Abstract
Summary Background Data:
Most patients who undergo Roux-en-Y gastric bypass (RYGB) experience rapid resolution of type 2 diabetes. Prior studies indicate that this results from more than gastric restriction and weight loss, implicating the rearranged intestine as a primary mediator. It is unclear, however, if diabetes improves because of enhanced delivery of nutrients to the distal intestine and increased secretion of hindgut signals that improve glucose homeostasis, or because of altered signals from the excluded segment of proximal intestine. We sought to distinguish between these two mechanisms.
Methods:
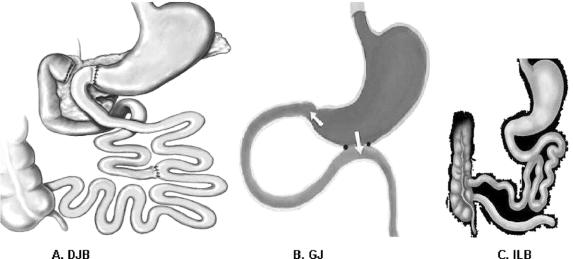
Goto-Kakizaki (GK) type 2 diabetic rats underwent duodenal-jejunal bypass (DJB), a stomach-preserving RYGB that excludes the proximal intestine, or a gastrojejunostomy (GJ), which creates a shortcut for ingested nutrients without bypassing any intestine. Controls were pair-fed (PF) sham-operated and untreated GK rats. Rats that had undergone GJ were then reoperated to exclude the proximal intestine; and conversely, duodenal passage was restored in rats that had undergone DJB. Oral glucose tolerance (OGTT), food intake, body weight, and intestinal nutrient absorption were measured.
Results:
There were no differences in food intake, body weight, or nutrient absorption among surgical groups. DJB-treated rats had markedly better oral glucose tolerance compared with all control groups as shown by lower peak and area-under-the-curve glucose values (P < 0.001 for both). GJ did not affect glucose homeostasis, but exclusion of duodenal nutrient passage in reoperated GJ rats significantly improved glucose tolerance. Conversely, restoration of duodenal passage in DJB rats reestablished impaired glucose tolerance.
Conclusions:
This study shows that bypassing a short segment of proximal intestine directly ameliorates type 2 diabetes, independently of effects on food intake, body weight, malabsorption, or nutrient delivery to the hindgut. These findings suggest that a proximal intestinal bypass could be considered for diabetes treatment and that potentially undiscovered factors from the proximal bowel might contribute to the pathophysiology of type 2 diabetes.
It is unclear if resolution of type 2 diabetes after Roux-en-Y gastric bypass is secondary to enhanced delivery of nutrients to the distal intestine or due to the exclusion of the duodenum and jejunum from the flow of nutrients. Here we provide evidence in support of the latter hypothesis, suggesting a role of the proximal small bowel in the pathogenesis of type 2 diabetes.
The majority of obese patients who undergo selected types of bariatric surgery experience resolution of type 2 diabetes and enjoy normal blood glucose and glycosylated hemoglobin levels, with discontinuation of all diabetes-related medications.1–4 Diabetes resolution occurs most commonly and rapidly after Roux-en-Y gastric bypass (RYGB) and biliopancreatic diversion (BPD),1,5,6 which share the common feature of a bypass of the proximal small intestine. Surgically induced decrease of caloric intake, weight loss, carbohydrate and fat malabsorption, or alterations in gut hormone release have all been suggested as possible explanations for the dramatic effect of bariatric surgery on diabetes.5–9
RYGB, the most commonly performed bariatric operation in the United States, involves two surgical alterations: restriction of gastric volume and diversion of ingested nutrients away from the proximal small intestine. We recently examined the effects of just the latter alteration by developing in rats an operation that leaves the stomach intact but diverts food away from the same amount of intestine as would be bypassed in a standard, proximal RYGB. Diabetic rats that underwent this duodenal-jejunal bypass (DJB) showed marked and durable improvement in their type 2 diabetes, independently of effects on caloric intake or weight loss.10 Substantially improved glucose homeostasis was observed from 1 week through 9 months following the operation. These findings suggest that the control of diabetes after gastrointestinal bypass surgery might result directly from the rerouting of nutrients through the bowel. The exact mechanisms of action of this procedure, however, remained unclear. Elucidating these mechanisms could help devise new medical and surgical therapies for type 2 diabetes and may also have important implications for understanding the disease.
Two hypothesis have been proposed to explain the effect of DJB (as well as RYGB or BPD in humans) on type 2 diabetes. The “hindgut hypothesis” holds that diabetes control results from the expedited delivery of nutrient chyme to the distal intestine, enhancing a physiologic signal that improves glucose metabolism.5,11–13 A potential candidate mediator of this effect is glucagon-like peptide 1 (GLP-1).11–13 This incretin hormone is secreted by L cells of the distal bowel in response to intestinal nutrients. It stimulates insulin secretion and exerts proliferative and antiapoptotic effects on pancreatic beta cells.16 If proven true, the hindgut hypothesis would spur further research on methods to enhance signaling by GLP-1 (or other distal gut peptides) to treat type 2 diabetes.
An alternative hypothesis is that the effect of selected bariatric operations on diabetes depends on exclusion of the duodenum and proximal jejunum from the transit of nutrients, possibly preventing secretion of a putative signal that promotes insulin resistance and type 2 diabetes (“foregut hypothesis”).5,6,14,15 Although no obvious candidate molecules can be identified with current knowledge, if proven true, this hypothesis might open new avenues in the search for the cause and cures of diabetes.
The purpose of this study was to investigate the mechanisms of surgical control of type 2 diabetes by testing the hindgut and the foregut hypotheses in Goto-Kakizaki rats, a nonobese animal model of type 2 diabetes.17 To test the hindgut hypothesis, we used a simple gastrojejunostomy (GJ), an operation that allows rapid delivery of nutrients to the distal bowel without excluding the duodenal-jejunal tract from nutrient passage. To test the foregut hypothesis, we used the DJB, which excludes the proximal small intestine from digestive continuity while also providing the same degree of shortcut for nutrient chyme to the hindgut as is present in a GJ. Secondary aims were to investigate the effect of DJB on intestinal glucose and fat absorption, as well as on levels of insulin and free fatty acids (FFA) in response to a standardized mixed meal.
RESEARCH DESIGN AND METHODS
Animals
Male 10-week-old GK rats were purchased from Taconic M&B A/S (Denmark). Animals had free access to tap water and were fed ad libitum with a 5% fat rat chow diet (Altromin 13/14). Age-matched male Wistar rats were used as nondiabetic controls. All animals were housed in individual cages under constant ambient temperature and humidity in a 12-hour light/dark cycle. These studies were approved by the Institutional Animal Care Committee of the IRCAD-EITS of Strasbourg, France.
Study Protocol
Rats were allowed 2 weeks for acclimation before the start of experiments.
Role of Excluding the Proximal Intestine Versus Rapid Delivery of Nutrients to the Distal Intestine
Twelve-week-old rats randomly underwent either DJB (Fig. 1A), GJ (Fig. 1B), sham operation plus pair-feeding to DJB rats (PF-sham), or no intervention (unoperated controls). All groups were fed the same type of diet. The main study outcome was glucose tolerance, as assessed by an oral glucose tolerance test performed 10 days after surgery. Food intake and body weight were also measured daily for the duration of the study. Three weeks after surgery, fasting and postprandial levels of FFA and insulin were measured before and at several time points after a standard mixed-meal test.
FIGURE 1. Interventions. A, Duodenal-jejunal bypass (DJB). This operation does not impose any restriction to the flow of food through the gastrointestinal tract. The proximal small intestine is excluded from the transit of nutrients, which are rapidly delivered more distally in the small bowel. Food exits the stomach and enters the small bowel at 10 cm from the ligament of Treitz, and digestive continuity is reestablished approximately 25% of the way down the jejunum. B, Gastrojejunostomy (GJ). This operation consists of a simple anastomosis between the distal stomach and the first quarter of the jejunum. The site of the jejunum that is anastomosed to the stomach is chosen at the same distance as in DJB (10 cm from the ligament of Treitz). Hence, the DJB and GJ share the feature of enabling early delivery of nutrients to the same level of small bowel. In contrast to DJB, the GJ does not involve exclusion of duodenal passage, and nutrient stimulation of the duodenum is maintained. C, Ileal bypass (ILB). This operation reduces intestinal fat absorption by preventing nutrients from passing through the distal ileum, where most lipids are absorbed.
Effect of DJB on Carbohydrate and Fat Absorption
A d-xylose test and measurement of fecal fat were performed 3 weeks postoperatively.
Role of Fat Malabsorption on Fasting and Postprandial Levels of FFA
An ileal bypass (ILB) was performed in matched GK rats (Fig. 1C). The ILB is an operation developed by Dr. Henry Buchwald in 197118 to treat hyperlipidemia. It induces significant fat malabsorption by excluding the distal ileum (where most lipids are absorbed) from the transit of nutrients. Plasma lipid profile (triglycerides [TG] and cholesterol) and FFA responses to the mixed-meal test in ILB animals were compared with those of DJB animals.
Effect of DJB in Nondiabetic Animals
Age-matched Wistar rats underwent either DJB or a sham operation plus pair-feeding to Wistar DJB animals.
Consequence of Duodenal Exclusion
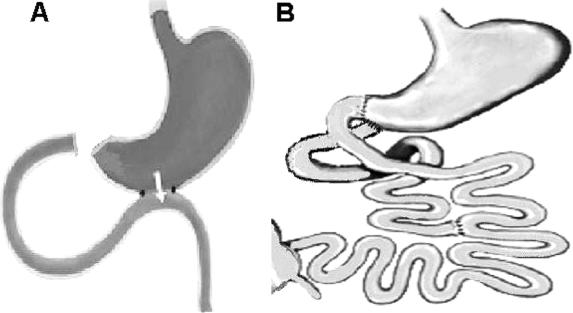
Rats who had undergone GJ were reoperated on 4 weeks after surgery to divide the duodenum from the stomach and exclude the proximal small intestine from antegrade passage of nutrients (Fig. 2A). The former GJ was left intact. Oral glucose tolerance was measured 1 week after the second operation and compared with preconversion glucose tolerance.
FIGURE 2. Reoperations. A, Duodenal jejunal exclusion after GJ. To verify the effects of excluding the intestinal foregut from the passage of food, GJ-treated rats underwent a second operation to divide the duodenum from the stomach, re-creating duodenal and proximal jejunal exclusion, as in DJB. After this operation, nutrients can only pass through the gastrojejunal anastomosis, and the duodenum and proximal jejunum are excluded from antegrade passage of food. B, Restoration of duodenal nutrient passage after DJB. To verify the effect of luminal nutrient stimulation of the proximal intestine, duodenal passage was restored in rats that had initially undergone DJB, by reanastomosing the proximal duodenum to the stomach but leaving the gastrojejunostomy intact. These rats have the same shortcut for nutrients to the hindgut as do DJB rats, but nutrient flow through their proximal small intestine is reestablished.
Verifying the Effect of Duodenal Nutrient Passage
Rats who had undergone DJB were reoperated on 4 weeks after the initial intervention, to restore duodenal passage by a gastroduodenal anastomosis between the side of the pyloric area and the proximal duodenal stump (Fig. 2B). Oral glucose tolerance was measured 1 week after the second operation and compared with preconversion glucose tolerance.
Interventions
Rats undergoing surgery were fasted overnight and anesthetized with 2% isoflurane and air/oxygen.
Duodenal-Jejunal Bypass (DJB)
As shown in Figure 1a, the gastric volume was maintained intact, while the entire duodenum and 10 cm of the proximal jejunum were bypassed. The stomach was divided from the beginning of the duodenum. A length of 10 cm from the ligament of Treitz was measured to chose the site for the gastrojejunal anastomosis, which was performed using a 6/0 resorbable suture. The continuity of biliopancreatic secretions was reconstructed by anastomosing the biliary limb to the alimentary limb of small bowel 15 cm distal to the gastrojejunal anastomosis in a Roux-en-Y fashion. Considering that the rat small bowel is approximately 100 cm long, this DJB represents a stomach-sparing bypass of approximately the same amount of intestine as is excluded in humans by “proximal RYGB.”
Sham Operation
Transections of the gastrointestinal tract were performed at all sites where enterotomies were performed for the DJB, but those where reanastomosed to maintain the physiologic circuit of food through the bowel.
Gastrojejunostomy (GJ)
The gastric volume was left unperturbed as in DJB, and a GJ was performed, to connect the prepyloric area of the stomach with the proximal portion of the jejunum through a latero-lateral anastomosis, allowing wide passage of nutrients rapidly in the distal bowel (Fig. 1b). As in DJB, the site of the jejunum for the GJ anastomosis was chosen by measuring the same distance of 10 cm from the ligament of Treitz. Therefore, in both DJB and GJ animals, the stomach empties into the same level of the small bowel. After this operation, however, duodenal nutrient passage is also maintained.
Duodenal-Jejunal Exclusion After GJ
Four weeks after the first operation of GJ, rats were reoperated on to perform a duodenal-jejunal exclusion (Fig. 2A). The proximal duodenum was divided from the stomach at the pylorus and closed with a running suture. The original gastrojejunal anastomosis was left intact. After this operation, nutrients can only pass through the gastrojejunal anastomosis. Thus, the entire duodenum and proximal jejunum are excluded from antegrade passage of nutrients, similar to a DJB.
Restoration of Duodenal Passage After DJB
To restore duodenal passage of nutrients after a DJB, the duodenal stump was dissected away from adhesions and reanastomosed to the pyloric area of the stomach in a latero-terminal fashion. The original gastrojejunal anastomosis was left intact (Fig. 2B), rendering an anatomy relatively similar to that of the GJ (Fig. 1B). This reversal represents a reciprocal experiment to that just described and depicted in Figure 2A.
Ileal bypass (ILB)
The distal ileum was interrupted at about 15 cm from the ileocecal valve (Fig. 1C). The proximal end of the bowel was anastomosed directly to the right colon, excluding the distal ileum from the transit of nutrients to cause fat malabsorption.
Outcome Measures
Body Weight Change and Food Intake Monitoring
Body weight and food intake were measured daily for the duration of the study. Sham-operated rats were pair-fed to their DJB pairs.
Oral Glucose Tolerance Test (OGTT)
After 12 to 14 hours of fasting, blood glucose was measured in conscious rats before (baseline) and at 10, 30, 60, 120, and 180 minutes after administration of 3 g/kg glucose by oral gavage. Blood was obtained from the tail and analyzed using a glucometer (One Touch Ultra, Lifescan, Johnson & Johnson, Milpitas, CA).
Mixed-Meal Test
After 12 to 14 hours of fasting a standardized mixed liquid meal (3.6 kcal/kg, 66% glucose, 16% lipids, 18% protein) was administered by oral gavage. Blood samples for determination of insulin and FFA were obtained from the tail of conscious rats at baseline and at 10, 30, 60, and 120 minutes after the meal. For plasma insulin measurement, blood was collected in EDTA tubes containing the gastrointestinal preservative. After centrifugation at 3000 rpm at 4°C for 12 minutes, plasma was immediately separated and stored at −80°C until being analyzed. Rat radioimmunoassay kits were used for measurement of insulin (Linco Research Inc., St. Charles, MI).
Lipid Profile Tests
Plasma total cholesterol, TG, and FFA were measured after 12 to 14 hours of fasting. Analytical methods were: 1) FFA, enzymatic method ACS-ACOD (Wako Chemicals, Dallas, TX); 2) TG, enzymatic method GPO-PAP (Roche Diagnostics); and 3) cholesterol, enzymatic method CHOD-PAP (Roche Diagnostics).
Oral d-xylose Loading Test
This test measures intestinal carbohydrate absorption by calculating the plasma concentration of d-xylose after ingestion of a known amount of d-xylose. Three milliliters of a solution containing d-xylose (0.8 g/kg body weight) was administered by oral gavage. Blood samples were drawn from the tail at baseline and at 60 minutes after d-xylose administration. Plasma xylose concentrations were measured by gas chromatography using an HP 5890 system (Hewlett-Packard GmbH, Waldbronn, Germany).
Fecal Fat Measurement
Feces were collected on the last morning of a 3-day period (animals were not fasted) from the cage pan. The fecal material was filtered to remove loose residual food, weighed for each animal, and stored at −80°C until being analyzed for total fat content using a near-infrared reflectance analyzer Infraalyser 450 (Bran + Luebbe SL, Nordersted, Germany). Percent fat absorption was calculated by subtracting fecal fat from the total amount of fat ingested over the 3 days of the test.
Statistical Analyses
Data are expressed as mean ± SD. Areas under curves (AUC) were calculated by trapezoidal integration. Statistical analyses were performed using the SPSS 12.0 system for Windows. Differences between means were evaluated using ANOVA or the Student t test as appropriate. The Least Significant Difference post hoc test was performed to assess significance of intergroup differences. Differences were considered significant at P < 0.05.
RESULTS
Before treatments, there were no significant differences among groups of GK rats in body weight or fasting glycemia.
Food Intake and Body Weight Gain
To control for the impact of surgical stress and decreased caloric intake on glucose tolerance in both GK and Wistar rats, sham-operated rats underwent pair-feeding (PF) to DJB-treated animals (Fig. 3). All operated animals ate less food and gained less weight compared with nonoperated controls (P < 0.05); however, no significant differences among surgical groups (DJB, GJ, PF-sham) were observed.
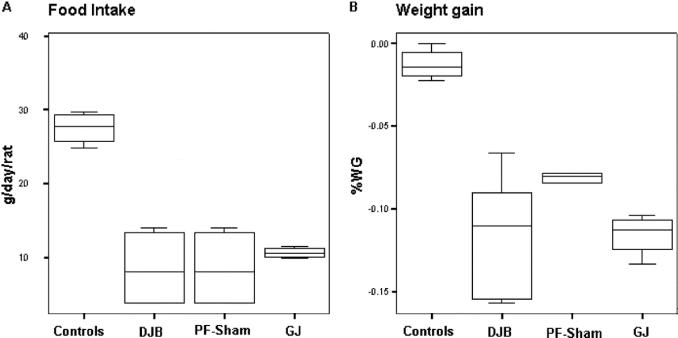
FIGURE 3. Food intake and body weight gain. Each box in the chart shows the median, quartiles, and extreme values. A, Food intake (g/day per rat) in GK rats. All operated animals ate less food compared with nonoperated controls, but no significant differences were found among surgical groups (DJB, GJ, PF-sham). GK-sham rats were pair-fed to the intake of GK-DJB rats; consequently, both groups had the same food intake throughout the study. B, Operated rats gained less weight than did nonoperated GK controls (*P < 0.05); however, there were no significant differences among DJB, GJ, and sham-PF animals.
Oral Glucose Tolerance
DJB-treated GK rats showed better glucose tolerance compared with all other study groups of GK animals, as shown by lower AUC glucose after an OGTT (P < 0.001) and lower 30- and 60-minute peak levels (P < 0.01 for both) (Fig. 4). Oral glucose tolerance (GT) in sham-operated pair-fed GK rats was not significantly different compared with nonoperated GK controls, suggesting that surgical stress and reduced caloric intake after DJB were not responsible for the enhancement of GT. Despite resulting in food intake and body weight that were similar to DJB, the GJ operation did not improve GT, as shown by similar AUC glucose and 30- and 60-minute peak levels compared with nonoperated GK controls and sham-PF animals (P = not significant). Surprisingly, in nondiabetic Wistar rats, the DJB increased glucose excursions during the OGTT, as shown by slightly higher AUC glucose compared with sham-operated pair-fed Wistar controls (P < 0.01).
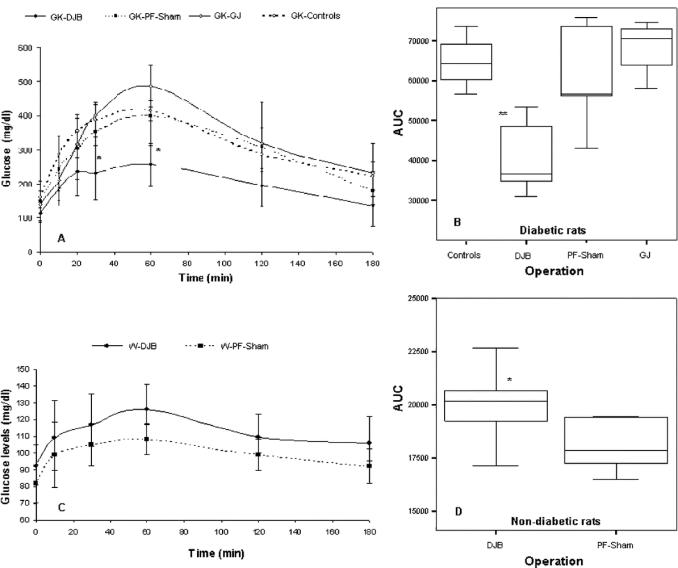
FIGURE 4. Glucose time course and AUC after a 3 g/kg oral glucose load (OGTT). Glucose time course (A) and AUC glucose (B) in GK diabetic rats, showing markedly improved glucose tolerance in DJB-treated animals. In contrast with the results of diabetic rats, glucose time course and AUC glucose in nondiabetic Wistar rats show that DJB slightly deteriorated glucose tolerance in normal animals. *P < 0.01, **P < 0.001.
Reoperation
Although the GJ procedure had no impact on the glucose tolerance, conversion of this operation to a duodenal-jejunal exclusion significantly improved GT, as shown by lower AUC glucose after an OGTT (P < 0.05; Fig. 5). Conversely, we attempted to convert DJB into GJ with restored duodenal nutrient passage. This operation had high mortality due to adhesions in the duodenal area that increased the risk of damage to the bowel wall and blood supply during mobilization of the duodenum stump. However, 2 rats that survived the second surgery underwent an OGTT 1 week postoperatively that showed a remarkable deterioration of glucose tolerance, as documented by a twofold greater AUC glucose compared with preconversion levels in the same rats. Together, these findings suggest a detrimental effect of duodenal passage of nutrients in diabetic rats.
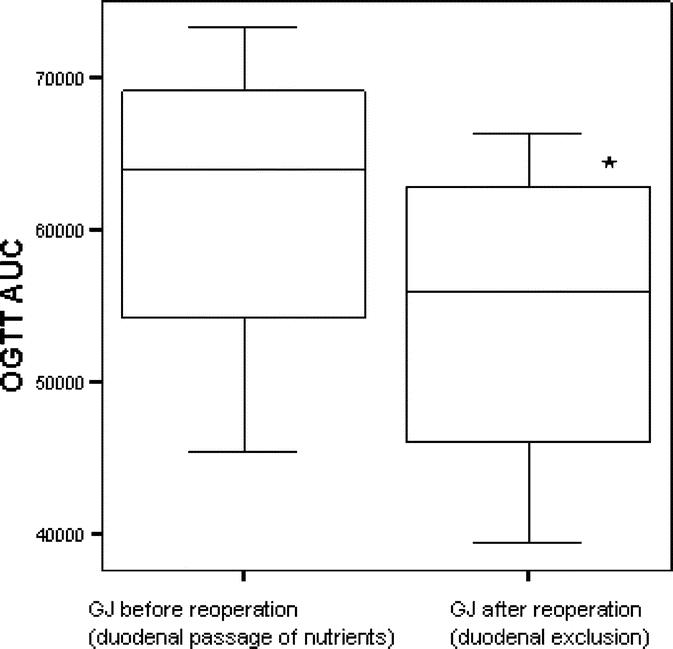
FIGURE 5. Glucose tolerance (AUC) after reoperation that excluded the duodenum from nutrient passage in GJ-treated rats. GJ-treated rats underwent a second operation to create duodenal exclusion, as in DJB. Glucose tolerance improved after duodenal exclusion, as shown by lower AUC glucose after an OGTT (*P = 0.02).
Intestinal Nutrient Absorption and Plasma Lipids
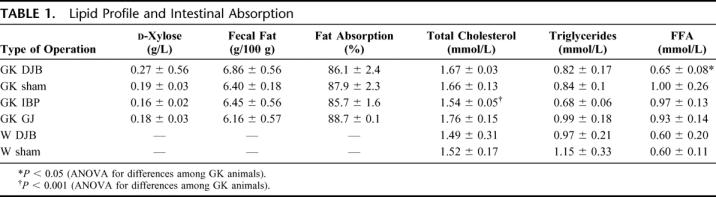
At the d-xylose test, there were no significant differences among groups, indicating that the DJB and GJ operations do not result in impaired intestinal absorption of glucose (Table 1). Whereas plasma cholesterol and TG in DJB animals were similar to those of PF-sham and GJ rats, the ILB yielded significantly lower levels of total cholesterol (P < 0.01).
TABLE 1. Lipid Profile and Intestinal Absorption
Insulin
DJB-treated rats did not show statistically significant differences in fasting or postmeal plasma insulin levels compared with PF-sham-operated animals. The GJ operation resulted in significant higher basal insulin levels and greater AUC insulin in response to the mixed meal stimulus, suggesting that the operation may have further deteriorated insulin resistance.
FFA
Fasting plasma FFA levels in GK DJB-treated rats were similar to those of nondiabetic animals and lower than those of all other GK groups (P < 0.01; Table 1). In nondiabetic rats, plasma concentrations of FFA decreased over baseline levels after a mixed-meal stimulus in both the sham- and the DJB-treated animals (90 minutes after stimulation, mean plasma FFA levels were 20% lower than baseline). GK-DJB treated rats tended to decrease FFA levels over baseline (−19% at 90 minutes after stimulation), whereas all other GK groups showed a trend toward stable or increased FFA concentrations after the mixed meal, although these differences were not statistically significant.
DISCUSSION
The results of our study show that exclusion of the proximal small intestine from contact with ingested nutrients is a critical component in the mechanism improving glucose tolerance after DJB in GK-diabetic rats. This observation supports the “foregut hypothesis” as an explanation for the improvement of type 2 diabetes after bariatric operations that bypass the proximal small intestine, such as RYGB and BPD. If mechanisms proposed by the “hindgut hypothesis” played a dominant role, GJ and DJB should have been equally effective in improving glucose tolerance since they equivalently expedite nutrient delivery to the hindgut. Instead, only DJB-treated rats showed improved glucose tolerance compared with controls, even though DJB and GJ rats had similar food intake and body weight, and neither operation caused significant malabsorption.
To confirm a primary role for the excluded proximal intestine in ameliorating diabetes, we showed that exclusion of duodenal nutrient passage in GK-diabetic rats that had initially undergone the GJ operation significantly improved glucose tolerance. Conversely, restoration of duodenal passage in rats initially receiving DJB reestablished impaired glucose tolerance.
Thus, in terms of intestinal rearrangements, bypass of the proximal intestine alone is necessary and sufficient to improve diabetes in GK rats.
The present study also provides further evidence to rule out decreased caloric intake as a critical mechanism for diabetes control after DJB in our rat model. Indeed, DJB-treated diabetic rats had better glucose tolerance than did pair-fed sham-operated animals with similar food intake and body weights. These results corroborate our earlier findings showing that DJB ameliorates type 2 diabetes in GK rats very rapidly and more effectively than does greater restriction of food intake in matched nonoperated animals.10 This finding agrees with clinical observations showing that very-low-calorie diets fail to improve diabetes in patients who later experience diabetes resolution from RYGB surgery.19 Moreover, diabetes typically resolves after RYGB long before significant weight loss has occurred.4
The results of our study do not support speculations that DJB ameliorates diabetes through intestinal malabsorption, since the d-xylose test and fecal fat assessment showed no reduction of carbohydrate or lipid absorption after this operation. Because the proportion of intestine removed from digestive continuity after our DJB procedure matches that bypassed in a typical proximal RYGB, our data also support clinical evidence arguing against a major role for malabsorption in the effects of human RYGB.20–23
An intriguing finding of our study is the small deterioration of glucose tolerance after DJB in nondiabetic animals, in contradiction with the marked improvement seen in diabetic rats. This discrepancy has two implications. On one hand, it provides further evidence against the hypothesis that DJB works primarily through early delivery of nutrients to the distal bowel. If that hypothesis were correct, one would expect improved (or at least unchanged) glucose tolerance even in normal rats, as a result of increased secretion of signals exerting a positive influence on glucose metabolism, such as GLP-1. On the other hand, this discrepancy demonstrates that, whereas duodenal passage of nutrients is necessary to maintain normal glucose tolerance in nondiabetic rats, it contributes instead to impaired glucose tolerance in diabetic animals.
Our data are consistent with results of clinical investigations evaluating the consequences of different reconstructions of gastrointestinal anatomy following gastrectomy for cancer or peptic ulcer. Several clinical studies, including a randomized trial24 comparing gastrectomy with duodenal exclusion (such as the Roux-en-Y reconstruction) versus gastrectomy with preservation of duodenal passage, showed that exclusion of the duodenum from the passage of food impairs glucose tolerance in nondiabetic subjects and results in lower plasma glucose-dependent insulinotropic polypeptide25 and insulin25–28 levels. This suggests that duodenal-jejunal exclusion may disrupt the physiologic entero-insular axis in nondiabetic individuals. Conversely, when gastrectomy with duodenal exclusion is performed in diabetic patients, it results in improvement or even resolution of diabetes,28,29 just as in the case of bariatric operations with duodenal exclusion.3–6
The results of our study and these clinical observations are consistent with the possibility that surgical bypass of the proximal small intestine reverses a humoral mechanism that originates in the proximal bowel and impairs glucose tolerance in diabetic individuals. This hypothesis characterizes type 2 diabetes as having a component of duodenal-jejunal dysfunction.
Although the underlying molecular mechanism of diabetes control after operations that exclude the intestinal foregut remains to be elucidated, our data suggest a possible role for changes in FFA metabolism. It is known that high concentrations of FFA lead to insulin resistance30,31 and impaired insulin secretion in animals and humans.31–33 DJB decreased both fasting and postprandial FFA levels, which could contribute to its positive influence on glucose tolerance. This effect of DJB on FFA does not seem to be due to intestinal fat malabsorption, since rats undergoing ILB did not show significant changes in FFA, even though the operation resulted in lower plasma TG and cholesterol concentrations compared with DJB-treated animals. This suggests that the DJB may counteract abnormalities of FFA metabolism in diabetic animals. However, reduction of FFA could also result from improved insulin sensitivity obtained through other mechanisms; hence, further research on the effect of DJB on lipid metabolism is needed to verify if changes in FFA metabolism take part in the molecular mechanism of diabetes control after DJB.
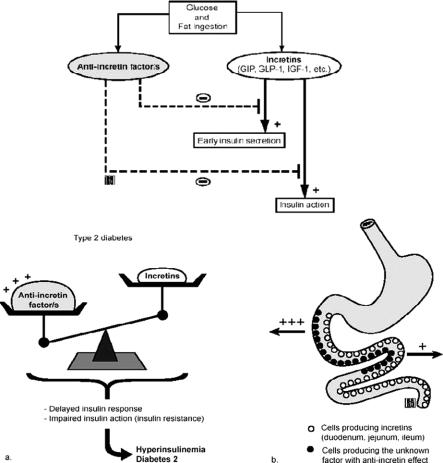
Whatever the exact molecular mechanism, our study suggests that the proximal small bowel contributes to the alterations of glucose metabolism in type 2 diabetes. We speculate that in normal conditions, stimulation of the proximal bowel with nutrients triggers both a glucose-dependent insulinotropic polypeptide response, which increases insulin secretion, as well as a concomitant counter-regulatory signal that controls insulin action to prevent hypoglycemia. In predisposed individuals, chronic stimulation with particular nutrients may create an imbalance between incretin and “anti-incretin” signals, resulting in insulin resistance and type 2 diabetes (Fig. 6).
FIGURE 6. Hypothesis regarding the possible contribution of the proximal intestine to the alterations of glucose metabolism in type 2 diabetes. The passage of nutrients through the intestinal foregut may trigger, in addition to the known incretin response, a concomitant counter-regulatory signal (“anti-incretin factor”) aimed to prevent hypoglycemia. This signal may interfere with pathways of the incretin system and/or act downstream to inhibit insulin action. In predisposed individuals, chronic stimulation with particular nutrients may create an imbalance between incretin and “anti-incretin” signals, resulting in insulin resistance and type 2 diabetes.
The results of this study have clinical implications. The evidence that duodenal-jejunal exclusion exerts a direct impact on glucose tolerance in diabetic subjects suggests that type 2 diabetes might be remedied specifically by surgical operations that bypass the proximal small bowel. A surgical option in some patients with type 2 diabetes may be suitable in the face of the worldwide epidemic of this disease and the limited efficacy of conventional therapeutic strategies in some instances.34,35 Our studies in nonobese diabetic rats and clinical observations of diabetes control after gastrointestinal bypass surgery in nonmorbidly obese individuals28–30,36 imply that surgery should not necessarily be reserved for people with a BMI greater than 35 kg/m2, as in current practice.37 Clearly, as with any invasive therapy, indications should be determined by an evaluation of the risk/benefit ratio. Clinical trials are warranted to define the ideal candidate patients for surgical treatment of type 2 diabetes.
CONCLUSION
This study demonstrates that exclusion of the proximal small intestine plays a major role in the beneficial effect of gastrointestinal bypass surgery on type 2 diabetes. Our findings suggest that the proximal small bowel is involved in pathogenic pathways of type 2 diabetes and open new avenues for research directed toward understanding and treatment of this condition.
ACKNOWLEDGMENTS
The authors thank Mrs. Anna Caprodossi for her skillful technical assistance with laboratory tests and to Mrs. Catherine Cers from “Websurg” for the illustrations.
Footnotes
Reprints: Francesco Rubino, MD, IRCAD- European Institute of Telesurgery, 1 Place de l'Hopital, 67091 Strasbourg, France. E-mail: f.rubino@lycos.com.
REFERENCES
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004 13;292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 3.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer PR, Burguera B, Ikramuddin S. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. [DOI] [PubMed] [Google Scholar]
- 6.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumbs A, Modlin IM, Ballantyne GH. Changes in insulin resistance following bariatric surgery: role of caloric restriction and weight loss. Obes Surg. 2005;15:462–473. [DOI] [PubMed] [Google Scholar]
- 8.Pinkney JH, Sjostrom CD, Gale EA. Should surgeons treat diabetes in severely obese people? Lancet. 2001;28:357:1357–1359. [DOI] [PubMed] [Google Scholar]
- 9.Pories WJ. Why does the gastric bypass control type 2 diabetes mellitus? Obes Surg. 1992;2:303–313. [DOI] [PubMed] [Google Scholar]
- 10.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason EE. The mechanism of surgical treatment of type 2 diabetes. Obes Surg. 2005;15:459–461. [DOI] [PubMed] [Google Scholar]
- 12.Patriti A, Facchiano E, Sanna A, et al. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes Surg. 2004;14:840–848. [DOI] [PubMed] [Google Scholar]
- 13.Mason EE. Ileal transposition and enteroglucagon/GLP1 in obesity (and diabetic?) surgery. Obes Surg. 1999;9:223–228. [DOI] [PubMed] [Google Scholar]
- 14.Pories WJ, Albrecht RJ. Etiology of type II diabetes mellitus: role of the foregut. World J Surg. 2001;25:527–531. [DOI] [PubMed] [Google Scholar]
- 15.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker DJ. Glucagon-like peptide 1 and the islet β-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. [DOI] [PubMed] [Google Scholar]
- 17.Galli J, Li LS, Glaser A, Ostenson CG, et al. Genetic analysis of non-insulin dependent diabetes mellitus in the GK rat. Nat Genet. 1996;12:31–37. [DOI] [PubMed] [Google Scholar]
- 18.Buchwald H, Varco RL. A bypass operation for obese hyperlipidemia patients. Surgery. 1971;70:62–70. [PubMed] [Google Scholar]
- 19.Lima J, Helena L, Oliveira S, et al. Rapid resolution of diabetes after gastric bypass. Obes Surg. 2005;15:448–449. [DOI] [PubMed] [Google Scholar]
- 20.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–2796. [DOI] [PubMed] [Google Scholar]
- 21.Brolin RE, LaMarca LB, Kenler HA, et al. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195–205. [DOI] [PubMed] [Google Scholar]
- 22.Faraj M, Jones P, Sniderman AD, et al. Enhanced dietary fat clearance in postobese women. J Lipid Res. 2001;42:571–580. [PubMed] [Google Scholar]
- 23.MacLean LD, Rhode BM, Nohr CW. Long- or short-limb gastric bypass? J Gastrointest Surg. 2001;5:525–530. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz A, Buchler M, Usinger K, et al. Importance of the duodenal passage and pouch volume after total gastrectomy and reconstruction with the Ulm pouch: prospective randomized clinical study. World J Surg. 1996;20:60–67. [DOI] [PubMed] [Google Scholar]
- 25.Bittner R, Bittner B, Beger HG. Homeostasis of glucose and gastric resection: the influence of the food passage through the duodenum (article in German). Z Gastroenterol. 1981;19:698–707. [PubMed] [Google Scholar]
- 26.Schattenmann G, Ebert R, Siiewert R, et al. Different response of gastric inhibitory polypeptide to glucose and fat from duodenum and jejunum. Scand J Gastroenterol. 1984;19:260–266. [PubMed] [Google Scholar]
- 27.Aldersberg D, Hammerschlag E. Post-gastrectomy syndrome. Surgery. 1947;21:720–729. [PubMed] [Google Scholar]
- 28.Friedman NM, Sancetta AJ, Magovern GJ. The amelioration of diabetes mellitus following subtotal gastrectomy. Surg Gynecol Obstet. 1955;100:201–204. [PubMed] [Google Scholar]
- 29.Forgacs S, Halmos T. Improvement of glucose tolerance in diabetics following gastrectomy [in German]. Z Gastroenterol. 1973;11:293–296. [PubMed] [Google Scholar]
- 30.Lewis GF, Carpentier A, Khosrow A, et al. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. [DOI] [PubMed] [Google Scholar]
- 31.Boden G. Role of fatty acids in the pathogenesis of insulin resistance in NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 32.Basu A, Jensen M. Fat metabolism in diabetes. In: Joslin's Diabetes Mellitus, 14th ed. Philadelphia: Lippincott Williams & Wilkins, 2005:265–274. [Google Scholar]
- 33.Mathews CE, Leiter EH. Rodent models for the study of diabetes. In: Joslin's Diabetes Mellitus, 14th ed. Philadelphia: Lippincott Williams & Wilkins, 2005:291–327. [Google Scholar]
- 34.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. [DOI] [PubMed] [Google Scholar]
- 35.Liebl A, Neiss A, Spannheimer A. Costs of type 2 diabetes in Germany: results of the CODE-2 study. Dtsch Med Wochenschr. 2001;126:585–589. [DOI] [PubMed] [Google Scholar]
- 36.Castagneto M, De Gaetano A, Mingrone G, et al. A surgical option for familial chylomicronemia associated with insulin-resistant diabetes mellitus. Obes Surg. 1998;8:191–198. [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health Consensus Development Conference. Gastrointestinal surgery for severe obesity. Am J Clin Nutr. 1992;55(suppl):615–619. [DOI] [PubMed] [Google Scholar]