastric varices (2), gastric erosions (1), portal hypertensive gastropathy (5), duodenal ulceration (1), Mallory Weiss tear (1), bleeding esophageal ulceration (1), esophagitis (1), and 1 site was unknown. Mean survival in these 104 patients (who rebled before eradication) was 23.8 months (median, 8.1 months) with a mean survival in Child-Pugh grades A, B, and C of 37.3, 37.6, and 11.0 months.
Eradication of Varices
Eradication and subsequent recurrence of esophageal varices after sclerotherapy was assessed in 182 of the 287 patients who survived and who were followed up for more than 3 months (Fig. 1). Of the remaining 105 patients, 90 died within 3 months of entering the study and 15 patients did not complete the 3 month follow-up period. These 15 patients either moved abroad or lived far from our center and attended other hospitals.

FIGURE 1. Eradication and recurrence of esophageal varices.
Esophageal varices were eradicated in 147 of the 182 patients (80.7%) after a median of 4.9 injections (range, 1–14) during a mean of 7.1 month (median, 4.6 months; range, 0.23–41.6 months) (Table 1). Mean survival in these 147 patients who had variceal eradication was 47.1 months (median, 36.8 months) with the mean survival in Child-Pugh grades A, B, and C of 40.2, 37.4, and 24.0 months, respectively. Esophageal varices remained eradicated in 69 patients (mean follow-up from eradication, 34.6 months; median, 23.6 months; range, 1–174 months). Mean survival in these 69 patients in whom varices remained eradicated was 39.6 months (median, 31.7 months) with the mean survival in Child-Pugh grades A, B, and C of 47.5, 42.4, and 30.0 months, respectively.
TABLE 1. Number of Injections and Time to Eradication, Recurrence, and Rebleeding

The 35 patients whose varices were not eradicated received a mean of 5.3 injections during a mean of 17 months. Twenty-four of the 35 patients died (15 liver failure, 2 bleeding esophageal varices, 3 hepatorenal failure, 2 other, 2 cause unknown). Five patients were lost to follow-up and 6 were alive at the end of the study.
Recurrent Bleeding After Variceal Eradication
Forty-five of the 78 patients with recurrence of esophageal varices after eradication presented with variceal bleeding. This occurred after a mean of 16 months (range, 0.5–172 months). Nine of these 45 patients also had additional bleeding episodes from other sites following the first variceal rebleed after eradication (7 due to portal hypertensive gastropathy and 2 bled from gastric ulcers). Twenty-two patients (48.8%) had several variceal rebleeding episodes.
Esophageal Complications
The 287 patients received 353 emergency and 1015 elective injection treatments during a total of 2565 endoscopy sessions. Minor complications of sclerotherapy were common after acute injection for active bleeding and included dysphagia, transient fever, and pulmonary atelectasis. A total of 747 complication events were documented in 234 patients during surveillance or unscheduled follow-up endoscopy after a prior variceal injection. If ulceration or stenosis was persistent and identified during a subsequent surveillance endoscopy session, this was documented as a separate additional event and recorded as such.
Mucosal ulceration at the injection site was found at follow-up endoscopy on 531 occasions in 199 patients. Subsequent sclerotherapy was delayed in patients who had mucosal ulceration greater than one quadrant of the esophageal circumference. An esophageal stricture at the injection site occurred in 25 patients after sclerotherapy, 9 of whom required esophageal dilatation on 29 occasions with relief of symptoms. Two patients developed an intramural esophageal hematoma after sclerotherapy which resolved on conservative therapy. Perforation of the esophagus occurred in 8 patients as a consequence of repeated sclerotherapy to control of recurrent acute variceal bleeding. Five of these 8 patients survived, including 3 who required surgery to treat the complication.
Survival Analysis
The cumulative survival of all 287 patients by life-table analysis was 67% at 1 year, 42% at 3 years, 26% at 5 years, and 13% at 10 years (Fig. 2). Survival according to Child-Pugh grade A was 68% at 3 years, 48% at 5 years, and 37% at 10 years. Survival of Child-Pugh grade B patients at 3 years was 54%, 34% at 5 years, and 17% at 10 years, and survival of Child-Pugh grade C patients was 21% at 3 years, 13% at 5 years, and 7% at 10 years (Fig. 3).

FIGURE 2. Cumulative survival rates by life-table analysis for all patients.
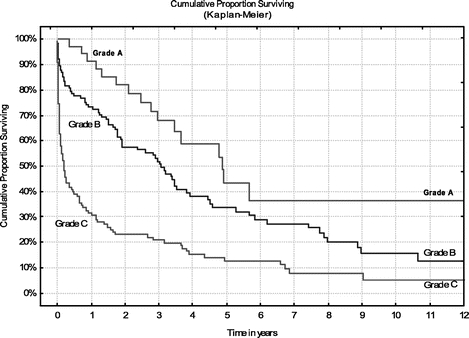
FIGURE 3. Cumulative survival rates by life-table analysis for patients at Child-Pugh risk grades A, B, and C.
Complete follow-up (median, 32.3 months; mean, 42.1 months; range, 3–198.9 months) was achieved in 133 patients of the 182 patients who survived more than 90 days. In 49 patients, follow-up was incomplete (median, 30.1 months; mean, 37.6 months; range, 3.5–104.8 months).
Causes of Death
A total of 201 (70%) of the 287 patients died during the course of the study. Liver failure was the commonest cause of death and occurred in 113 patients. Hepatorenal failure was the cause of death in 23 patients. Twelve patients died of pneumonia, 37 of multiorgan failure, often precipitated by bleeding varices, and 3 died of bleeding from other sites. Eleven patients died of other causes. These were carcinoma in 5 (lung 1, bladder 1, hepatocellular carcinoma 2, oropharynx 1), myocardial infarction 1, cerebrovascular accident 1, perforated gastric ulcer 1, acute pancreatitis 1, respiratory failure 1, and motor vehicle accident 1. In 2 patients, the cause of death could not be established.
DISCUSSION
Alcoholic liver disease is a major cause of morbidity and mortality worldwide.1,2 In Western countries, up to 50% of end-stage liver disease has alcohol as the main etiologic factor.3 The mortality from alcoholic cirrhosis is higher than nonalcoholic cirrhosis, and survival at 5 and 10 years is only 23% and 7% in some studies, with 25% of patients dying within 1 year.3,4 Thus, the mortality rate of alcoholic cirrhosis is greater than many of the major categories of carcinoma such as breast, colon, and prostate. Uncontrolled bleeding from varices and the consequences of ensuing liver decompensation are the commonest causes of death in alcoholic cirrhotics.1–4 Accurate long-term data detailing variceal recurrence or rebleeding after eradication, and survival in alcoholic cirrhotic patients are scant.36–39 In this prospective study, the long-term efficacy of sclerotherapy was evaluated by using the specific endpoints of recurrent bleeding, variceal eradication, and survival in a large group of consecutive alcoholic cirrhotic patients treated at a single surgical center.
Ultimate survival and outcome of treatment in this consecutive cohort of patients was disappointing. Although varices were eradicated in 82% of patients who survived more than 3 months, recurrent varices ultimately developed in 57% of patients, half of whom had further variceal bleeding. Several important and unresolved problems related to the role of repeated sclerotherapy in the long-term management of patients with esophageal varices remain. There is increasing recognition that an important limitation of long-term sclerotherapy is the substantial incidence of rebleeding, which is a particular feature of the early phase after endoscopic therapy has begun.24,28 The most common source of recurrent bleeding before variceal eradication in this study was from patent residual varices, which occurred in 31.7% of our patients. Urgent repeat endoscopy is essential since in 87.5% of patients with recurrent bleeding, varices were the source and were treated by sclerotherapy, which was effective in 113 variceal rebleeds in 91 patients. In 13 patients, a nonvariceal source of bleeding was identified. Serial sclerotherapy successfully eradicated esophageal varices in 80% of our patients. Although new varices formed following initial obliteration in 78 of 147 patients, this was associated with rebleeding in 45 of the 147 patients, which would support the validity of the concept of variceal eradication as a specific end point of treatment.
The number of sclerotherapy sessions required to achieve variceal obliteration has varied considerably within reported series and between centers. While there is some evidence to suggest that the technique of injection sclerotherapy might affect the number of sessions necessary to achieve obliteration,27,31 this alone does not explain the substantial differences found between patients. While the risk of rebleeding diminishes with time as the variceal channels are obliterated, some recurrent bleeds are major and may contribute to deaths from liver failure. Any protocol for long-term endoscopic management of variceal hemorrhage requires a firm definition of treatment failure, which allows alternative treatment options to be instigated. That such a definition is difficult to formulate is reflected in the major discrepancies in the proportion of treatment failures in the larger controlled trials.20,44–46 We think that patients who develop life-threatening variceal bleeding after an adequate course of treatment should be regarded as failures of long-term treatment, and in these a transjugular intrahepatic portosystemic shunt gives the best results.47 Other logistic problems with long-term sclerotherapy include the need for lifelong follow-up with repeated injections because varices recur in time. Surviving patients place an increasing burden on hospital resources, even when sclerotherapy is performed on an outpatient basis.
Injection sclerotherapy is an invasive endoscopic procedure that requires a high level of manipulative skill and mature judgment. Complications related to injection occur mostly during acute major or recurrent bleeding when varices are large and the patient is restless or uncooperative.32 The incidence of complications varies widely in reported studies because of variations in patient population, the type and severity of the underlying liver disease, and the different injection techniques used.32 In addition, the incidence of sclerotherapy-induced complications is higher when carefully documented in prospective studies.32 In the present study, complications in the 287 alcoholic patients undergoing both emergency and elective sclerotherapy were frequent, occurring in one fourth of all sclerotherapy treatments and in more than two thirds of patients overall. Asymptomatic esophageal ulceration at the injection site was the most common complication and was detected at follow-up endoscopy. Ulceration is usually of little consequence in the overall management of the patient, but occasionally subsequent sclerotherapy had to be delayed. Our present policy is to use smaller volumes of sclerosant as varices decrease in size in an attempt to reduce the extent of ulceration. When ulceration involves more than one quadrant of the esophageal circumference, further injection should be delayed until healing has occurred to prevent an esophageal stricture.43 Serious complications were esophageal strictures and perforation. Esophageal stricture was significant and persistent in 9 of the 25 patients with this complication and all 9 responded to endoscopic balloon dilatation. Perforation of the esophagus in this study was confined to emergency injection for complex recurrent bleeding48 and had a substantial mortality.
Overall survival for the entire cohort was 42% at 3 years and 26% at 5 years. Even in Child-Pugh grade A patients, there was an inexorable decline in the survival rate. Several factors, including variceal size and wall tension, portal pressure gradient of >12 mm Hg and endoscopic variceal stigmata have been documented to predict the risk of bleeding and overall prognosis.49 The cause of portal hypertension, continued consumption of alcohol, and the degree of liver decompensation are further important predictors of rebleeding and mortality.50,51 An obvious shortcoming of this paper is the lack of data detailing continued alcohol consumption, abstinence, or recidivism and their relationship to rebleeding and survival. Patients’ voluntary admission or recall of continued alcohol use is, however, notoriously inaccurate and may be unintentionally or intentionally misleading and was therefore not pursued in this study. Others have similarly indicated that any study evaluating continued alcohol consumption should be viewed with caution because of the difficulties in confirming abstinence.52 The adverse effects of alcohol on many tissues appear to be dose-related, and abstinence at any stage in the disease should be encouraged. However, the benefits to be expected in term of rebleeding and mortality may be modest. Nevertheless, only abstinent patients are considered for liver transplantation in most major centers, and this offers the best hope of improving long-term prognosis in this patient group.53,54
The range of treatment options for bleeding esophageal varices has expanded markedly during the past 2 decades. The treatment of acute and recurrent variceal bleeding is best accomplished by a skilled, knowledgeable, and well-equipped team using a multidisciplinary integrated approach. Optimal management should provide the full spectrum of treatment options, including pharmacologic therapy, endoscopic treatment, interventional radiologic procedures, surgical shunts, and liver transplantation.30 Endoscopic variceal ligation has now replaced injection sclerotherapy in the elective treatment of esophageal varices in most centers. Data from randomized controlled trials show more rapid eradication of varices with lower rates of recurrent bleeding and fewer complications such as strictures and perforation.29 However, a recent survey by the American College of Gastroenterology International GI Bleeding Registry shows that sclerotherapy is still used as frequently as banding for endoscopic intervention during index bleeding and more frequently than banding for control of variceal rebleeding.31 Likely reasons include convenience, cost, and widespread availability. It is noteworthy that several recent randomized controlled trials comparing band ligation with sclerotherapy have reported a higher recurrence rate of varices in patients undergoing band ligation.30 If this observation is confirmed by further studies, the increased recurrence rate of varices may reduce or abolish the advantage of band ligation over sclerotherapy in the long term.29 There is further evidence that endoscopic sclerotherapy is the more cost-effective treatment per life-year gained if active variceal hemorrhage is present at the index endoscopy procedure.55 This outcome appears related to the significantly higher treatment failure rate experienced with endoscopic ligation for active variceal bleeding.55 The long-term data in this study have documented the variceal recurrence and rebleeding rate using a standard sclerotherapy technique. No comparable data exist for variceal ligation, and ligation will require similar long term data to validate and vindicate its current status as the preferred endoscopic technique.
Our current management policy is for patients to have regular endoscopic therapy to achieve early variceal eradication, appreciating that factors such as esophageal ulceration and poor patient compliance may interfere with the endoscopic therapy program. After eradication of the varices, patients have surveillance endoscopy at 6- and then 12-month intervals and, if recurrent varices are identified, a comprehensive endoscopic treatment schedule is instituted again. Ultimately, the use of sequential combined endoscopic techniques with variceal banding initially when varices are large followed by sclerotherapy when varices are small may enhance the endoscopic management of esophageal varices in terms of reducing complications, facilitating earlier eradication, and preventing recurrence.30
Footnotes
Reprints: Jake E. J. Krige, MB ChB, FRCS(Ed), FACS, FCS(SA), Department of Surgery, J45 OMB, University of Cape Town Health Sciences Faculty, Anzio Road, Observatory 7925, Cape Town, South Africa. E-mail: jake@curie.uct.ac.za.
REFERENCES
- 1.Orholm M, Sorensen TI, Bentsen K, et al. Mortality of alcohol abusing men prospectively assessed in relation to history of abuse and degree of liver injury. Liver. 1985;5:253–260. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF, DeBakey S, Zobeck TS. Surveillance Report No. 18: Liver Cirrhosis Mortality in the United States, 1973–1988. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism, Division of Biometry and Epidemiology, 1991. [Google Scholar]
- 3.Propst A, Propst T, Zangerl G, et al. Prognosis and life expectancy in chronic liver disease. Dig Dis Sci. 1995;40:1805–1815. [DOI] [PubMed] [Google Scholar]
- 4.Chedid A, Mendenhall CL, Gartside P, et al. Prognostic factors in alcoholic liver disease: VA Cooperative Study Group. Am J Gastroenterol. 1991;86:210–216. [PubMed] [Google Scholar]
- 5.Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800–809. [PubMed] [Google Scholar]
- 6.Saunders JB, Walters JRF, Davies P, et al. A 20-year prospective study of cirrhosis. Br Med J. 1981;282:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332–354. [DOI] [PubMed] [Google Scholar]
- 8.Williams SGT, Westaby D. Recent advances in the endoscopic management of variceal bleeding. Gut. 1995;36:647–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krige JE, Beckingham IJ. ABC of diseases of liver, pancreas, and biliary system. Portal hypertension-1: varices. BMJ. 2001;322:348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terblanche J, Burroughs AK, Hobbs KEF. Controversies in the management of bleeding esophageal varices. N Engl J Med. 1989;320:1393–1398 and 1469–1475. [DOI] [PubMed] [Google Scholar]
- 11.Terblanche J. The surgeon’s role in the management of portal hypertension. Ann Surg. 1989;209:381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terblanche J, Krige JEJ, Bornman PC. The treatment of esophageal varices. Annu. Rev. Med. 1992;43:69–82. [DOI] [PubMed] [Google Scholar]
- 13.Fleischer DE. Etiology and prevalence of severe persistent upper gastrointestinal bleeding. Gastroenterology. 1983;84:538–543. [PubMed] [Google Scholar]
- 14.De Dombal FT, Clarke JR, Clamp SE, et al. Prognostic factors in upper GI bleeding. Endoscopy. 1986;18(supplement 2):6–10. [DOI] [PubMed] [Google Scholar]
- 15.Terblanche J. The long-term management of patients after an oesophageal variceal bleed: the role of sclerotherapy. Br J Surg. 1985;72:88–90. [DOI] [PubMed] [Google Scholar]
- 16.Terblanche J, Bornman PC, Kahn D, et al. Failure of repeated injection sclerotherapy to improve long-term survival after oesophageal variceal bleeding: a five-year prospective controlled clinical trial. Lancet. 1983;2:1328–1332. [DOI] [PubMed] [Google Scholar]
- 17.Terblanche J, Northover JMA, Bornman PC, et al. A prospective controlled trial of sclerotherapy in the long-term management of patients after esophageal variceal bleeding. Surg Gynecol Obstet. 1979;148:323–333. [PubMed] [Google Scholar]
- 18.Bendtsen F, Jensen LS. Bleeding oesophageal varices. Scand J Gastroenterol Suppl. 1996;216:1–9. [DOI] [PubMed] [Google Scholar]
- 19.Terblanche J. The use of sclerotherapy for the management of oesophageal varices in portal hypertension. Surg Endosc. 1988;2:149–155. [DOI] [PubMed] [Google Scholar]
- 20.Westaby D. Prevention of recurrent variceal bleeding: endoscopic techniques. Gastrointest Endosc Clin North Am. 1992;2:121–133. [Google Scholar]
- 21.Howard ER, Stringer MD, Mowat AP. Assessment of injection sclerotherapy in the management of 152 children with oesophageal varices. Br J Surg. 1988;75:404–408. [DOI] [PubMed] [Google Scholar]
- 22.Graffeo M, Buffoli F, Lanzani G, et al. Survival after endoscopic sclerotherapy for esophageal varices in cirrhotics. Am J Gastroenterol. 1994;89:1815–1822. [PubMed] [Google Scholar]
- 23.Copenhagen Esophageal Varices and Sclerotherapy Project. Sclerotherapy after first variceal hemorrhage in cirrhosis: a randomized multi-center trial. N Engl J Med. 1984;311:1594–1600. [DOI] [PubMed] [Google Scholar]
- 24.Westaby D, Macdougall BR, Williams R. Improved survival following injection sclerotherapy for esophageal varices: final analysis of a controlled trial. Hepatology. 1985;5:827–830. [DOI] [PubMed] [Google Scholar]
- 25.Korula J, Balart LA, Radvan G, et al. A prospective, randomized controlled trial of chronic esophageal variceal sclerotherapy. Hepatology. 1985;5:584–589. [DOI] [PubMed] [Google Scholar]
- 26.Soderlund C, Ihre T. Endoscopic sclerotherapy versus conservative management of bleeding oesophageal varices: a 5-year prospective controlled trial of emergency and longterm treatment. Acta Chir Scand. 1985;151:449–456. [PubMed] [Google Scholar]
- 27.Kitano S, Koyanagi N, Iso Y, et al. Prevention of recurrence of esophageal varices after endoscopic injection sclerotherapy with ethanolamine oleate. Hepatology. 1987;7:810–815. [DOI] [PubMed] [Google Scholar]
- 28.Terblanche J, Kahn D, Bornman PC. Long-term injection sclerotherapy treatment for esophageal varices: a 10 year prospective evaluation. Ann Surg. 1989;210:725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tait IS, Krige JE, Terblanche J. Endoscopic band ligation of oesophageal varices. Br J Surg. 1999;86:437–446. [DOI] [PubMed] [Google Scholar]
- 30.Krige JE, Shaw JM, Bornman PC. The evolving role of endoscopic treatment of esophageal varices. World J Surg. 2005;29:966–973. [DOI] [PubMed] [Google Scholar]
- 31.Sorbi D, Gostout CJ, Peura D, et al. An assessment of the management of acute bleeding varices: a multicenter prospective member-based study. Am J Gastroenterol. 2003;98:2424–2434. [DOI] [PubMed] [Google Scholar]
- 32.Krige JEJ, Terblanche J, Bornman PC. Complications of endoscopic variceal sclerotherapy. In: Sivak MV, ed. Gastroenterologic Endoscopy, 2nd ed. Philadelphia: Saunders, 1999. [Google Scholar]
- 33.Krige JEJ, Bornman Shaw JM, et al. Complications of endoscopic variceal therapy. S Afr J Surg. 2005;43:177–194. [PubMed] [Google Scholar]
- 34.Terblanche J, Stiegmann GV, Krige JEJ, et al. Long-term management of variceal bleeding: the place of varix injection and ligation. World J Surg. 1994;18:185–192. [DOI] [PubMed] [Google Scholar]
- 35.Terblanche J. Has sclerotherapy altered the management of patients with variceal bleeding? Am J Surg. 1990;160:37–42. [DOI] [PubMed] [Google Scholar]
- 36.Pugh S, Lewis S, Smith PM. Bleeding oesophageal varices in alcoholic cirrhosis: long-term follow-up of endoscopic sclerotherapy. Q J Med. 1993;86:241–245. [PubMed] [Google Scholar]
- 37.Waked I, Korula J. Analysis of long-term endoscopic surveillance during follow-up after variceal sclerotherapy from a 13-year experience. Am J Med. 1997;102:192–199. [DOI] [PubMed] [Google Scholar]
- 38.Hartigan PM, Gebhard RL, Gregory PB. Sclerotherapy for actively bleeding esophageal varices in male alcoholics with cirrhosis: Veterans Affairs Cooperative Variceal Sclerotherapy Group. Gastrointest Endosc. 1997;46:1–7. [DOI] [PubMed] [Google Scholar]
- 39.Tomikawa M, Hashizume M, Okita K, et al. Endoscopic injection sclerotherapy in the management of 2105 patients with esophageal varices. Surgery. 2002;131(suppl):171–175. [DOI] [PubMed] [Google Scholar]
- 40.Bornman PC, Kahn D, Terblanche J, et al. Rigid versus fiberoptic endoscopic injection sclerotherapy: a prospective randomized controlled trial in patients with bleeding esophageal varices. Ann Surg. 1988;208:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahn D, Krige JE, Terblanche J, et al. A 15-year experience of injection sclerotherapy in adult patients with extrahepatic portal venous obstruction. Ann Surg. 1994;219:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krige JE, Bornman PC, Goldberg PA, et al. Variceal rebleeding and recurrence after endoscopic injection sclerotherapy: a prospective evaluation in 204 patients. Arch Surg. 2000;135:1315–1322. [DOI] [PubMed] [Google Scholar]
- 43.Krige JEJ, Terblanche J. Injection sclerotherapy of oesophageal varices. In: Jamieson GG, DeBas HT, eds. Rob and Smiths’ Operative Surgery: Surgery of the Upper Gastrointestinal Tract, 5th ed. London: Chapman and Hall, 1994:10–20. [Google Scholar]
- 44.Warren WD, Henderson JM, Millikan WJ, et al. Distal splenorenal shunt versus endoscopic sclerotherapy for long-term management of variceal bleeding: preliminary report of a prospective, randomized trial. Ann Surg. 1986;203:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rikkers LF, Burnett DA, Volentine GD, et al. Shunt surgery versus endoscopic sclerotherapy for long-term treatment of variceal bleeding: early results of a randomized trial. Ann Surg. 1987;206:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teres J, Bordas JM, Bravo D, et al. Sclerotherapy vs. distal splenorenal shunt in the elective treatment of variceal hemorrhage: a randomized controlled trial. Hepatology. 1987;7:430–436. [DOI] [PubMed] [Google Scholar]
- 47.Azoulay D, Castaing D, Majno P, et al. Salvage transjugular intrahepatic portosystemic shunt for uncontrolled variceal bleeding in patients with decompensated cirrhosis. J Hepatol. 2001;35:590–597. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg PA, Krige JEJ, Bornman PC, et al. Free esophageal perforation after endoscopic sclerotherapy for bleeding varices. Dis Esoph. 1995;8:188–192. [Google Scholar]
- 49.Gluud C, Henriksen JH, Nielsen G. Prognostic indicators in alcoholic cirrhotic men. Hepatology. 1988;8:222–227. [DOI] [PubMed] [Google Scholar]
- 50.Madonia S, D’Amico G, Traina M, et al. Prognostic indicators of successful endoscopic sclerotherapy for prevention of rebleeding from oesophageal varices in cirrhosis: a long-term cohort study. Dig Liver Dis. 2000;32:782–791. [DOI] [PubMed] [Google Scholar]
- 51.Vorobioff J, Groszmann RJ, Picabea E, et al. Prognostic value of hepatic venous pressure gradient measurements in alcoholic cirrhosis: a 10-year prospective study. Gastroenterology. 1996;111:701–709. [DOI] [PubMed] [Google Scholar]
- 52.Orrego H, Blake JE, Blendis LM, et al. Reliability of assessment of alcohol intake based on personal interviews in a liver clinic. Lancet. 1979;8156:1354–1356. [DOI] [PubMed] [Google Scholar]
- 53.Schenker S, Perkins HS, Sorrell MF. Should patients with end-stage alcoholic liver disease have a new liver? Hepatology. 1990;11:314–319. [DOI] [PubMed] [Google Scholar]
- 54.Bird GL, O’Grady JG, Harvey FA, et al. Liver transplantation in patients with alcoholic cirrhosis: selection criteria and rates of survival and relapse. BMJ. 1990;301:15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gralnek IM, Jensen DM, Kovacs TO, et al. The economic impact of esophageal variceal hemorrhage: cost-effectiveness implications of endoscopic therapy. Hepatology. 1999;29:44–50. [DOI] [PubMed] [Google Scholar]


