Abstract
Objective:
To assess body composition, eating pattern, and basal metabolic rate in patients undergoing obesity surgery in a randomized trial.
Introduction:
There is limited knowledge regarding how different bariatric surgical techniques function in terms of altering body composition, dietary intake, and basic metabolic rate.
Methods:
Non-superobese patients were randomized to laparoscopic Roux-en-Y gastric bypass (LGBP, n = 37) or laparoscopic vertical banded gastroplasty (LVBG, n = 46). Anthropometry, dual-energy x-ray absorptiometry (DEXA), computed tomography (CT), indirect calorimetry, and reported dietary intake were registered prior to and 1 year after surgery.
Results:
Follow-up rate was 97.6%. LGBP patients had significantly greater reduction of waist circumference and sagittal diameter compared with LVBG. DEXA demonstrated a larger reduction of body fat in all compartments after LGBP, especially at the trunk (P<0.001). CT demonstrated more reduction of the visceral fat (P=0.016). Patients were able to eat all types of food after LGBP, although about 30% claimed they avoided fats. LGBP patients decreased their proportion of dietary fat significantly more than those operated on with LVBG (P = 0.005), who consumed more sweet foods and avoided whole meat and vegetables. Lean tissue mass (LTM) was proportionally less reduced, especially in men, after LGBP. The decreases in BMR postoperatively reflected the lower body mass in a pattern that did not differ among the groups.
Conclusion:
LGBP patients demonstrated better outcomes compared with LVBG patients in terms of body composition. Energy expenditure developed as expected postoperatively. A “steering” away from fatty foods after LGBP may be an important mechanism of action in gastric bypass.
Non-superobese patients were operated with laparoscopic gastric bypass or laparoscopic vertical banded gastroplasty in a randomized clinical trial. The outcome 1 year after surgery concerning anthropometry favored gastric bypass with a body composition reflecting a lower metabolic risk. No differences were found concerning the development of basic metabolic rate, while there were large differences in eating patterns where patients after gastric bypass “normalized” their diet and reduced their intake of calories, especially from fat foods.
There are as yet no indications of decrease in the global obesity epidemic.1 The etiology is probably multifactorial, to which a genetic disposition together with unlimited access to high calorie foods and a lifestyle promoting physical inactivity contribute. Morbidity and mortality are strongly correlated to the level of obesity,2–4 and the list of diseases with a causal factor in obesity continues to expand. Obesity surgery is the only option that has been demonstrated to result in efficient, sustained long-term weight loss in the treatment of severe obesity.5
Most authors use the amount of weight loss (or percent excess weight loss [%EWL]) as the only tool to evaluate the efficacy of bariatric surgical procedures. Generally, there is a strong correlation between the level of weight loss and improvements in metabolic risk factors and quality of life (QoL).6,7 However, there is limited knowledge about the differences between the surgical options (restrictive, malabsorptive, and combined) in the crucial task of reducing the metabolic risk and thereby contribute to curing morbidity and preventing mortality.
Previous studies of the difference between Roux-en-Y Gastric bypass (GBP) and the restrictive bariatric procedures, such as vertical banded gastroplasty (VBG), have reported a superior outcome in terms of weight loss and a better dietary pattern after GBP.8–12 Studies of body composition have demonstrated a reduction of body fat as well as a decrease in lean tissue mass (LTM) following bariatric surgery.13–16 The question of whether or not higher energy expenditure than expected could be a contributing factor to weight loss after obesity surgery has been disputed in previous studies.17–20 No study has to our knowledge prospectively compared changes in body composition and/or energy expenditure related to dietary intake in a randomized clinical trial between different bariatric surgical techniques. Based on a prospective randomized trial, we have recently reported that laparoscopic GBP (LGBP) resulted in a weight reduction that was clearly superior to the effect of laparoscopic vertical banded gastroplasty (LVBG). Furthermore, remedial surgical interventions were more frequent following LVBG.21
The patients enrolled in the above mentioned clinical trial21 were also examined with regard to other outcome variables not directly related to the operative procedures. In this paper, we report the results concerning effects on body composition, energy expenditure and dietary intake.
MATERIALS AND METHODS
Patients
During the period March 2000 to April 2001, 100 patients with BMI < 50 kg/m2 were recruited to the study from an existing waiting list for obesity surgery. Indications for surgery followed the National Institutes of Health (NIH) criteria, ie, a BMI > 40 kg/m2 or a BMI > 35 kg/m2 with obesity-related morbidity.22
After informed consent had been obtained, the patients were randomized to LGBP (n = 50) or LVBG (n = 50) in a computerized program that stratified for weight, body mass index (BMI), age, and associated morbidity. Before inclusion to the study, 13 patients decided to have either an LGBP (n = 9) or a LVBG (n = 4). They were excluded from the study, as were 4 patients who turned out to have a BMI > 50 kg/m2.
At the 1-year follow-up, 4 patients (randomized and operated on with LVBG) have been reoperated (conversion to GBP) and 2 women were pregnant. Those patients were also excluded from analysis, as was one patient in each group who was lost to follow-up. Consequently, 36 patients operated on with LGBP and 39 patients with LVBG were available for the 1-year follow-up.
Patients who were initially recruited and examined, but according to the above-mentioned reasons excluded from the study, have been followed according to the protocol but are only presented as a dropout analysis.
All participants underwent the same anthropometric, energy expenditure, and body composition measurements prior to and 1 year after surgery. Furthermore, all patients answered questionnaires regarding dietary intake and foods avoided before and 1 year after the operation. The time from preoperative examinations to surgery was a maximum of 6 weeks and at the 1 year follow up there was a tolerance of ±6 weeks.
Surgical Technique
The operations were performed by either of 2 surgeons. Our operational technique for LVBG and LGBP has been presented elsewhere.23,24 Briefly, the LVBG was performed as described by Mason and MacLean with a small gastric pouch (10–20 mL) and a divided vertical staple line. The outflow was reinforced with a prestretched Gore-Tex band with a circumference of 5.0 cm calibrated over a gastric tube of 9-mm diameter. The LGBP technique includes an antecolic-antegastric Roux-en-Y construction with a small gastric pouch (10–20 mL). The gastro-enteroanastomosis was constructed with a 45-mm straight stapler and supplementary hand suturing. The length of the Roux-limb was 75 cm and the afferent jejunal limb had a length of about 30 cm from the ligament of Treitz to the entero-enteroanastomosis.
Anthropometry
All anthropometric measurements were performed with the subjects dressed in underwear after an overnight fast. Height was measured to the nearest 1.0 cm with the subject standing with his or her back to a wall-mounted stadiometer in bare feet. Weight was measured to the nearest 0.1 kg with calibrated scales. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2). Sagittal diameter (anteroposterior) was measured using a carpenter's spirit level and a ruler. The subjects were examined in the recumbent position on a firm examining table. The spirit level was placed perpendicular to the length axis of the body over the abdomen at the level of the iliac crest, and the distance from the examining table to the horizontal level was measured during normal expiration. Waist was measured at the level of the iliac crests and hip at the femoral great trochanters.
Dual Energy X-Ray Absorptiometry (DEXA)
In this three-compartment model, bone mineral content (BMC), fat, and LTM25 were measured, as was bone mineral density (BMD). The DEXA scanner used was a LUNAR DPX-L (Scanexport Medical, Helsingborg, Sweden) with software version 1.31 and with the extended analysis program for total body analysis (LUNAR Radiation, Madison, WI). The scanner uses a constant potential x-ray source and a K-edge filter to achieve a congruent beam of stable dual-energy radiation. Quality assurance tests were conducted on a daily basis, as recommended by the manufacturer. The participants were examined in the supine position. The manufacturer does not recommend examinations of individuals heavier than 135 kg because the sum of BMC, body fat, and LTM gradually lags behind weight at higher body weights. The precision error as determined for double examinations in 10 healthy subjects repositioned between the examinations was: 1.7% for body fat, 0.7% for LTM, 1.9% for total BMC, and 1.5% for total body BMD.
Computed Tomography (CT)
Tissues areas were determined using a HiSpeed Advantage (HSA) CT system (version RP2, GE Medical Systems, Milwaukee, WI) with the following settings: 120 kV, slice thickness 5 mm, and fixed filtration. Four scans were obtained from each participant. Scan 1 was obtained in the midthigh region, scan 2 at the fourth lumbar vertebra level (L4), scan 3 at midliver level, and scan 4 at the fourth cervical vertebra level (C4). From scan 1, the tissue areas of the right leg were reported. The images were transferred to a separate UNIX-based analyzing unit. Tissue areas were determined as previously described26 with the following precision errors calculated from double determinations: sc adipose tissue (AT) (0.5%), the sum of visceral AT (1.2%).
Basic Metabolic Rate (BMR)
BMR of subjects was determined by indirect calorimetry (Sensor Medics 2900, mixing chamber principle). Metabolic rate was calculated from registrations of the CO2 and O2 concentrations in the inhaled and exhaled air during a 30-minute period, awake, in a supine position after an initial resting period of 30 minutes. Subjects were encouraged to be normally active but avoid vigorous exercises on the day prior to examination. The subjects fasted from 22:00 hours previous evening until after the BMR measurement in the morning. Calibration of the registration instrument was performed daily.
Dietary Assessment
The Swedish Obese Subjects study questionnaire was used for dietary assessment. This questionnaire has been described in detail by Lindroos et al,27 and the validity has been demonstrated to be satisfactory. The questionnaires included 49 questions on ordinary food consumption patterns during the past 3 months, with the emphasis on portion size and day of week. Amounts of snack foods and candies were quantified using sizes for preconfectioned packages as sold in Sweden. Bread-type, thickness, and contents of sandwiches were described in detail, owing to the large contribution of sandwiches in the Swedish diet. The amounts of food reported by the subjects were converted into grams, from which daily intake of energy and 29 different nutrients were computed. In addition, a short questionnaire form was used to explore whether the patient avoided certain foods. Included were direct questions (eg, Do you eat whole meat?) and an open question (ie, Do you avoid eating any foods? Why?).
Statistics and Ethics
Dietary, anthropometric, energy expenditure, and body composition data 1 year after surgery are presented as the mean difference (Δ), thus with postoperative values subtracted from preoperative values for each individual and the calculated mean of these differences for the separate groups (reduction = positive value).
Data presented are generally means with standard deviation. Significance of difference was calculated using 2-tailed paired and unpaired Student t test or Fisher exact test, and a P < 0.05 was considered statistically significant. Software packages used were Excel and SPSS, Microsoft Corporation.
The local Ethics Committee at Sahlgrenska University Hospital, Göteborg, approved the study protocol.
RESULTS
Baseline
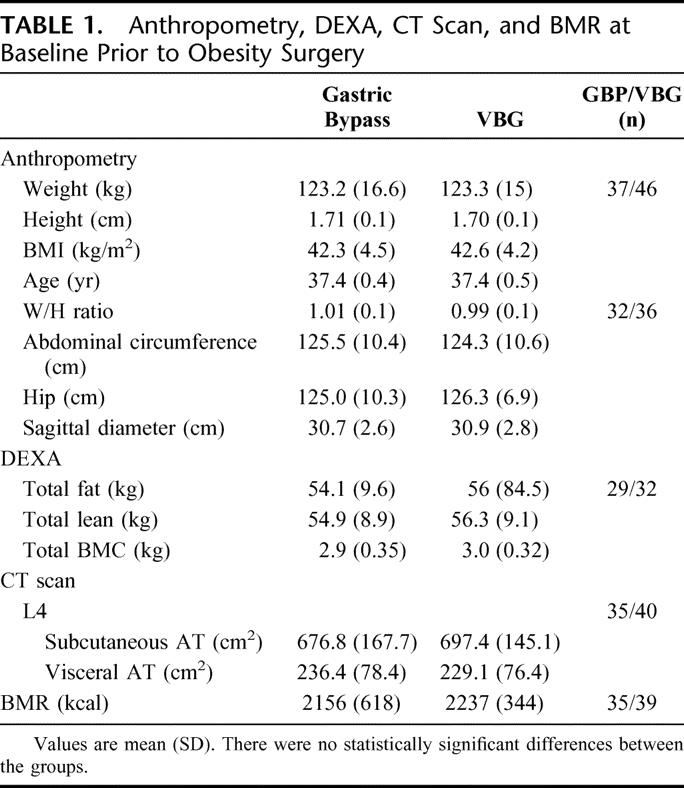
There were no differences between the groups before surgery in terms of age, sex, weight, height, BMI or comorbidity. Neither was there any differences concerning preoperative anthropometry and body composition or BMR (Table 1). Reported energy intake differed between the groups at the baseline: 2690 (1040) versus 3493 (1628) after LGBP and LVBG, respectively. Dietary composition did though not differ (percent energy from protein, carbohydrates and fat of total energy intake).
TABLE 1. Anthropometry, DEXA, CT Scan, and BMR at Baseline Prior to Obesity Surgery
Anthropometry
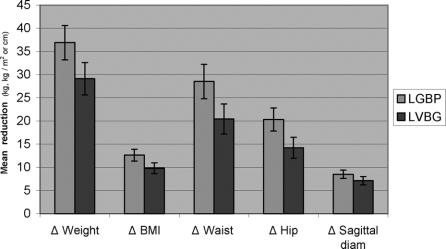
One year postoperatively, patients in the LGBP group had reduced their weight, BMI, waist circumference, hip circumference, and sagittal diameter significantly more than in the LVBG group as demonstrated in Figure 1.
FIGURE 1. Means (CI) of individual reductions (Δ) in anthropometric values from prior to 1 year after obesity surgery with laparoscopic gastric bypass (LGBP) or laparoscopic VBG (LVBG).
DEXA
Surgical treatment resulted in dramatic reductions of total fat, favoring LGBP over LVBG (26.9 ± 9.4 kg and 20.2 ± 8.6 kg, respectively 1 year after surgery, P = 0.005). Trunkal fat reduction contributed most to this difference. The LTM was significantly less reduced among men who were operated with LGBP compared with LVBG while there was no difference in the female group (Table 2).
TABLE 2. Anthropometry, DEXA, and CT Scan 1 Year After Laparoscopic Gastric Bypass (LGBP) and Laparoscopic Vertical Banded Gastroplasty (LVBG)
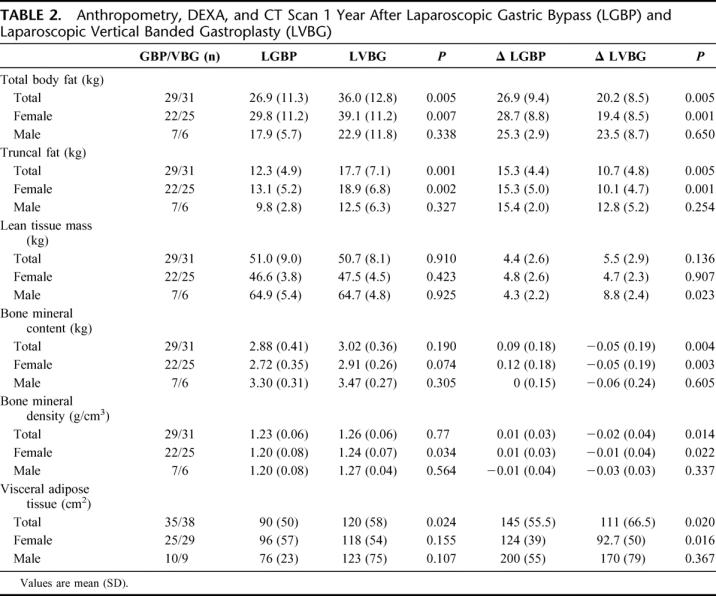
A decrease of total BMC was found in the LGBP group (90 ± 180 g), while the LVBG group increased it (50 ± 190 g) until 1 year after surgery (P = 0.0028 between groups). Regarding BMD there were only minor reductions from prior to and 1 year after surgery, still revealing supernormal values for both groups 1 year after surgery (Z-score 103 and 104%, where 100% is the estimated normal value) (Table 2).
CT
One year postoperatively, LGBP patients had significantly larger reductions of adipose tissue as shown at the abdominal scan. The larger reduction in visceral adipose tissue after LGBP was, however, proportionally equivalent to the reduction of subcutaneous adipose tissue.
BMR
One year postoperative mean values of BMR did not differ between the groups (P = 0.81), neither did the reductions (Δ) in BMR (nor when adjusted for the expected decrease). The mean reductions 1 year after surgery were 498 ± 273 kcal and 481 ± 234 kcal, respectively, after LGBP and LVBG (P = 0.773).
Dietary Intake
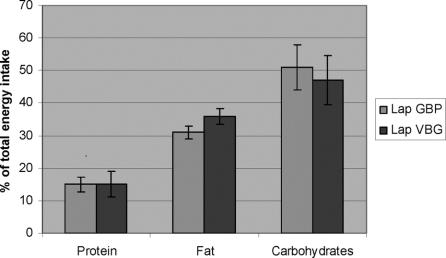
There were substantially reductions in reported energy intakes in both groups 1 year after surgery. The reduction of ingested energy (mathematically adjusted for the baseline differences in energy intake) was 1465 ± 942 kcal and 1087 ± 940 kcal, respectively, in the LGBP and LVBG groups (P = 0.105 for difference between groups). The proportions of total energy intake from protein, fat, and carbohydrates 1 year after surgery are shown in Figure 2. There was only a minor contribution from alcohol (1%–2%) in total dairy energy intake. LGBP patients reported a significant lower proportion of calories ingested as fat (30.5% ± 5.5% vs. 35.2% ± 6.3% after LVBG, P = 0.0014) and higher proportion of calories from carbohydrates (52.0% ± 6.9% vs. 47.7% ± 7.6%, P = 0.0149) as compared with LVBG patients.
FIGURE 2. The proportion of total energy intake from protein, fat, and carbohydrates 1 year after obesity surgery with laparoscopic gastric bypass (LGBP) or laparoscopic VBG (LVBG). Data are mean (CI).
As shown in Figure 3, LVBG patients reported a higher proportion of their total energy intake from foods high in sugar and fat than LGBP patients, who instead reported a higher relative intake from fruits and vegetables.
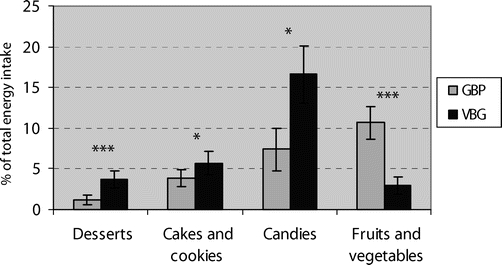
FIGURE 3. The proportion of total energy intake from various food groups 1 year after obesity surgery with laparoscopic gastric bypass (LGBP) or laparoscopic VBG (LVBG). *P < 0.05. ***P < 0.001.
A large number of LVBG patients avoided whole meat (about two thirds), bread (about one third), and fruits and vegetables (about one third). The corresponding figure in the LGBP group was 10% or less, whereas almost one third of the LGBP patients consciously avoided fatty foods, mainly due to not feeling good after eating it (Figure 4).
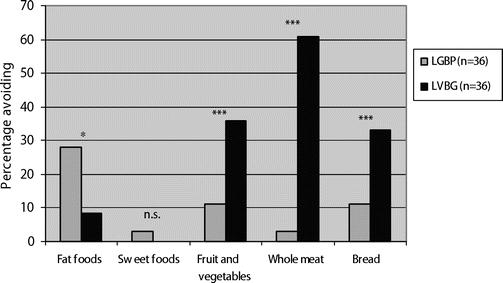
FIGURE 4. The proportion of patients that reported avoidance of various food groups 1 year after obesity surgery with laparoscopic gastric bypass (LGBP) or laparoscopic VBG (LVBG). *P < 0.05. ***P < 0.001.
An analysis of gender differences in the pattern of dietary intake preoperative and postoperative did not reveal any major differences compared with the figures presented for the entire group.
Dropout
Overall follow-up rate of patients included in the study was 97.6% (1 patient in each group was lost to follow-up). All patients who were excluded from analysis were analyzed. The dropout analysis revealed a coherent pattern with those included.
Anthropometry
The preoperative and postoperative anthropometric data of 1 patient in the VBG group were lost to registration.
DEXA
Thirteen patients (7 in VBG and 6 in GBP groups) with preoperative weight of >135 kg were not investigated owing to technical reasons described above.
CT
The 1-year follow-up was not usable in 2 patients (1 in each group) owing to technical problems.
DISCUSSION
Several surgical techniques been used in the history of obesity surgery. There is a wide disparity in the application of surgical techniques in different regions. Gastric bypass is the “gold standard” in North America, while bariatric surgeons elsewhere have mainly preferred restrictive techniques as standard. Evidence for tailored bariatric surgery, especially in non-superobese patients, is lacking. The proposed selection of “sweet-eaters”28 has been difficult to confirm in other studies.8,29
Laparoscopic techniques for most bariatric procedures have been developed during the last decade.30–34 Restrictive obesity surgery has been regained in popularity, possibly to some extent because restrictive operations are easier than complex to perform using laparoscopy. In laparoscopic complex bariatric operations (GBP, Duodenal switch), a great variations in the frequency of perioperative complications have been reported.24,33,35–38 However, it seems possible to establish a complication rate at the same level or better than been reported in association with open surgery.39,40 Laparoscopic techniques generally offers the patient a rapid recovery with less impact on respiratory function41 and few wound complications (infection, dehiscence, incision hernia).42,43
The goal of performing obesity surgery is 2-fold. On the one hand, the weight loss per se improves the quality of life of patients. There is a strong correlation between the level of weight loss and improvements of most aspects of quality of life.7 On the other hand, and from the medical professions point of view perhaps more important, obesity surgery is supposed to reduce the metabolic impairments associated with obesity. The ultimate goal from this point of view is to reduce morbidity and prevent mortality. However, we have only limited evidence-based knowledge about how different bariatric operations succeed in this respect.
The present study shows that the greater weight loss after LGBP than LVBG was attributable to a larger reduction in total body fat, where reduction of truncal fat contributed most. Waist circumference and sagittal diameter improved more after LGBP than LVBG. Even if there were some differences in the magnitude of these changes between males and females, the pattern of larger reduction after LGBP was coherent between the genders.
This seems significant, since especially the waist circumference and the sagittal diameter are independent risk factors for cardiovascular events andmortality.44–46 Visceral fat is probably a major contributor to the metabolic syndrome;47,48 therefore, the better reduction achieved in visceral fat seems advantageous. Although there were differences between the groups in the level of improvements, it must be stressed that virtually all patients in both groups postoperatively decreased their body weight and had a body composition associated with lower metabolic risk.
Another finding was a better-maintained LTM in the weight loss following LGBP compared with LVBG in men for which the reason remains to be investigated.
BMC decreased following LGBP and LVBG. Bone mass loss has previously been shown after gastric bypass49 and vertical banded gastroplasty,50 as well as after “nonsurgical” weight loss.50 This may support the consistent use of vitamin D and calcium supplements. The clinical significance of this is, however, unclear since obese patients generally have high BMC and BMD.
The resting energy expenditure developed as expected postoperatively in both groups, ie, a reduction reflecting the amount of weight lost. This is consistent with recently presented series18 and does not support theories about a postoperative increase in energy expenditure in hypometabolic LGBP patients as a mechanism of action in the weight loss following surgery.17
The most obvious effect of all bariatric surgical procedures is an alteration in the patients eating patterns. Studies have indicated that restrictive surgery (such as LVBG) tends to increase the patients' use of liquid high calorie diets (sweet-eating)8,29 and also induce avoidance of foods that are difficult to digest (ie, whole meat and raw vegetables). The findings in this study confirm this unfortunate development.
In addition, we recorded a reduction in dietary fat following LGBP to be the single most pronounced differing factor in the dietary composition between the groups. A low proportion of dietary fat is associated to less forming of body fat and thereby a lower body weight. This could actually be an effect making gastric bypass being more effective than restrictive procedures even in the short term. As proposed for obesity treatment in general,51 one of the most important factors in achieving an adequate and sustained weight loss after bariatric surgery may be to reduce the total energy intake by reducing dietary fat.
“Dumping” symptoms are generally supposed to be common after GBP and are proposed to be secondary to sugar intake. Some of the “dumping” symptoms (such as nausea, fatigue, malaise) are unspecific and therefore difficult to relate to a specific causal factor. Few of the patients operated with LGBP in this study reported that they avoided sweet foods, while close to 30% of patients who underwent LGBP claimed that they avoided eating fatty foods. This indicates that some of what is claimed to be “dumping” symptoms in the literature could be induced by eating fatty foods.
Patients in this series who were converted from the original VBG construction to a GBP should have been assessed in the LVBG group 1 year after surgery from a strict intention-to-treat view. However, since the aim of this study was to compare the outcome of the different surgical techniques, and the decision to reoperate was an active intervention, we decided to define reconstruction on the original bariatric construction as an endpoint.
CONCLUSION
We have demonstrated an advantageous outcome concerning body composition and dietary intake after LGBP as compared with LVBG. We found better reduction of total body fat, including visceral adipose tissue, following LGBP. Through an unclear mechanism, the lean tissue mass was better preserved in the weight loss following LGBP in men. Postoperatively, we found no difference in the decrease of the basic metabolic rate between the groups. Dietary intake after LGBP demonstrated a “steering” toward an advantageous diet with an ability to eat all foods, except for an avoidance of fat which, in fact, can be one mechanism of action in gastric bypass.
ACKNOWLEDGMENTS
The authors thank participating investigators Anna Laurenius, RD, who critically reviewed the study proposal and provided and cared for study patients and John Brandberg, MD, who collected body composition data. The authors also thank the skillful personnel at Utvecklingslab, Sahlgrenska University Hospital for excellent collaboration.
Footnotes
Supported in parts by grant from the Research Council of Västra Götalands Region, Sweden.
Reprints: Torsten Olbers, MD, PhD, Department of Surgery, Sahlgrenska University Hospital, SE-41345 Göteborg, Sweden. E-mail: torsten.olbers@vgregion.se.
REFERENCES
- 1.World Health Organization. Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 2.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. [DOI] [PubMed] [Google Scholar]
- 3.Sjostrom LV. Morbidity of severely obese subjects. Am J Clin Nutr. 1992;55(suppl 2):508–515. [DOI] [PubMed] [Google Scholar]
- 4.Sjostrom LV. Mortality of severely obese subjects. Am J Clin Nutr. 1992;55(suppl 2):516–523. [DOI] [PubMed] [Google Scholar]
- 5.The SBU on overweight and obesity: huge increase of overweight-related diseases. Lakartidningen. 2002;99:3188–3192. [PubMed] [Google Scholar]
- 6.Sjostrom CD, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–484. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS): an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998;22:113–126. [DOI] [PubMed] [Google Scholar]
- 8.Brolin RL, Robertson LB, Kenler HA, et al. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg. 1994;220:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall JC, Watts JM, O'Brien PE, et al. Gastric surgery for morbid obesity: the Adelaide Study. Ann Surg. 1990;211:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard L, Malone M, Michalek A, et al. Gastric bypass and vertical banded gastroplasty: a prospective randomized comparison and 5-year follow-up. Obes Surg. 1995;5:55–60. [DOI] [PubMed] [Google Scholar]
- 11.MacLean LD, Rhode BM, Forse RA, et al. Surgery for obesity: an update of a randomized trial. Obes Surg. 1995;5:145–150. [DOI] [PubMed] [Google Scholar]
- 12.Naslund I. Gastric bypass versus gastroplasty: a prospective study of differences in two surgical procedures for morbid obesity. Acta Chir Scand Suppl. 1987;536:1–60. [PubMed] [Google Scholar]
- 13.Gahtan V, Goode SE, Kurto HZ, et al. Body composition and source of weight loss after bariatric surgery. Obes Surg. 1997;7:184–8. [DOI] [PubMed] [Google Scholar]
- 14.Das SK, Roberts SB, Kehayias JJ, et al. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. Am J Physiol Endocrinol Metab. 2003;284:E1080–E1088. [DOI] [PubMed] [Google Scholar]
- 15.Adami GF, Barreca A, Gianetta E, et al. Body composition in subjects with surgically obtained stable body weight normalization. Int J Obes. 1989;13:55–58. [PubMed] [Google Scholar]
- 16.Giusti V, Suter M, Heraief E, et al. Effects of laparoscopic gastric banding on body composition, metabolic profile and nutritional status of obese women: 12-months follow-up. Obes Surg. 2004;14:239–245. [DOI] [PubMed] [Google Scholar]
- 17.Flancbaum L, Choban PS, Bradley LR, et al. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery. 1997;122:943–949. [DOI] [PubMed] [Google Scholar]
- 18.Das SK, Roberts SB, McCrory MA, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. [DOI] [PubMed] [Google Scholar]
- 19.Feurer ID, Crosby LO, Buzby GP, et al. Resting energy expenditure in morbid obesity. Ann Surg. 1983;197:17–21. [PMC free article] [PubMed] [Google Scholar]
- 20.Buscemi S, Caimi G, Verga S. Resting metabolic rate and postabsorptive substrate oxidation in morbidly obese subjects before and after massive weight loss. Int J Obes. 1996;20:41–46. [PubMed] [Google Scholar]
- 21.Olbers T, Fagevik Olsen MF, et al. Randomized clinical trial on laparoscopic Roux-en-Y gastric bypass versus laparoscopic vertical banded gastroplasty for obesity. Br J Surg. 2005;92:557–562. [DOI] [PubMed] [Google Scholar]
- 22.NIH Consensus statement. Bioelectrical impedance analysis in body composition measurement. National Institutes of Health Technology Assessment Conference Statement. December 12–14, 1994. Nutrition. 1996;12:749–762. [PubMed] [Google Scholar]
- 23.Olbers T, Lonroth H, Dalenback J, et al. Laparoscopic vertical banded gastroplasty: an effective long-term therapy for morbidly obese patients? Obes Surg. 2001;11:726–730. [DOI] [PubMed] [Google Scholar]
- 24.Olbers T, Lonroth H, Fagevik-Olsen M, et al. Laparoscopic gastric bypass: development of technique, respiratory function, and long-term outcome. Obes Surg. 2003;13:364–370. [DOI] [PubMed] [Google Scholar]
- 25.Thomsen TK, Jensen VJ, Henriksen MG. In vivo measurement of human body composition by dual-energy X-ray absorptiometry (DXA). Eur J Surg. 1998;164:133–137. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury B, Sjostrom L, Alpsten M, et al. A multicompartment body composition technique based on computerized tomography. Int J Obes Relat Metab Disord. 1994;18:219–234. [PubMed] [Google Scholar]
- 27.Lindroos AK, Lissner L, Sjostrom L. Validity and reproducibility of a self-administered dietary questionnaire in obese and non-obese subjects. Eur J Clin Nutr. 1993;47:461–481. [PubMed] [Google Scholar]
- 28.Sugerman HJ, Londrey GL, Kellum JM, et al. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg. 1989;157:93–102. [DOI] [PubMed] [Google Scholar]
- 29.Lindroos AK, Lissner L, Sjostrom L. Weight change in relation to intake of sugar and sweet foods before and after weight reducing gastric surgery. Int J Obes Relat Metab Disord. 1996;20:634–643. [PubMed] [Google Scholar]
- 30.Belachew M, Legrand MJ, Defechereux TH, et al. Laparoscopic adjustable silicone gastric banding in the treatment of morbid obesity: a preliminary report. Surg Endosc. 1994;8:1354–1356. [DOI] [PubMed] [Google Scholar]
- 31.Lonroth H, Dalenback J, Haglind E, et al. Vertical banded gastroplasty by laparoscopic technique in the treatment of morbid obesity. Surg Laparosc Endosc. 1996;6:102–107. [PubMed] [Google Scholar]
- 32.Lonroth H, Dalenback J, Haglind E, et al. Laparoscopic gastric bypass: another option in bariatric surgery. Surg Endosc. 1996;10:636–638. [PubMed] [Google Scholar]
- 33.Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg. 2000;10:514–523; discussion 524. [DOI] [PubMed]
- 34.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4:353–357. [DOI] [PubMed] [Google Scholar]
- 35.Baltasar A, Bou R, Miro J, et al. Laparoscopic biliopancreatic diversion with duodenal switch: technique and initial experience. Obes Surg. 2002;12:245–248. [DOI] [PubMed] [Google Scholar]
- 36.See C, Carter PL, Elliott D, et al. An institutional experience with laparoscopic gastric bypass complications seen in the first year compared with open gastric bypass complications during the same period. Am J Surg. 2002;183:533–538. [DOI] [PubMed] [Google Scholar]
- 37.Suter M, Giusti V, Heraief E, et al. Laparoscopic Roux-en-Y gastric bypass: initial 2- year experience. Surg Endosc. 2003;17:603–609. [DOI] [PubMed] [Google Scholar]
- 38.Westling A, Gustavsson S. Laparoscopic vs open Roux-en-Y gastric bypass: a prospective, randomized trial. Obes Surg. 2001;11:284–292. [DOI] [PubMed] [Google Scholar]
- 39.Higa KD, Ho T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: technique and 3-year follow-up. J Laparoendosc Adv Surg Tech A. 2001;11:377–382. [DOI] [PubMed] [Google Scholar]
- 40.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000;10:233–239. [DOI] [PubMed] [Google Scholar]
- 41.Olsen MF, Josefson K, Dalenback J, et al. Respiratory function after laparoscopic and open fundoplication. Eur J Surg. 1997;163:667–672. [PubMed] [Google Scholar]
- 42.Lujan JA, Frutos MD, Hernandez Q, et al. Laparoscopic versus open gastric bypass in the treatment of morbid obesity: a randomized prospective study. Ann Surg. 2004;239:433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–289; discussion 289–291. [DOI] [PMC free article] [PubMed]
- 44.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. [DOI] [PubMed] [Google Scholar]
- 45.Ohrvall M, Berglund L, Vessby B. Sagittal abdominal diameter compared with other anthropometric measurements in relation to cardiovascular risk. Int J Obes Relat Metab Disord. 2000;24:497–501. [DOI] [PubMed] [Google Scholar]
- 46.Bigaard J, Tjonneland A, Thomsen BL, et al. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res. 2003;11:895–903. [DOI] [PubMed] [Google Scholar]
- 47.Bosello O, Zamboni M. Visceral obesity and metabolic syndrome. Obes Rev. 2000;1:47–56. [DOI] [PubMed] [Google Scholar]
- 48.Bjorntorp P. Visceral fat accumulation: the missing link between psychosocial factors and cardiovascular disease? J Intern Med. 1991;230:195–201. [DOI] [PubMed] [Google Scholar]
- 49.Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–1065. [DOI] [PubMed] [Google Scholar]
- 50.Guney E, Kisakol G, Ozgen G, et al. Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. Obes Surg. 2003;13:383–388. [DOI] [PubMed] [Google Scholar]
- 51.Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: satiation, satiety, and patterns of eating. J Am Diet Assoc. 1997;97(suppl 7):63–69. [DOI] [PubMed] [Google Scholar]