Abstract
Objective:
To describe the field of surgical innovation from a historical perspective, applying new findings from research in technology innovation.
Background:
While surgical innovation has a rich tradition, as a field of study it is embryonic. Only a handful of academic centers of surgical innovation exist, all of which have arisen within the last 5 years. To this point, the field has not been well defined, nor have future options to promote surgical innovation been thoroughly explored. It is clear that surgical innovation is fundamental to surgical progress and has significant health policy implications. A process of systematically evaluating and promoting innovation in surgery may be critical in the evolving practice of medicine.
Methods:
A review of the academic literature in technology innovation was undertaken. Articles and books were identified through technical, medical, and business sources. Luminaries in surgical innovation were interviewed to develop further relevance to surgical history. The concepts in technology innovation were then applied to innovation in surgery, using the historical example of surgical endoscopy as a representative area, which encompasses millennia of learning and spans multiple specialties of care.
Results:
The history of surgery is comprised largely of individual, widely respected surgeon innovators. While respecting individual accomplishments, surgeons as a group have at times hindered critical innovation to the detriment of our profession and patients. As a clinical discipline, surgery relies on a tradition of research and attracting the brightest young minds. Innovation in surgery to date has been impressive, but inconsistently supported.
Conclusion:
A body of knowledge on technology innovation has been developed over the last decade but has largely not been applied to surgery. New surgical innovation centers are working to define the field and identify critical aspects of surgical innovation promotion. It is our responsibility as a profession to work to understand innovation in surgery, discover, translate, and commercialize advances to address major clinical problems, and to support the future of our profession consistently and rationally.
While novel technology is as old as the discipline of surgery, the field and study of surgical innovation are new. Recent research in technology innovation is just now being applied to surgery to develop rational strategies to promote innovation and surgical technology development. A discussion of critical factors to be considered is initiated and a possible course for the emerging field of surgical innovation suggested.
Over the last decade, the concepts and principles of innovation have largely been defined through research and publication in the business literature.1–3 These concepts in innovation may now be applied to other professions. Surgery, as one of the oldest and most respected fields, built upon continuous innovation, has a unique culture and deep tradition. While some aspects of research in the broad field of innovation are directly applicable to surgery, many unique aspects of our craft and practice require specialized thought. As such, perhaps it is the surgeon’s responsibility to describe and study innovation as it applies to our field.
“The abdomen, the chest and the brain will forever be shut from the intrusion of the wise and humane surgeon.” So opined Sir John Ericksen, Surgeon Extraordinaire to Queen Victoria in 1837. Today our surgeon colleagues will perform an average of 80,000 operations each day, many in the abdomen, the chest, or the brain. Innovations that took us from then to now can be thought of in several broad categories (Fig. 1).

FIGURE 1. Broad categories of innovation.
While innovation in surgery has a rich tradition, the field and study of surgical innovation are new. Ten peer-reviewed articles focusing specifically on surgical innovation have been published in the last 10 years, 5-fold the total number of previous publications. An increasing number of surgical leaders think that innovation may be the only way to maintain the quality of their profession.4 To date, attempts have been made to systematically evaluate broader concepts in technology innovation as they apply to surgery. Within a context of surgical history, specifically that of surgical endoscopy, we have tried to reference current concepts in the broadest context of technology innovation to the field of surgical innovation. Each section of this article will describe an aspect of innovation followed by support from historical references.
Our goal in writing this article is to initiate a dialogue on surgical innovation practice and policy that builds upon emerging concepts in technology innovation research. Current thought will be reviewed and new terms and concepts provided that may be relevant to the study of innovation in surgery as the field matures.
A comprehensive evaluation of surgical innovation would include discussion of ethics, economics, policy, and education, all important aspects. However, to maintain the focus of this article, those discussions will be left for future communications, and this manuscript will focus on fundamental concepts of how innovation is defined, assessed, critiqued, and encouraged.
Innovation Defined
Innovation is a broad term defined as the act of introducing something new5 or the use of a new idea or method.6 In some instances, it is used synonymously with invention, although innovation is more precisely defined as something thought up or mentally fabricated. Importantly, no technology or its application is entirely new, as no inventor works within a vacuum.7 All definitions of innovation involve both new ideas and an act of use or practice. The coupling of new ideas and hands-on use is also a central tenet of surgery, partially explaining the historical success of surgeons as innovators and the progress, which their innovation created. These new ideas may come in the form of technology, technique, or a combination. The process by which surgical innovation applies new ideas to “hands on” clinical needs is analogous to the process by which translational research applies basic research to clinical problems.
Research is not the same as innovation. Advancement in the basic sciences, such as immunology, biochemistry, and physiology, represents critically important progress. This research contributes to the fundamental knowledge base and supports future invention. However, basic science research is not the same as innovation as it does not require application or intended use. The distance between the two can be thought of as the translational gap.
Fundamental Concepts in Innovation
Many terms used within innovation research are new or lack universal definitions. Several terms that are representative of concepts important to surgical innovation require definition and clarity. The terms are fundamentally based on technology, market forces, or clinical impact. These terms are not mutually exclusive and often have overlapping meaning. Table 1 and Figures 2 and 3 provide graphic representations of these terms and their use.
TABLE 1. Key Features of Innovation Nomenclature
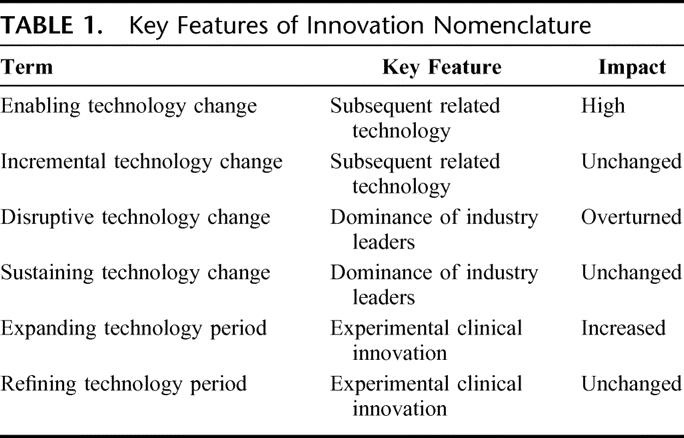
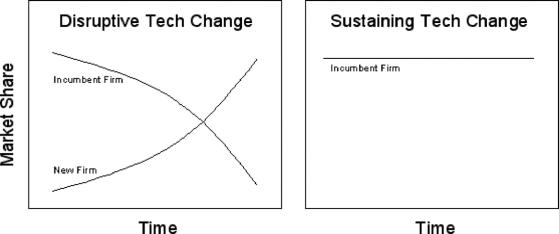
FIGURE 2. Graphic representation of disruptive versus sustaining technology change.
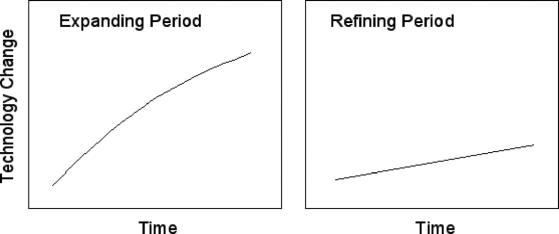
FIGURE 3. Graphic representation of expanding versus refining innovation period.
Technology innovation may be enabling or incremental. Enabling technology refers to an innovation that supports further developments within a field. An enabling procedure similarly supports development of new procedures within a field. For example, instrument sterilization represented an enabling technology as it supported countless advances within surgery. Similarly, vascular anastomotic technique perfected by Carel, was an enabling procedure, promoting a series of advances in surgical technique and innovation, from vascular repair to organ transplantation. On the other hand, an incremental technology change is an innovation which marginally improves upon currently available technology and does not lead to a significant technology shift. A new surgical clip with better holding strength and placement characteristics would represent an incremental change.
Over the last decade, a variety of market-based terms have arisen to describe the commercial impact of innovation. A disruptive technology change is defined as an innovation which topples industry leaders, leading to a significant loss in their market share. In surgery, industry leaders may be defined as the large corporations that often determine technology promotion. In a broader interpretation, industry leaders may be clinical leaders within a medical specialty who often determine technology adoption. In this case, specialty market share changes according to patient volume.
Generally, disruptive innovations are technologically straightforward and begin by catering to an emerging market focused on a new set of product attributes. When introduced, the technology is often inferior to the existing options or otherwise undesirable to customers, causing it to be ignored by industry leaders. As the technology develops, the growth curve of the disruptive innovation surpasses its previous competitor within a segment of the market2 (Fig. 2). As an example, percutaneous transluminal balloon angioplasty in its early development was dangerous and inferior to open coronary artery bypass. However, it ultimately proved to be disruptive within the field of cardiothoracic surgery, causing a shift in market share toward interventional cardiology.
The opposite of a disruptive change is a sustaining technology change. This change is defined as an improvement, generally made by current industry leaders, to maintain the rate of growth within an existing technology niche.2 The advances may be large and even enabling to new technology or procedures but are not disruptive to existing market forces. An example of a sustaining technology change is the invention of the coronary stent. This was an incremental change that improved outcomes within an existing market but did not topple either corporate or clinical industry leaders (Fig. 2).
In evaluating the history of surgical innovation, the existing terms in technology innovation are useful as they define the nomenclature. Indeed, as the field of technology innovation has matured over the last decade, many of these terms have only recently been defined and brought into common usage. For further insight into these concepts, seminal works within the business literature by Utterback,1 Christensen,2 and Roberts3 are recommended.
It should be recognized that these concepts and terms grew fundamentally out of business analysis to better understand market forces and industry trends. Indeed, the best known publication in the field focuses primarily on the computer disk drive industry.2 While these concepts represent a foundation, it is clear that physicians and surgeons often have a different view of technology change than corporate executives. Most surgeons innovate on a daily basis, tailoring therapies and operations to the intrinsic uniqueness of every patient and their disease.
It is the unsolved problem, or the repetitive failures of existing therapies that stimulate surgeons to find a better way. As a scrub tech, Tom Fogarty witnessed first hand the inadequacies of a groin to popliteal arteriotomy to extract an embolus. Indeed, such a procedure was frequently followed by several more and then by an amputation. Throughout history, surgeons have been the most prolific medical device innovators. Our lexicon in innovation must reflect a history that has often been less dependent upon market forces than upon patient outcome, peer review, and peer esteem. It should be noted that the impetus for surgical innovation may be changing as surgical care and health care, as a whole, are managed with fiscal performance as at least one primary outcome measure.
In examining the history of surgery, surgical nuances are not well described or accounted for in the current innovation literature. The history of surgical innovation has followed an ebb-and-flow pattern. New enabling technology is developed, generally building upon the work of others. The technology may be slowly or quickly adopted, but ultimately leads to a rapid expansion of medical capabilities and procedures. This rapid expansion leads to a slower period of technology refinement and consolidation of approach. Where initially physicians may try different techniques building upon a given enabling technology, ultimately, one or several will be widely adopted and others will be discarded. Since some surgeons are technologically savvy and relish new technology, they are often early adopters, at times making the process of acceptance rapid and underpinning a strong phase of expansion. On the other hand, since many surgeons tend to be risk-averse, long periods of avoidance of change may be seen.
New nomenclature in surgical innovation should focus on recognizing the clinical impact of novel technology, more than market impact. It should also characterize the ebb and flow of technology along a continuum and describe such phases of innovation. An expanding period of innovation can be defined as a time when technology develops rapidly and patient care is significantly altered. Most enabling technologies and enabling procedures support a network of new technology development and fall within expanding periods. A refining period of innovation can be defined as a time when existing technologies are improved upon, but patient care is changed little by these improvements. A refining innovation generally either increases efficiency, lessens the labor or device costs for a procedure, or slightly improves outcome. Most disruptive technologies are refining in that they use or improve upon an existing technology while reducing unit cost.2 Incremental technologies also fit within the category of refining. The terms expanding and refining periods are independent of industry leaders (Fig. 3), reflecting historical context and the overall impact of an innovation on patients and providers. It is our opinion that both phases and types of innovation should be recognized as important.
Much of the information presented thus far views innovation as it has been described from a business perspective. To further understand each of these concepts as they are applied to the field of surgery, the history of surgical endoscopy will be examined. Rather than attempting to describe all important innovations leading to surgical endoscopy, critical elements of its history will be used as a reference to elucidate concepts in innovation. Since most surgical specialties contributed to advances in surgical endoscopy, the focus will be on surgical innovation as a whole rather than specific subspecialties. For additional historical background, books and articles exclusively focused on the subject are recommended.8–10 It should be noted that within the history of surgical endoscopy, many aspects are considered great successes while others have been called failures. It is not our intention to applaud or criticize any specific individual or field, but rather to learn from overall trends in this technology evolution.
Rise of the Surgical Endoscopic Technique
The history of endoscopic surgery has been described in multiple phases, generally categorized by progress in underlying technologies: light sources, flexible instruments, and interventional tools. These attributes were developed in parallel and have been well described.11,12
The first iteration of approach was described by Hippocrates in the 4th century B.C. Within his school on the island of Kos, he described the first rectal speculum,13 an enabling technology that supported subsequent endoscopic developments such as the pelvic speculum.12
Not much changed until the advent of focused lighting. In 1805, Philip Bozzini, invented the Lichtleiter, a lighting apparatus supporting visualization of the bladder and rectum through mirror reflected candle-light.13 This enabling technology supported critically important future advances in lighting. Antoine Jean Desormeaux built on Bozzini’s external light source to create and present the first functional cystocope,11 earning him the title the “Father of Endoscopy.”8 Although the dates are frequently confused within the literature, Desormeaux’s work was widely disseminated after an initial presentation in Paris in 185514 through subsequent French and English publications in 186515 and 1867.16 Desormeaux’s innovation was an enabling procedure, replicated and improved upon countless times over the following decade. Not coincidentally, this period also saw the invention of functional esophagoscopy17 and hysteroscopy.18 The combination of enabling technology and procedure stimulated an expanding period in endoscopic innovation with the development of multiple new technologies and a significant impact on patient care.
This period of rapid activity and technology change within endoscopy gradually gave way to an ebb period where technology was refined. Incremental changes, such as the separation of the lens from the introducing sheath and the use of different telescopes by Boisseau du Rocher,11 led to evolution of more usable endoscopic devices. This refining period was critical to support development of functional technology and predicate the next expanding period of endoscopic evolution.
In 1901, Georg Kelling introduced a cystoscope into a dog’s abdominal cavity, performing the first laparoscopy.19 The enabling technology of electric lighting combined with the enabling procedure of laparoscopy stimulated a period of experimentation with body cavity visualization.12 Although Kelling’s work generated interest and roughly 2 decades of related experimentation, the following 50 years were largely refining for endoscopic technique. During this slow refining period, a great deal of attention was focused on the rapidly expanding fields of cardiothoracic and trauma surgery. During the 1960s and 1970s, Kurt Semm helped rekindle interest and enable advances in surgical endoscopy by inventing devices for automatic insufflation, thermocoagulation, and endoscopic irrigation.
By the 1970s and 1980s, a new period of surgical endoscopy expansion had begun. Modern minimal access surgery was largely ushered in through arthroscopy for orthopedics and laparoscopy for gynecologic surgery. It is beyond the scope and focus of this article to discuss the many endoscopic pioneers in urologic, gynecologic, orthopedic, thoracic, and general surgery deserving of mention. For detailed information, articles focused on a given surgical specialty are recommended.20–22 To illustrate fundamental concepts in innovation, an advance in surgical endoscopy which has been exceedingly well documented, the laparoscopic cholecystectomy, will be examined. While a relatively late development in surgical endoscopy, the advent of laparoscopic cholecystectomy represented an inflection point for interventional technique and propelled an expanding period of procedural and technologic advancement.
Taking advantage of advances in orthopedic, urologic, and gynecologic technology, Philippe Mouret and Francois Dubois, in 1987, used newly designed endoscopic equipment to perform the first laparoscopic cholecystectomies.23 News of the procedures spread, attracting attention worldwide and leading to rapid research and progress in surgical endoscopy. Newly developed video endoscopy acted as an enabling technology24 while the operation represented an enabling procedure. The work was disruptive to industry leaders, both corporate and clinical, many of whom initially viewed the process as costly and time-consuming and some of whom were unable to transition to the modern era of laparoscopic intervention. Of note, with disruptive innovation, industry leaders frequently do not recognize the value of a novel technology, providing opportunity to smaller industry firms and individuals.
Innovation Assessed
To assess how innovation occurs, it is necessary to understand individual innovators. In this section, the history of surgical endoscopy will be used to assess select innovators as well as the environment within which they operated. Why have surgeons throughout history excelled at innovation and led in the development of medical devices? Why are some surgeons successful in innovation while others fail? These questions will be evaluated by first focusing on traits common to surgeons and subsequently introducing several new concepts in innovation.
Surgeon Innovator
Surgeons are fundamentally decision makers. While within most corporations, only top executives make significant leadership decisions, all surgeons face clinical decisions on an hourly basis, many with significant impact and consequence. Furthermore, surgeons in private practice are decision makers within their “firms.” Within the corporate context, Clark and Wheelwright refer to this as heavyweight versus lightweight teams. A heavyweight team includes decision makers who feel comfortable taking risks and making relatively independent decisions.25 Because of necessary frameworks for risk reduction, these individuals are rare within the corporate environment. Based on personality and training, most surgeons leading teams naturally fall within the category of heavyweight.
Surgeons have historically been idea generators and creative practitioners within their craft. In the technology life cycle, as initially described by Utterback, the first phase of idea generation and evaluation is fluid and requires vision and flexibility.1 When asked where the greatest weakness in product innovation is, many managers indicate the idea generation phase.26,27 The surgeon’s training requires daily situation assessment, decision analysis, and frequent development of new processes. Each clinical case offers unique challenges and requires a degree of creativity. For this reason, surgeons often excel at the creative, fuzzy front end of technology development.
Surgeons understand clinical needs and may anticipate future advances and opportunities. A lead user is defined as a technology user whose present strong needs will become general in a marketplace months or years in the future.28 Many surgeons are lead users within the field of surgical intervention and instinctively recognize emerging market opportunities. On the other hand, companies often have a difficult time recognizing or competing in emerging markets. Many corporate planning systems do not focus the attention of senior management on unanticipated successes, particularly in new markets.29 Furthermore, because promotion often depends on short-term accomplishments, managers must distance themselves from the uncertainties inherent in product development while technical personnel protect themselves against the loss of corporate commitment.30 Because of corporate structure and funding processes, a company’s leaders may only be made aware of an emerging market years after a surgeon recognizes the clinical opportunity. This, in part, explains why surgeons have been successful within startup companies in creating disruptive technology. Furthermore, surgeons have the advantage, as thought leaders within a field, to promote a procedure or invention based on clinical outcomes. This practice has come under intense scrutiny with a renewed public and academic focus on conflict of interest. However, it is clear that in the history of surgical innovation, individual proponents of a new process or technology have been essential in its development and adoption.30,31
Although an innovator’s personality is critical to new technology development and adoption, context can be just as important.32 For surgical innovation, we will use the term context to refer to time and place. The timing of an invention determines not only interest level from the general community, but also technology availability and cost-effectiveness. For an enabling procedure to lead to expansion within a field, the availability of a cluster of new technologies is often required. The place of invention is also surprisingly important. An innovator within a small city is far less likely to have the intellectual interaction and academic connections necessary to have his or her invention noticed.14 While time may determine technology availability, place will often determine financial and intellectual resources. The externalities, or synergistic benefits associated with location, product adoption, and personal network, are increasingly being recognized. The importance of medical technology clusters has been highly publicized, with the largest U.S. clusters currently thriving in the San Francisco Bay Area and Boston.
To further elucidate these concepts and highlight specific traits and context that support development of enabling innovations, the first widely publicized endoscopy and, more than a century later, the first widely publicized laparoscopic surgery will be examined.
Surgeon Innovators in Context
Antonin Jean Desormeaux, who presented the first description of a cystoscope to the Academy of Medicine in Paris in 1855,11,33 has been described as the “Father of Endoscopy” for the discoveries and technology that grew out of his work.34 Both the person and context were critical to his innovation.
The context for Desormeaux’s work was Paris in the 1850s and 1860s. This represented an expanding technology period for medicine and surgery. Dr. William Morton had first demonstrated anesthesia in 1846. Charles Darwin published Origin of the Species in 1859.35 Joseph Lister’s seminal paper on antisepsis would be published in 1867 in Lancet.36 During this time period, France was a global center for medical advances. Within the century, French scientists and physicians invented the modern medical clinic, the stethoscope, and the germ theory of disease.37
Desormeaux, as a person, was practical in his innovation. Working twenty-five years after development of the incandescent light bulb,13 he rejected the electricity storing batteries, which required an assistant to carry and adjust them. Instead, he modified Philip Bozzini’s Lichtleiter33 to serve as a focusing mechanism for natural light source. To this, he fitted a series of endoscopic tubes.16 Desormeaux took great interest in spreading his ideas and is well known for his monograph8 and the cystocope description he presented to the Academy of Medicine in Paris.38 These public descriptions are cited as having stimulated American instrument makers to commence the production of endoscopes.23
The context provided Desormeaux necessary light focusing technology and lenses as well as a cluster of medical technology innovators to work with in his endeavors. His personality led him to both publish and publicize his work. Together, this helped Desormeaux to not only develop the first effective endoscope, but also to popularize his findings and stimulate expansion of the field.
A modern day story of innovation, as already referenced, is the introduction of laparoscopic cholecystectomy. In early 1987, Philippe Mouret, a general surgeon in Lyon, France, operated on a woman suffering from both a gynecologic disorder and gallstones. He turned the laparoscope upward and performed the first laparoscopic cholecystectomy.12 While Mouret had not published his experience, Francois Dubois of Paris learned of the procedure and met with Mouret. Later that year, Dubois began experiments in both animals and humans and presented his work at an academic meeting in Paris. Dubois worked with a French colleague, Jacques Perissat, to publicize efforts in laparoscopic cholecystectomy. Hosting surgeons and presenting frequently at meetings, they were able to propel visibility of the procedure until more surgeons had rallied to the cause.12 Subsequent years saw rapid expansion of technology available for laparoscopy as well as surgeons interested in performing ever more complex operations.
The context for Dubois’ innovation was Paris in 1987. At this time, communication was easily attained throughout the world. Dubois was actively involved in clinical research and publication and was connected within an academic network. Perhaps more importantly, Dubois was physically near Mouret in Lyon (which remains an important attribute even today) and able to visit him in person.38 The timing also coincided with a series of technical advances. Illumination had been advanced during the 1960s and 1970s with proximal light sources and “cold light.” In 1983, Welch Allyn introduced the first endoscope with video rather than fiberoptic transmission.37 The first miniature solid state camera was introduced in 198639 and thus made feasible a connection with a scope, a seminal contribution by our colleague Nezhat.40,41 Video transmission allowed a better field of vision, now enabling assistants to see and work simultaneously, further supporting an expanding period of endoscopic innovation.
Personal traits also supported Dubois as an effective innovator. As a university professor, he exhibited an interest in novel approaches to clinical care and actively published his results.12 When he heard of an interesting use of laparoscopy, he arranged to meet Mouret and discuss the procedure with him. As Dubois performed and studied the procedure, he also engaged his colleagues within academic forums. He became the social focus of a network, collaborating with local colleagues such as Perissat and inviting others to learn from his technique.42
Personality and the right context combined to support Desormeaux and Dubois as stunningly effective innovators, both in developing new technology and bringing it to practice. These elements highlight basic aspects of the surgeon innovator. The proper context may currently exist for rapid expansion in nanotechnology, regenerative medicine, and robotics. With the context in place, these fields require effective clinical innovators to bring about new therapies and improved patient care.
Innovation Critiqued
While it is clear that surgeons throughout history have acted as innovators critical to the development of new technology and procedures, many innovators find their actions ineffective in influencing the surgical community. Health care has been described as the most entrenched, change-averse industry in the United States.13 Within health care, surgical culture is often seen to be particularly traditional, overly emphasizing our past. It is understandable that the overall tone would be conservative within a field where a radical or novel approach can translate to significant morbidity or mortality. Still, countless opportunities may be lost as innovators and innovations are ignored or actively rejected by consensus within our field.
To use the framework of surgical endoscopy to understand how innovation can be rejected, the same events from the previous section will be examined to elucidate difficulties with the introduction of new technology.
Desormeaux was able to build and generate interest in an effective endoscope in 1865. As it turns out, recent advances in electricity and anesthesia were not actually critical to his efforts. With adequate technology available at the time, why could Phillipe Bozzini, who invented the first effective lighting system for endoscopy in 1805, not popularize his approach? Indeed, Bozzini demonstrated the invention to the Alert Faculty in Vienna, who rejected it as a “magic lantern.”8 He was censured for “undue curiosity” by the Medical Faculty of Vienna, and his innovation was never put into practice.43 While there are a variety of reasons this occurred, it is indicative that the surgical community failed to grasp the importance of the invention and support the innovator.
More than 100 years later, in the mid-1980s, laparoscopic technique had little use in major operations. While it may be hypothesized that the 1986 introduction of the computer chip television camera made major laparoscopic operations more feasible,37 it is not clear why more minor procedures were not popularized, nor is it clear why there was such a high level of antagonism from the surgical community when the procedure was introduced. Gynecologic, orthopedic, and urologic surgeons had used endoscopy for minor procedures for decades. The first laparoscopic appendectomy was performed by Dekok in 1977.44 At least one surgeon claims to have performed laparoscopic cholecystectomy years before Mauret and Dubois.45 Yet, use of the laparoscope for major operations in the early 1980s was negligible. Once again, there was a significant lack of support for innovators within the field.
While it is clear that the surgeon innovator’s personal characteristics are critical to technology development and diffusion, context is equally important. Included in context is community support for surgical innovation.
There are countless historical examples of the surgical community’s reluctance to accept change. From the 1805 comment that Bozzini’s Lichtleiter was a “magic lantern” to the 1992 Caversham Conference where the “tidal wave” of new technology was noted to be “threatening western health-care systems,”45 the surgical community has been consistently antagonistic to innovators. Comments within the same conference included that “surgical innovation is part of that threat” and “surgery needs to be on its guard against fashion.”46 These thoughts are not unreasonable. As eloquently described by Stirratt et al in the same time period, “new surgical procedures must be tested, and that means clinical testing by mortality and morbidity, and psychologic and social testing by outcome for the individual patient and the community.”46 It may also be noted that laparoscopic cholecystectomy was not evaluated initially by randomized controlled trials but was propelled by anecdotes and case series.47
Nevertheless, for a field that is proud of its innovative roots, and in fact, dependent upon them, we often are stingy in our praise for novel ideas and procedures. It is clear that personal characteristics of the innovator and acceptance of the surgical community are just as critical to innovation as technology and technique. Both enabling and incremental technology changes are important, but both require a fertile ground within which they can take root.
Innovation Encouraged
Using the history of surgical endoscopy as a guide, fundamental concepts in technology innovation as they apply to surgery have been examined. Several new concepts and terms unique to innovation within surgery have also been introduced. While surgical endoscopy reflects only a small subset of innovation within surgery, it is representative of the larger picture. Most will find applicability of these concepts and terms to other stories or personal experiences in innovation.
As a specialty, we are just now beginning to analyze and understand what has made us the leaders in medical device innovation for the last 2 millennia and which elements have hindered our progress. It is ironic that this research comes during the same time period when many surgeons feel under attack from all sides. HMOs and advocacy groups call for practice standardization. The legal system encourages conservative patient management. Financial realities require cost/ benefit assessment, and in growing commoditization. Taken alone, each of these elements may be a positive overall force in patient care. However, taken together, they mandate restriction of creativity, experimentation, and ultimately innovation.
Surgeons are increasingly under pressure to achieve a fiscal report card that is black and not red. Workload increases, reimbursement decreases, and extra time, which was traditionally dedicated to teaching, research, and innovation, becomes harder to find. Fiscal responsibility is a necessary feature of the modern economy, but increasing efficiency and operative productivity may not be a sustainable strategy within the profession.
Does this represent a doomed future? We think that, as technology becomes more prevalent and accessible to the common inventor, there is unprecedented opportunity for the surgeon innovator. The current potential for advancement in therapeutic intervention is only rivaled by the mid-1800s with the advent of anesthesia and antisepsis. Rapid advances in imaging, minimally invasive technique, and robotic technology suggest we are at a threshold for a new era in patient care. There has never been more capital applied to medical technology and devices or more interest in surgical technology development. The successful surgeon innovator of today clearly must be savvy not only in medicine, but also in technology and business. As we continue to draw bright, hard-working, and talented individuals to our ranks, this is well within our grasp.
As a field, we have generated some of the leading innovators in history. We have also discouraged and rejected critical innovations. During this era of unprecedented opportunity and formidable roadblocks, it is time we as a profession take an active role in promoting innovation. In this effort, we must understand historical advances, recognize our successes and mistakes, clarify current challenges and resources, and work to promote a supportive environment within our field. If not us, then who else will take this role? We think that our future depends upon a clear understanding of innovation and rational and strong support of innovators within our specialty.
Footnotes
Partial salary support (to D.J.R.) was received from the Lucile Packard Foundation for Children’s Health, Palo Alto, CA.
Reprints: Thomas M. Krummel, MD, FACS, Department of Surgery, Lucille Packard Children’s Hospital, 701B Welch Road, Stanford, CA 94305-5748. E-mail: tkrummel@stanford.edu.
REFERENCES
- 1.Utterback JM. Mastering the Dynamics of Innovation. Boston: Harvard Business School Press, 1994. [Google Scholar]
- 2.Christensen CM. The Innovator’s Dilemma. New York: HarperCollins, 1997:15–16. [Google Scholar]
- 3.Roberts EB. Innovation: Driving Product, Process, and Market Change. San Francisco: Jossey-Bass, 2002. [Google Scholar]
- 4.Cosgrove DM. The innovation imperative. J Thorac Cardiovasc Surg. 2000;120:839–842. [DOI] [PubMed] [Google Scholar]
- 5.The American Heritage Dictionary of the English Language, 4th ed. Houghton Mifflin, 2004. [Google Scholar]
- 6.Jones JW, McCullough LB, Richman BW. Ethics of surgical innovation to treat rare diseases. J Vasc Surg. 2004;39:918–919. [DOI] [PubMed] [Google Scholar]
- 7.History of Technology. Encyclopaedia Britannica, 2004. [Google Scholar]
- 8.Gorden A. The History and Development of Endoscopic Surgery. London: Saunders, 1993. [Google Scholar]
- 9.Gotz F, et al. The history of laparoscopy. In: Color Atlas of Laparoscopic Surgery. New York: 1993. [Google Scholar]
- 10.Modlin IM, Kidd M, Lye KD. From the lumen to the laparoscope. Arch Surg. 2004;139:1110–1126. [DOI] [PubMed] [Google Scholar]
- 11.Lau WY, Leow CK, Li AK. History of endoscopic and laparoscopic surgery. World J Surg. 1997;21:444–453. [DOI] [PubMed] [Google Scholar]
- 12.Litynski GS. Endoscopic surgery: the history, the pioneers. World J Surg. 1999;23:745–753. [DOI] [PubMed] [Google Scholar]
- 13.Rosin D. History. In: Minimal Access Medicine and Surgery. Oxford: Radcliffe Medical, 1993:1–9. [Google Scholar]
- 14.Desormeaux A.J. De l’Endoscopie, instrument proper a éclairer certaines cavités intérieures de l’économie. In: Compte rendu des Séances de l’Academie des Sciences. 1855. [Google Scholar]
- 15.Desormeaux AJ. De l’Endoscope et de Ses Applications au Diagnostic et au Traitement des Affections de l’Urèthre et de la Vessie. Paris: Balliere, 1865. [Google Scholar]
- 16.Desormeaux AJ. The endoscope and its application to the diagnosis and treatment of affections of the genitourinary passages. Chicago Med J. 1867;24:177–194. [PMC free article] [PubMed] [Google Scholar]
- 17.Bevan L. The oesophagoscope. Lancet. 1868;1:470. [Google Scholar]
- 18.Pantaleoni D. On endoscopic examination of the cavity of the womb. Medical Press Circular, 1869:26. [Google Scholar]
- 19.Kelling G. Die Tamponade der Bauchhohle mit Luft zur Stillung lebensgefahrlicher Intestinalblutungen. Med Wochenschr. 1901;48:1480. [Google Scholar]
- 20.Shah J. Endoscopy through the ages. Br J Urol Int. 2002;89:645. [DOI] [PubMed] [Google Scholar]
- 21.Treuting R. Minimally invasive orthopedic surgery: arthroscopy. Ochsner J. 2000;2:158–163. [PMC free article] [PubMed] [Google Scholar]
- 22.Mancuso S. Endoscopy in gynecology. Rays. 1998;23:603–604. [PubMed] [Google Scholar]
- 23.Paolucci B, Schaeff B, Stuttgart G. Gasless Laparoscopy in General Surgery and Gynecology: Diagnostic and Operative Procedures. 1996. [Google Scholar]
- 24.Spaner SJ, Warnock GL. A brief history of endoscopy, laparoscopy, and laparoscopic surgery. J Laparoendosc Adv Surg Tech. 1997;7:369–373. [DOI] [PubMed] [Google Scholar]
- 25.Clark K, Wheelwright S. Organizing and leading heavyweight development teams. Calif Manage Rev. 1992;34:9–28. [Google Scholar]
- 26.Smith PG, Reinertsen DG. Developing Products in Half the Time. New York: Van Nostrand Reinhold, 1991. [Google Scholar]
- 27.Von Hippel E. Lead users: a source of novel product concepts. Manage Sci. 1986;32:791–805. [Google Scholar]
- 28.Drucker P. Innovation and Entrepreneurship. New York: Harper & Row, 1985. [Google Scholar]
- 29.Schon DA. The Reflective Practitioner: How Professionals Think in Action. New York: Basic Books, 1983. [Google Scholar]
- 30.Denis JL, Hebert Y, Langley A, et al. Explaining diffusion patterns for complex health care innovations. Health Care Manage Rev. 2002;27:60–73. [DOI] [PubMed] [Google Scholar]
- 31.Greer AL. Scientific knowledge and social consensus. Controlled Clin Trials. 1994;15:431–436. [DOI] [PubMed] [Google Scholar]
- 32.David TE. Innovation in surgery. J Thorac Cardiovasc Surg. 2000;119(suppl):38–41. [DOI] [PubMed] [Google Scholar]
- 33.Shah J. Endoscopy through the ages. Br J Urol Int. 2002;89:645. [DOI] [PubMed] [Google Scholar]
- 34.Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray, 1859. [PMC free article] [PubMed] [Google Scholar]
- 35.Lister J. On the antiseptic principle of the practice of surgery. Lancet. 1867;2. [Google Scholar]
- 36.Picard JF. American patronage and French Medicine: from the Rockefeller philanthropy to INSERM. In: John Shaw Billings Society for the History of Medicine. 1995. [Google Scholar]
- 37.Kalbasi H, Moddaressi Y. History and development of laparoscopic surgery. J Assoc Iranian Endosc Surgeons. 2001;1:1. [Google Scholar]
- 38.Sircus W. Milestones in the evolution of endoscopy: a short history. J R Coll Physicians (Edinb). 2003;33:124–134. [PubMed] [Google Scholar]
- 39.Nezhat C. Videolaseroscopy and laser laparoscopy in gynaecology. Br J Hosp Med. 1987;38:219–224. [PubMed] [Google Scholar]
- 40.Dubois FP, Berthelot G. Coelioscopic cholecystectomy: preliminary report of 36 cases. Ann Surg. 1990;211:60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubois F, Berthelot G, Levard H. Laparoscopic cholecystectomy: historic perspective and personal experience. Surg Laparosc Endosc. 1991;1:52–57. [PubMed] [Google Scholar]
- 42.Christensen CM, Bohmer R, Kenagy J. Will disruptive innovations cure health care? Harvard Business Rev. 2000;78:102–112. [PubMed] [Google Scholar]
- 43.Filipi CJ, Fitzgibbons RJ, Salerno GM. Historical review: diagnostic laparoscopy to laparoscopic cholecystectomy and beyond. Surg Laparosc. 1991;3:21. [PubMed] [Google Scholar]
- 44.Clarke HC. History of endoscopic and laparoscopic surgery. World J Surg. 2001;25:967–968. [DOI] [PubMed] [Google Scholar]
- 45.Stirrat G, Ramsay B. Surgical innovation under scrutiny. Lancet. 1993;342:187–188. [PubMed] [Google Scholar]
- 46.Stirratt G, et al. The challenge of evaluating surgical procedures. Ann R Coll Surg Engl. 1992;74:80–84. [PMC free article] [PubMed] [Google Scholar]
- 47.Banta HD. Minimally Invasive Therapy (MIT) in Five European Countries. Amsterdam: Elsevier, 1993. [DOI] [PubMed] [Google Scholar]