Abstract
We have developed an eight-plasmid DNA transfection system for the rescue of infectious influenza A virus from cloned cDNA. In this plasmid-based expression system, viral cDNA is inserted between the RNA polymerase I (pol I) promoter and terminator sequences. This entire pol I transcription unit is flanked by an RNA polymerase II (pol II) promoter and a polyadenylation site. The orientation of the two transcription units allows the synthesis of negative-sense viral RNA and positive-sense mRNA from one viral cDNA template. This pol I–pol II system starts with the initiation of transcription of the two cellular RNA polymerase enzymes from their own promoters, presumably in different compartments of the nucleus. The interaction of all molecules derived from the cellular and viral transcription and translation machinery results in the generation of infectious influenza A virus. The utility of this system is proved by the recovery of the two influenza A viruses: A/WSN/33 (H1N1) and A/Teal/HK/W312/97 (H6N1). Seventy-two hours after the transfection of eight expression plasmids into cocultured 293T and MDCK cells, the virus yield in the supernatant of the transfected cells was between 2 × 105 and 2 × 107 infectious viruses per milliliter. We also used this eight-plasmid system for the generation of single and quadruple reassortant viruses between A/Teal/HK/W312/97 (H6N1) and A/WSN/33 (H1N1). Because the pol I–pol II system facilitates the design and recovery of both recombinant and reassortant influenza A viruses, it may also be applicable to the recovery of other RNA viruses entirely from cloned cDNA.
Influenza A virus, a member of the family Orthomyxoviridae, is a negative-sense RNA virus with a segmented genome. Its genetic composition allows this virus to evolve by reassortment of gene segments from different strains. Of the 15 hemagglutinin (HA) and 9 neuraminidase (NA) subtypes circulating in aquatic birds, only the H1N1, H2N2, and H3N2 subtypes are known to have caused pandemics in humans (1). There is evidence that pigs can serve as an intermediate host (“mixing vessel”) for the generation of new strains that are pathogenic in humans (2). The H5N1 influenza A outbreak in Hong Kong in 1997 showed that highly pathogenic influenza A viruses can also be transmitted directly from avian species to humans (3–6). The potential of influenza A viruses to generate new pathogenic strains from a vast number of circulating strains in the natural reservoir indicates that disease control requires monitoring these viruses and developing improved antiviral therapies and vaccines.
For influenza A virus, reverse-genetics systems have allowed the manipulation of the viral genome (7, 8). Unlike positive-strand viruses (i.e., poliovirus), the negative-sense viral RNAs (vRNAs) of influenza A viruses are not infectious. Only vRNA molecules encapsidated with the four viral polymerase complex proteins (PB1, PB2, PA, nucleoprotein) are able to initiate a viral replication and transcription cycle. After the ribonucleoproteins (RNPs) penetrate the cell nucleus, the associated proteins begin to transcribe the (−)vRNAs into mRNAs and (+)cRNAs. These cRNAs serve as templates for the synthesis of vRNAs. The first reverse-genetics system to be developed for influenza A virus was the RNP-transfection method (9, 10). After in vitro transcription of virus-like vRNA by T3 or T7 RNA polymerase and reconstitution of viral ribonucleoprotein (vRNP) molecules, genetically altered RNP segments were introduced into eukaryotic cells by transfection. Infection with influenza helper virus resulted in the generation of viruses possessing a gene derived from cloned cDNA. The establishment of the RNA polymerase I (pol I)-driven synthesis of vRNA molecules in vivo allowed the intracellular production of RNP complexes (11). In this system, virus-like cDNA was inserted between the pol I promoter and terminator sequences (12). Unlike the mRNA transcripts synthesized by RNA polymerase II (pol II), pol I-generated RNAs lack both a 5′ cap and a 3′ poly(A) tail. Functional vRNP molecules could be generated either by infection with helper virus or by cotransfection of protein expression plasmids encoding PB1, PB2, PA, or nucleoproteins (11, 13–15). Recent studies demonstrated that the plasmid-driven expression of all eight vRNAs from a pol I promoter and the coexpression of the polymerase complex proteins result in the formation of infectious influenza A virus (16, 17). Because the generation of influenza A virus entirely from plasmids requires no infection with helper virus, no selection system is needed; therefore, all gene segments can be manipulated without technical limitations. Previously, we established a new transcription system that allows the recovery of infectious virus if the PB1 cDNA is inserted between a pol I promoter and a pol II promoter (ref. 18, Fig. 1). Cotransfection of a pol I–pol II promoter PB1 construct with plasmids expressing vRNA and viral proteins resulted in the production of virus.
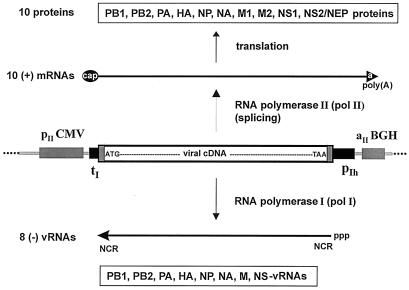
Figure 1.
Schematic representation of the pol I–pol II transcription system for synthesis of vRNA and mRNA. The cDNA of each of the eight influenza virus segments is inserted between the pol I promoter (pIh) and the pol I terminator (tI). This pol I transcription unit is flanked by the pol II promoter (pIICMV) of the human cytomegalovirus and the polyadenylation signal (aIIBGH) of the gene encoding bovine growth hormone. After transfection of the eight expression plasmids, two types of molecules are synthesized. From the human pol I promoter, negative-sense vRNA is synthesized by cellular pol I. The synthesized vRNA contains the noncoding regions (NCR) at the 5′ and 3′ ends. Transcription by pol II yields mRNAs with 5′ cap structures and 3′ poly(A) tails; these mRNAs are translated into viral proteins. The ATG of the viral cDNA is the first ATG downstream of the pol II transcription start site.
Here, we describe a plasmid-based transfection system for the rescue of influenza A virus entirely from cloned cDNA. Unlike established plasmid-based systems, this system for the generation of influenza A virus consists of the construction and transfection of only eight plasmids, each containing one copy of the viral cDNA. This reverse-genetics system reduces the number of plasmids required for the recovery of influenza A viruses and allows the generation of reassortant viruses.
Materials and Methods
Cloning of Plasmids.
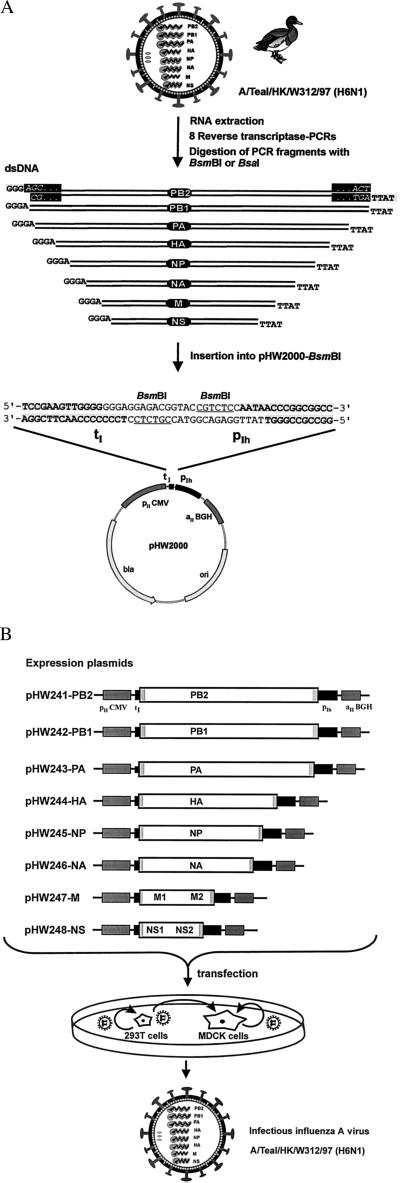
The plasmid pHW2000 (Fig. 3A) is derived from pHW12 (18). This cloning vector contains 225 bp of the human pol I promoter and 33 bp of the murine terminator sequence separated by two BsmBI sites. The pol I promoter and terminator elements are flanked by a truncated immediate–early promoter of the human cytomegalovirus (starting approximately 350 bp upstream of the transcription start site as found in pcDNA3, Invitrogen) and by the polyadenylation signal of the gene encoding bovine growth hormone. The eight plasmids containing the cDNA of the virus A/WSN/33 (H1N1) [pHW181-PB2, pHW182-PB1, pHW183-PA, pHW184-HA, pHW185-NP, pHW186-NA, pHW187-M, and pHW188-NS (NS, were constructed by inserting ApaI–SalI fragments (with viral cDNA and pol I promoter and terminator sequences) of the plasmids pPolI-WSN-PB2, pPolI-WSN-PB1, pPolI-WSN-PA, pPolI-WSN-NP, pPolI-WSN-HA, pPolI-WSN-NA (16), pHW127-M, and pHW128-NS (18) into the ApaI–SalI vector fragment of pHW2000. The eight plasmids containing the cDNA of A/Teal/HK/W312/97 (H6N1) (pHW241-PB2, pHW242-PB1, pHW243-PA, pHW244-HA, pHW245-NP, pHW246-NA, pHW247-M, and pHW248-NS) were constructed by reverse transcriptase–PCR (RT-PCR) amplification of the viral RNA. The primers used in the PCR reaction contained segment-specific sequences at their 3′ ends and BsmBI or BsaI restriction site sequences at their 5′ ends. After digestion of the PCR products with BsmBI or BsaI, the fragments were cloned into the vector pHW2000 (Fig. 3A). The sequences of the primers we used are available on request. To ensure that the viral cDNAs derived from RT-PCR amplification in the expression plasmids did not have unwanted mutations, the inserted cDNAs were sequenced.
Figure 3.
Schematic representation of the method developed for the construction and transfection of the eight expression plasmids to recover A/Teal/HK/W312/97 (H6N1). (A) Viral RNA was extracted from virus particles. RT-PCR was performed with primers containing segment-specific nucleotides and sequences for the type IIs restriction endonucleases BsmBI or BsaI. The eight viral PCR fragments were digested with BsmBI or BsaI and inserted into pHW2000 (linearized with BsmBI). This insertion resulted in eight expression constructs where the viral cDNAs are precisely fused to the pol I promoter and terminator (the viral terminal sequences AGC… ACT are shown for the PB2 segment in the black rectangles). (B) The eight expression plasmids with a pol I promoter and a pol II promoter contain one copy of each of the viral cDNAs of the eight segments. The open reading frames for the 10 viral proteins are flanked by the segment-specific noncoding regions (gray boxes). Because the used human pol I promoter shows high activity only in cell lines derived from humans or related species, we cocultured human 293T cells together with the standard cell line used for influenza A (MDCK cells). Viruses produced in the 293T cells after transfection can then infect MDCK cells and replicate.
Viruses and Cell Culture.
Influenza viruses A/WSN/33 (H1N1) and A/Teal/HK/W312/97 (H6N1) were propagated in 10-day-old eggs. Madin–Darby canine kidney (MDCK) cells were maintained in MEM containing 10% FBS. 293T human embryonic kidney cells were cultured in Opti-MEM I (Life Technologies, Gaithersburg, MD) containing 5% FBS. For the transfection experiments, six-well tissue culture plates were used. The day before transfection, confluent 293T and MDCK cells in a 75 cm2 flask were trypsinized, and 10% of each cell line was mixed in 18 ml OptiMEM I; 3 ml of this cell suspension was seeded into one well of a six-well plate. The cocultured MDCK and 293T cells (0.2 − 1 × 106 cells per well each) were used for the transfection experiments. TransIT LT-1 (Panvera, Madison, WI) was used according to the manufacturer's instructions to transfect the cells. Briefly, 2 μl of TransIT LT-1 per 1 μg of DNA was mixed, incubated at room temperature for 45 min, and added to the cells. Six hours later, the DNA-transfection mixture was replaced by Opti-MEM I. Thirty hours after transfection, 1 ml of Opti-MEM I containing TPCK-trypsin was added to the cells; this addition resulted in a final concentration of TPCK-trypsin of 0.5 μg/ml in the cell supernatant. The virus titer of the cell supernatant was determined by titration of the supernatant on MDCK cells.
RNA Isolation and RT-PCR.
Viral RNA was isolated from virus particles with the RNeasy-Kit (Qiagen, Valencia, CA), which was used according to the manufacturer's instructions. For characterization of recombinant influenza viruses, the Access RT-PCR kit (Promega) was used according to the protocol provided. The following primers were used in the RT-PCR experiments: Bm-NS#1 (5′-TAT TCG TCT CAG GGA GCA AAA GCA GGG TG-3′) and Bm-NS#2 (5′-ATA TCG TCT CGT ATT AGT AGA AAC AAG GGT GTT TT-3′). RT-PCR experiments were performed by using the PTC-200 DNA engine (MJ Research, Watertown, MA). The amplification program started with 1 cycle at 48°C for 45 min and 1 cycle at 94°C for 2 min. These cycles were followed by 40 cycles at 94°C for 20 sec, 52°C for 30 sec, and 72°C for 40 sec; the program ended with one cycle at 72°C for 5 min. The PCR products were analyzed by agarose gel electrophoresis and sequenced with the primer Bm-NS#1. The Center for Biotechnology at St. Jude Children's Research Hospital determined the sequence of template DNA by using rhodamine or dRhodamine dye-terminator cycle sequencing ready reaction kits with AmpliTaq DNA polymerase FS [Perkin–Elmer/Applied Biosystems (PE/ABI)] and synthetic oligonucleotides. Samples were subjected to electrophoresis, detection, and analysis on PE/ABI model 373, model 373 Stretch, or model 377 DNA sequencers.
Results
Establishment of the pol I–pol II System for the Generation of A/WSN/33 (H1N1).
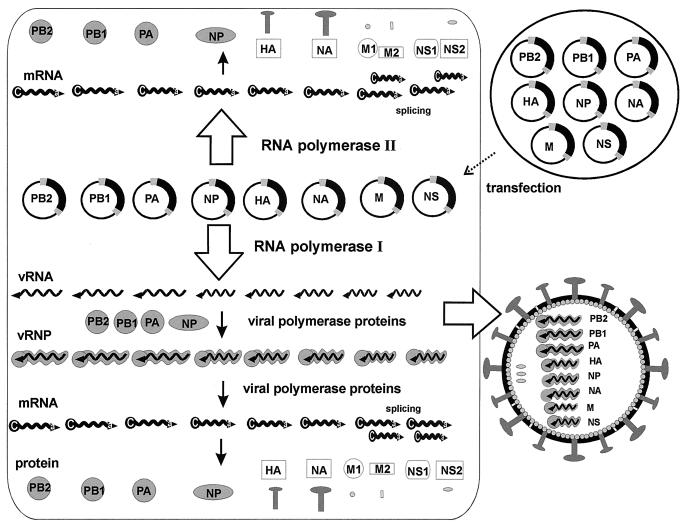
Because the genome of influenza A virus contains eight segments, we reasoned that the insertion of all eight influenza A cDNAs between a pol I promoter and a pol II promoter should result in the transcription of the eight vRNAs, all viral mRNAs, and in the synthesis of all 10 viral proteins (Fig. 1). After assembly of all vRNPs with the structural proteins, infectious influenza A virus should then be formed (Fig. 2).
Figure 2.
The eight-plasmid pol I–pol II system for the generation of influenza A virus. Eight expression plasmids containing the eight viral cDNAs inserted between the human pol I promoter and the pol II promoter (see Fig. 1) are transfected into eukaryotic cells. Because each plasmid contains two different promoters, both cellular pol I and pol II will transcribe the plasmid template, presumably in different nuclear compartments, which will result in the synthesis of viral mRNAs and vRNAs. After synthesis of the viral polymerase complex proteins (PB1, PB2, PA, nucleoproteins), the viral replication cycle is initiated. Ultimately, the assembly of all viral molecules directly (pol II transcription) or indirectly (pol I transcription and viral replication) derived from the cellular transcription and translation machinery results in the interaction of all synthesized molecules (vRNPs and the structural proteins HA, NA, M1, M2, NS2/NEP) to generate infectious influenza A virus.
To test whether we could rescue infectious influenza A virus with this cDNA-bidirectional transcription system, we constructed the eight expression plasmids (pHW181-PB2, pHW182-PB1, pHW183-PA, pHW184-HA, pHW185-NP, pHW186-NA, pHW187-M, and pHW188-NS) containing the eight cDNAs of A/WSN/33 (H1N1). We cotransfected eight plasmids (1 μg of each plasmid) (Table 1) into transiently cocultured 293T-MDCK cells. Both cell lines were cocultured in one cell-culture well the day before transfection to ensure conditions for high DNA transfection efficiency (293T cells) and for replication efficiency (MDCK cells) of influenza A viruses. After 48 and 72 h, the MDCK cells showed a virus-induced cytopathic effect, but no cytopathic effect was observed after transfection of seven plasmids without the PB1-expression construct (Table 1). The virus titer of the supernatant of transfected cells was determined at different times after transfection by titration in MDCK cells. Twenty-four hours after transfection, the cell supernatant contained 1 × 103 viruses per milliliter; 2 × 107 infectious viruses were generated 72 h after transfection (Table 1). The recovered viruses were passaged two times on MDCK cells. To verify that the generated virus was the designed A/WSN-virus, we produced the cDNA for the nonstructural (NS) gene by RT-PCR (Fig. 4A, lane 8). The generation of two fragments after digestion with the restriction endonuclease NcoI (Fig. 4B, lane 8) and sequence analysis of the amplified fragment confirmed that the recovered virus was indeed the designed A/WSN virus. These findings show that the pol I and pol II-driven synthesis of vRNA and mRNA from eight templates results in the generation of infectious influenza A virus.
Table 1.
Plasmid sets used for the recovery of A/WSN/33 (H1N1) and A/Teal/HK/W312/97 (H6N1) viruses
| Segment | A/WSN/33 (H1N1) | A/Teal/HK/W312/97 (H6N1) | ||
|---|---|---|---|---|
| 1 | pHW181-PB2 | pHW181-PB2 | pHW241-PB2 | pHW241-PB2 |
| 2 | — | pHW182-PB1 | — | pHW242-PB1 |
| 3 | pHW183-PA | pHW183-PA | pHW243-PA | pHW243-PA |
| 4 | pHW184-HA | pHW184-HA | pHW244-HA | pHW244-HA |
| 5 | pHW185-NP | pHW185-NP | pHW245-NP | pHW245-NP |
| 6 | pHW186-NA | pHW186-NA | pHW246-NA | pHW246-NA |
| 7 | pHW187-M | pHW187-M | pHW247-M | pHW247-M |
| 8 | pHW188-NS | pHW188-NS | pHW248-NS | pHW248-NS |
| Virus titer* | ||||
| 24 h | 0 | 1 × 103 | 0 | 0 |
| 48 h | 0 | 2 × 106† | 0 | 2 × 103 |
| 72 h | 0 | 2 × 107† | 0 | 2 × 105† |
The numbers represent infectious virus particles per milliliter of the supernatant of transfected cells as determined 24, 48, and 72 h after transfection.
Cytopathic effect in the cocultured MDCK cells was observed.
Figure 4.
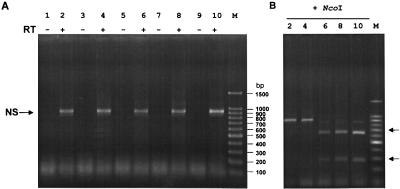
Characterization of the recovered viruses by RT-PCR. (A) RNA was extracted from virus particles after two passages of the supernatant of transfected cells (see Tables 1 and 2) on MDCK cells. RT-PCR was performed with primers specific for the NS gene segment and with vRNA extracted from virions. The NS primers we used were not strain specific, thus allowing the amplification of any influenza A NS segment. The reaction products were subjected to electrophoresis on a 2% agarose gel. To ensure that the amplified DNA fragments were derived from vRNA and not from plasmid DNA carried over from transfected cells, one reaction was performed without the addition of reverse transcriptase (RT−). Lanes 1 and 2, recombinant A/Teal/HK/W312/97 (Table 1); lanes 3 and 4, M reassortant (Table 2); lanes 5 and 6, NS reassortant (Table 2); lanes 7 and 8, recombinant A/WSN/33 virus (Table 1); lanes 9 and 10, quadruple reassortant (Table 2). (B) NcoI digestion of the fragments shown in A (only the WSN-NS-cDNA has an NcoI site). The identity of the NS fragments was also verified by sequence analysis of the amplified product (not shown).
Recovery of A/Teal/HK/W312/97 (H6N1) by Cotransfecting Eight Plasmids.
The influenza virus A/WSN/33 (H1N1), originally derived from the human influenza pandemic strain from 1918 (19, 20), has been passaged in mouse brain and is well adapted for growth in cell culture. To evaluate the efficiency of the eight-plasmid system for the generation of a virus from cloned cDNA that is not already adapted for growth in cell culture, we sought to generate the virus A/Teal/HK/W312/97 (H6N1) from cloned cDNA alone. This H6N1 virus was isolated from a dead teal during the H5N1 outbreak in Hong Kong in 1997. Genetic analysis of this virus revealed that it has seven segments with more than 97% nucleotide homology to the pathogenic H5N1 virus strains (E.H. and R.G.W., unpublished results). RNA was extracted from infected allantoic fluid, and the RT-PCR-amplified cDNAs were inserted into pHW2000; this insertion resulted in eight expression plasmids (Fig. 3). Seventy-two hours after transfection of pHW241-PB2, pHW242-PB1, pHW243-PA, pHW244-HA, pHW245-NP, pHW246-NA, pHW247-M, and pHW248-NS (1 μg each) into cocultured 293T-MDCK cells, a virus-induced cytopathic effect was observed in MDCK cells (Table 1). The virus yield was 2 × 105 infectious viruses per milliliter of the supernatant of the transfected cells. As shown in Fig. 4 A and B, lane 2, the identity of the recovered virus was verified by characterization of the NS segment. These results illustrate that this plasmid system requires the cloning of only eight cDNAs into one plasmid vector and that the transfection of the eight expression plasmids allows the recovery of an influenza A virus not already adapted to growth in mammalian cells.
Rescue of Reassortant Influenza A Viruses.
We sought to test the utility of the eight-plasmid system for the generation of reassortant viruses. Because this DNA transfection system does not require any selection system, the recovery of reassortant viruses should be achievable by appropriate combinations of expression plasmids in the transfection reactions. Seven expression plasmids containing the cDNA of A/Teal/HK/W312/97 (H6N1) were cotransfected with one expression plasmid containing the cDNA of A/WSN/33 (H1N1) (Table 2). High virus yields were obtained for the reassortant viruses containing seven segments of the teal virus and the M segment or NS segment of the WSN virus. Lower virus yields were obtained for the neuraminidase (NA) and HA-reassortant viruses (Table 2). Because we were able to rescue single reassortant viruses with the eight-plasmid system, we tested whether we could rescue a virus with multiple segments derived from one virus. Therefore, we transfected four expression plasmids containing the cDNA of the RNP-complex genes of the H6N1 virus (pHW241-PB2, pHW242-PB1, pHW243-PA and pHW245-NP) together with the plasmids pHW184-HA, pHW186-NA, pHW187-M, and pHW188-NS containing the cDNA of the WSN virus (Table 2). We recovered 4 × 106 viruses per milliliter of the cell supernatant. As shown in Fig. 4 (lane 10), the amplified NS segment of the quadruple reassortant virus was cleaved by NcoI; thus, the NS segment is derived from the WSN virus. These results show that the eight-plasmid transfection system allows the recovery of single and quadruple reassortant viruses.
Table 2.
Generation of reassortant influenza A viruses between A/Teal/HK/W312 (H6N1) and A/WSN/33 (H1N1) by cotransfecting eight plasmids
| Segment* | HA | NA | M | NS | HA-NA-M-NS |
|---|---|---|---|---|---|
| 1 | pHW241-PB2 | pHW241-PB2 | pHW241-PB2 | pHW241-PB2 | pHW241-PB2 |
| 2 | pHW242-PB1 | pHW242-PB1 | pHW242-PB1 | pHW242-PB1 | pHW242-PB1 |
| 3 | pHW243-PA | pHW243-PA | pHW243-PA | pHW243-PA | pHW243-PA |
| 5 | pHW245-NP | pHW245-NP | pHW245-NP | pHW245-NP | pHW245-NP |
| 4 | pHW184-HA | pHW244-HA | pHW244-HA | pHW244-HA | pHW184-HA |
| 6 | pHW246-NA | pHW186-NA | pHW246-NA | pHW246-NA | pHW186-NA |
| 7 | pHW247-M | pHW247-M | pHW187-M | pHW247-M | pHW187-M |
| 8 | pHW248-NS | pHW248-NS | pHW248-NS | pHW188-NS | pHW188-NS |
| Virus titer† | 2 × 102 | 2 × 103 | 2 × 105 | 2 × 107 | 4 × 106 |
Plasmids containing the cDNA of A/WSN/33 (H1N1) are shown in bold.
The numbers represent infectious virus particles per milliliter of supernatant of transfected cells as determined 72 h after transfection.
Discussion
The fact that we were able to rescue influenza A virus after transfection of the eight expression plasmids containing the cDNA of A/Teal/HK/W312/97 (H6N1) or A/WSN/33 (H1N1) proves that the pol I–pol II transcription system provides sufficient amounts of vRNA and viral proteins for the formation of infectious influenza A virus. Two types of mRNAs that differ in their noncoding regions are synthesized (Fig. 1). The mRNA type encoding all viral proteins is directly transcribed by pol II. In addition to the influenza virus sequences of the noncoding regions, these mRNAs contain sequences from the pol I promoter and the murine terminator regions. Importantly, the pol I–pol II expression system we developed contained only 33 bp of the murine terminator sequences. Previous studies using the reporter genes chloramphenicol acetyltransferase and green fluorescent protein showed that sequences in the 174-bp terminator region reduced pol II-mediated expression of protein (21). A second mRNA type is generated after the initiation of the viral replication and transcription process (Fig. 2). This mRNA is synthesized by the viral polymerase proteins and contains a 5′ cap structure derived from cellular RNAs by cap snatching preceding the influenza virus noncoding sequences. The structural proteins translated from both mRNAs associate with the RNP complexes to form new virus particles. After the budding of transfectant viruses, the generated virus particles can then replicate in the 293T cells and in the cocultured MDCK cells.
Recently, plasmid-based systems that are designed to express the eight vRNAs and viral mRNAs from separate plasmids have been developed. In the system developed by Neumann et al. (16), the eight cDNAs were inserted between a human pol I promoter sequence (407 bp) and a murine terminator sequence (174 bp). The expression of the four RNP-complex proteins was driven by the human cytomegalovirus promoter. Transfection of 12 plasmids into 106 293T cells resulted in virus recovery of more than 103 plaque-forming units (pfu); this efficiency could be increased to 5 × 107 pfu after the transfection of 17 plasmids. Fodor et al. (17) developed a system in which the eight cDNAs were inserted between a human pol I promoter sequence (250 bp) and a genomic ribozyme sequence of hepatitis δ virus to ensure the precise 3′ end of the vRNA. For the expression of the polymerase complex genes, plasmids containing the adenovirus type 2 major late promoter were used. After transfection of the 12 expression plasmids into Vero cells, only one or two infectious viral particles were rescued from 106 transfected cells. Unlike these approaches, our method deployed the eight cDNAs in eight plasmids that contained 225 bp of the pol I promoter sequences and 33 bp of the terminator sequences. In the pol I–pol II transcription system, all 10 viral proteins are expressed from a truncated immediate–early promoter of the human cytomegalovirus. The fact that the expression of all structural proteins with the 17-plasmid system (16) and with the 8-plasmid system (this study) resulted in a higher efficiency of virus recovery than did cotransfection of plasmids expressing the RNP complex proteins (16, 17) supports the idea that the generation of infectious influenza A virus is enhanced by providing the HA, NA, M1, M2, and NS2 proteins early after transfection.
The viral replication cycle involves a complex interaction between the viral proteins with each other and with cellular factors (22). Thus, for the generation of infectious virus, the plasmid-driven synthesis of viral molecules should provide optimal concentrations of viral proteins for the initiation of the replication cycle and for the formation of virus-like particles. Although the eight-plasmid system proved to be efficient, it might be possible to further increase the production of virus. It was shown that the ratio of transfected plasmids expressing the RNP complex proteins and the expression of the M1 protein influences the transcriptase activity (14, 23). The efficiency of the formation of virus-like particles also depends on the concentration of structural viral proteins (24–26). The efficiency of the generation of infectious virus with the pol I–pol II system might therefore be increased further by varying the plasmid concentrations used in the transfection reaction or by using expression plasmids with different pol II promoters. Because the splicing efficiency mediated by cellular factors influences the ability of influenza A virus to replicate (27), the use of cell lines other than 293T may increase the virus yield for certain influenza A strains. The high virus yield of the quadruple reassortant (Table 2) is consistent with the finding that the rapid replication of A/WSN/33 (H1N1) in cultured cells is mediated by the HA, NA, and M segments (19, 28, 29).
The fact that we could generate viable reassortants (Table 2) between the avian H6N1 virus and the human H1N1 virus indicates that this H6N1 virus can acquire gene segments from a distantly related virus. Genetic analysis suggested that the pathogenic H5N1 viruses were generated by reassortment (30). H5N1-like gene segments are found in the H6N1 (E.H. and R.G.W., unpublished results) and H9N2 subtypes (31), a finding indicating that these viruses may have been precursors of the pathogenic H5N1 viruses. Reassortment events that could create new pathogenic influenza viruses are likely to occur in the future. However, the ability to generate and manipulate these viruses by the simplified method developed in this study will help researchers better understand the biological properties of these new viruses and develop efficient vaccines to protect a population against them. The length of the time period between the emergence of a new pathogenic strain and the preparation of a vaccine is a crucial variable in the effectiveness of a vaccination program. The ability to generate viruses by cloning only eight plasmids reduces the time needed for the generation of potential vaccine candidates and improves existing reverse-genetics systems by simplifying virus creation and reducing the overall cost of production of a vaccine.
The concept of introducing viral cDNA between a pol I promoter and a pol II promoter into eukaryotic cells for the recovery of virus might also be applicable for the generation of other members of the family Orthomyxoviridae. For influenza B virus, this strategy would require the construction and cotransfection of eight plasmids; for influenza C, seven; and for Thogotovirus, six. The in vivo transcription of 5′-capped mRNA as well as vRNA from the same cDNA template may also simplify plasmid-based systems for other RNA viruses or even facilitate the establishment of pol I–pol II systems for viruses from other families (e.g., Arenaviridae, Bunyaviridae).
Acknowledgments
We thank L. Zhang, S. Krauss, and D. Walker for excellent technical support. We thank C. Scholtissek for reading the manuscript and for helpful suggestions. We also thank Flo Witte and Julia Cay Jones for scientific editing. These studies were supported by Public Health Research Grants AI95357, AI29680, and AI 44386 from the National Institute of Allergy and Infectious Diseases, by Cancer Center Support CORE Grant CA-21765, and by the American Lebanese Syrian Associated Charities.
Abbreviations
- HA
hemagglutinin
- MDCK cells
Madin–Darby canine kidney cells
- NA
neuraminidase
- NS
nonstructural
- M
matrix
- pol I
RNA polymerase I
- pol II
RNA polymerase II
- RNP
ribonucleoprotein
- RT-PCR
reverse transcriptase–PCR
- vRNA
viral RNA
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100133697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100133697
References
- 1.Webster R G, Bean W J, Gorman O T, Chambers T M, Kawaoka Y. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scholtissek C, Bürger H, Kistner O, Shortridge K F. Virology. 1985;147:287–294. doi: 10.1016/0042-6822(85)90131-x. [DOI] [PubMed] [Google Scholar]
- 3.Claas E C, Osterhaus A D, van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 4.Suarez D L, Perdue M L, Cox N, Rowe T, Bender C, Huang J, Swayne D E. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, et al. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 6.Shortridge K F. Vaccine. 1999;17,(Suppl 1):S26–S29. doi: 10.1016/s0264-410x(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 7.Palese P, Zheng H, Engelhardt O G, Pleschka S, Garcia-Sastre A. Proc Natl Acad Sci USA. 1996;93:11354–11358. doi: 10.1073/pnas.93.21.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann G, Kawaoka Y. Adv Virus Res. 1999;53:265–300. doi: 10.1016/s0065-3527(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 9.Luytjes W, Krystal M, Enami M, Pavin J D, Palese P. Cell. 1989;59:1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- 10.Enami M, Luytjes W, Krystal M, Palese P. Proc Natl Acad Sci USA. 1990;87:3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann G, Zobel A, Hobom G. Virology. 1994;202:477–479. doi: 10.1006/viro.1994.1365. [DOI] [PubMed] [Google Scholar]
- 12.Zobel A, Neumann G, Hobom G. Nucleic Acids Res. 1993;21:3607–3614. doi: 10.1093/nar/21.16.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flick R, Neumann G, Hoffmann E, Neumeier E, Hobom G. RNA. 1996;2:1046–1057. [PMC free article] [PubMed] [Google Scholar]
- 14.Pleschka S, Jaskunas R, Engelhardt O G, Zurcher T, Palese P, Garcia-Sastre A. J Virol. 1996;70:4188–4192. doi: 10.1128/jvi.70.6.4188-4192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Konig M, Hobom G, Neumeier E. Virology. 1998;246:83–94. doi: 10.1006/viro.1998.9169. [DOI] [PubMed] [Google Scholar]
- 16.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez D R, Donis R, Hoffmann E, et al. Proc Natl Acad Sci USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fodor E, Devenish L, Engelhardt O G, Palese P, Brownlee G G, Garcia-Sastre A. J Virol. 1999;73:9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann E, Neumann G, Hobom G, Webster R G, Kawaoka Y. Virology. 2000;267:310–317. doi: 10.1006/viro.1999.0140. [DOI] [PubMed] [Google Scholar]
- 19.Goto H, Kawaoka Y. Proc Natl Acad Sci USA. 1998;95:10224–10228. doi: 10.1073/pnas.95.17.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid A H, Fanning T G, Hultin J V, Taubenberger J K. Proc Natl Acad Sci USA. 1999;96:1651–1656. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann E. Ph.D. Thesis. Giessen, Germany: Justus Liebig University; 1997. [Google Scholar]
- 22.Ludwig S, Pleschka S, Wolff T. Viral Immunol. 1999;12:175–196. doi: 10.1089/vim.1999.12.175. [DOI] [PubMed] [Google Scholar]
- 23.Perez D R, Donis R O. Virology. 1998;249:52–61. doi: 10.1006/viro.1998.9318. [DOI] [PubMed] [Google Scholar]
- 24.Mena I, Vivo A, Perez E, Portela A. J Virol. 1996;70:5016–5024. doi: 10.1128/jvi.70.8.5016-5024.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Puertas P, Mena I, Castillo M, Vivo A, Perez-Pastrana E, Portela A. J Gen Virol. 1999;80:1635–1645. doi: 10.1099/0022-1317-80-7-1635. [DOI] [PubMed] [Google Scholar]
- 26.Neumann G, Watanabe T, Kawaoka Y. J Virol. 2000;74:547–551. doi: 10.1128/jvi.74.1.547-551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau S C, Scholtissek C. Virology. 1995;212:225–231. doi: 10.1006/viro.1995.1473. [DOI] [PubMed] [Google Scholar]
- 28.Schulman J L, Palese P. J Virol. 1977;24:170–176. doi: 10.1128/jvi.24.1.170-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda J, Bucher D J, Ishihama A. J Virol. 1994;68:8141–8146. doi: 10.1128/jvi.68.12.8141-8146.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Subbarao K, Cox N J, Guo Y. Virology. 1999;261:15–19. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- 31.Guan Y, Shortridge K F, Krauss S, Webster R G. Proc Natl Acad Sci USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]