Abstract
Summary Background Data:
Use of synbiotics has been reported to benefit human health, but clinical value in surgical patients remains unclear.
Objective:
To investigate the effect of perioperative oral administration of synbiotics upon intestinal barrier function, immune responses, systemic inflammatory responses, microflora, and surgical outcome in patients undergoing high-risk hepatobiliary resection.
Methods:
Patients with biliary cancer involving the hepatic hilus (n = 101) were randomized before hepatectomy, into a group receiving postoperative enteral feeding with synbiotics (group A); or another receiving preoperative plus postoperative synbiotics (group B). Lactulose-mannitol (L/M) ratio, serum diamine oxidase (DAO) activity, natural killer (NK) cell activity, interleukin-6 (IL-6), fecal microflora, and fecal organic acid concentrations were determined before and after hepatectomy. Postoperative infectious complications were recorded.
Results:
Of 101 patients, 81 completed the trial. Preoperative and postoperative changes in L/M ratio and DAO activity were similar between groups. Preoperatively in group B, NK activity, and lymphocyte counts increased, while IL-6 decreased significantly (P < 0.05). Postoperative serum IL-6, white blood cell counts, and C-reactive protein in group B were significantly lower than in group A (P < 0.05). During the preoperative period, numbers of Bifidobacterium colonies cultured from and total organic acid concentrations measured in feces increased significantly in group B (P < 0.05). Postoperative concentrations of total organic acids and acetic acid in feces were significantly higher in group B than in group A (P < 0.05). Incidence of postoperative infectious complications was 30.0% (12 of 40) in group A and 12.1% (5 of 41) in group B (P < 0.05).
Conclusions:
Preoperative oral administration of synbiotics can enhance immune responses, attenuate systemic postoperative inflammatory responses, and improve intestinal microbial environment. These beneficial effects likely reduce postoperative infectious complications after hepatobiliary resection for biliary tract cancer.
Effects of perioperative synbiotic administration were investigated in a randomized controlled trial involving patients undergoing hepatobiliary resection for biliary cancer. Synbiotic use was associated with enhanced immune responses, attenuated systemic inflammatory responses, an improved intestinal microbial environment, and reduced postoperative infectious complications.
Much of the immune system is situated in the gastrointestinal tract.1–4 Disruption of gut barrier function and intestinal microbial imbalance can result in systemic inflammation and ultimately induce septic complications after surgery.5–7 Despite improvements in surgical techniques and refinements in perioperative care, the high incidence of postoperative infectious complications in patients undergoing hepatobiliary resection for biliary cancer remains a major problem.8–12 Enteral feeding is one effective treatment among several strategies aiming to minimize postoperative septic complications concerning bacterial translocation.13–15 A recently proposed refinement of enteral feeding suggests dietary supplements including probiotics and prebiotics as having have a potential to prevent bacterial translocation.16–19
Probiotics, viable bacteria that benefit the host by improving intestinal microbial balance, now are used widely in food supplements.20 Prebiotics are nondigestable food constituents that selectively alter growth or activity of one or a limited number of bacterial species in the colon in a manner that potentially improves the health of the host.20–22 Used in combination, probiotics and prebiotics are called synbiotics.22 While synbiotic combinations are considered to have beneficial effects on human health, their clinical value in surgical patients remains unclear given a paucity of applicable clinical studies.23–25 We recently assessed clinical usefulness of synbiotics in biliary cancer patients who underwent hepatobiliary resection, finding that postoperative synbiotic administration reduced infectious complications after hepatectomy, mainly by correcting an intestinal microbial imbalance.26 Based on this observation, we hypothesized that additional preoperative administration of synbiotics should reduce infectious complications still more effectively.
The present prospective randomized study was designed to investigate the effect of preand postoperative administration of synbiotics on intestinal permeability, integrity, immune responses, systemic inflammatory responses, and microflora, as well as surgical outcome, in a clinical setting.
MATERIALS AND METHODS
This study involved 101 patients with biliary cancer (perihilar cholangiocarcinoma or gallbladder cancer involving the hepatic hilus) who were scheduled to undergo combined liver and extrahepatic bile duct resection with hepaticojejunostomy at the Nagoya University Hospital between May 2003 and April 2005. Patients scheduled to undergo hepatopancreatoduodenectomy were not included. The 101 subjects were randomized before surgery to either group A, receiving postoperative enteral feeding with synbiotics, or group B, receiving synbiotic treatment preoperatively as well as postoperatively. Written informed consent for participation was obtained from each patient before enrollment in the study, which was approved by the Human Research Review Committee of the Nagoya University Hospital.
All patients received a regular diet preoperatively; no patient received parenteral or enteral nutritional supplementation. As preoperative synbiotic treatment, the following agents were administered orally: one 80 mL bottle of Yakult 400 (Yakult Honsha, Tokyo, Japan) containing at least 4 × 1010 living Lactobacillus casei strain Shirota; one 100-mL bottle of Bifiel (Yakult Honsha) containing at least 1 × 1010 living Bifidobacterium breve strain Yakult; and galactooligosaccharides (Oligomate 55, Yakult Honsha; 15 g/day). Group B patients underwent this synbiotic treatment of 2 weeks before hepatectomy, while group A patients did not. In patients with obstructive jaundice treated with percutaneous transhepatic biliary drainage (PTBD), all bile drained externally from the biliary drainage catheter was replaced orally or via a nasoduodenal tube, aiming to maintain intestinal integrity.27 All patients underwent intestinal preparation with an iso-osmotic solution (2 L) given the day before operation, and received antibiotic prophylaxis as a single intravenous drip infusion 30 minutes before surgery.
An 8-Fr catheter was placed through a jejunal limb during surgery for enteral feeding in both groups. This enteral feeding (Impact, 1 kcal/mL; Novartis Consumer Health, Bern, Switzerland) was initiated on postoperative day 1 at 100 kcal/day and was increased gradually to 400 kcal/day by day 5. Oral intake was begun on day 4 or 5, with enteral feeding decreasing gradually as oral intake increased. Parenteral nutrition also was supplied via a central venous catheter placed in the operating room immediately before surgery. This central venous catheter was removed 7 to 10 days postoperatively. Synbiotics used after hepatectomy were Yakult BL Seichōyaku (Yakult Honsha) containing 1 × 108 living Lactobacillus casei strain Shirota, and 1 × 108 living Bifidobacterium breve strain Yakult, per gram; as well as galactooligosaccharides (Oligomate 55, Yakult Honsha). Yakult BL Seich???yaku (3 g /day) and Oligomate 55 (15 g/day) were administered through the feeding catheter from postoperative day 1 to day 14. Patients in both groups underwent this postoperative synbiotic treatment.
A lactulose-mannitol test was performed 1 day before the trial, 1 day before hepatectomy, and 2, 7, and 21 days after hepatectomy. Blood was sampled before and after surgery for standard laboratory tests and measurements of serum diamine oxidase (DAO) activity, natural killer (NK) cell activity, and interleukin-6 (IL-6) concentration. Feces were sampled 1 day before the trial, 1 day before hepatectomy, and 7 and 21 days after hepatectomy for bacteriologic examination.
Lactulose-Mannitol Test
Intestinal permeability was assessed by the lactulose-mannitol test. The test solution consisted of 10 g of lactulose (Sigma-Aldrich, Tokyo, Japan) and 5 g of mannitol (Sigma-Aldrich), which were mixed in 60 mL of physiologic saline. After an overnight fast, subjects voided fully and the test solution was administered orally. In the next 6 hours, the subjects were at rest and were allowed no food or water. All urine was collected for 6 hours. A 10-mL sample was taken from this pooled urine collection and frozen at −20°C until analysis. Urinary lactulose and mannitol concentrations were measured by gas-liquid chromatography.28 Finally, the urinary lactulose-mannitol ratio (L/M ratio) was calculated.
DAO Activity
Blood samples were centrifuged at 1000g for 10 minutes at 4°C, and sera were stored at −80°C until measurement. Serum DAO activity was determined using Takagi's method,29 a simple, sensitive colorimetric assay based on a coupled reaction involving peroxidase and a novel chromogen, 10-(carboxymethyl-aminocarbonyl)-3, 7-bis (dimethylamino) phenothiazine sodium (DA-67; Wako Pure Chemical Industries, Osaka, Japan). Briefly, 1.5 mL of cadaverine solution [525.3 mg of cadaverine hydrochloride dissolved in 100 mL of piperadine-N, N′-bis (2-ethanesulfonic acid) buffer (25 mmol/L, pH 7.2, containing 0.5% Triton X-100)] and 0.1 mL of the serum sample were incubated at 37°C for 30 minutes. Then 1.5 mL of a solution containing DA-67 (40.8 mg/mL), ascorbate oxidase (from cucumber; 5 units/mL), and peroxidase (Type X from horseradish; 6 purpurogallin units/mL) was added, followed by incubation for another 60 minutes. Light absorption was measured by spectrophotometry (UVIDEC-40; Japan Spectroscopic, Tokyo, Japan) at a wavelength of 668 nm.
NK Cell Activity and IL-6 Concentration
NK cell activity of peripheral blood mononuclear cells was measured by a europium (Eu+) release assay as described previously.30 Serum IL-6 concentrations were measured by a two-step sandwich enzyme-linked immunosorbent assay (ELISA) method using kit (R&D Systems, Minneapolis, MN), as described previously.31
Fecal Bacteriologic Examination
Feces were collected in a test tube maintained anaerobically in an atmosphere of 7% H2 and 5% CO2 in N2. The test tube was refrigerated until culture. VL-G roll tube agar32 supplemented with 0.2% cellobiose and 0.2% maltose (modified VL-G roll tube agar) was used to determine total anaerobe counts. Various media were used for selective isolation of different microorganisms: modified VL-G roll tube agar to which 80 ìg/mL vancomycin and 1 ìg/mL kanamycin were added for Bacteroidaceae; CW agar (Nikken Bio Medical Laboratory, Kyoto, Japan) for lecitinase-positive Clostridium; MPN agar33 for Bifidobacterium; COBA agar34 for Enterococcus; LBS agar (Becton Dickinson and Company, Cockeysville, MD) supplemented with 0.8% Laboratory Lemco powder (Oxoid, Basingtoke, UK) for Lactobacillus; Staphylococcus medium no.110 agar (Nissui Pharmaceutical, Tokyo, Japan) for Staphylococcus and Bacillus; DHL agar (Nissui Pharmaceutical) for Enterobacteriaceae; NAC agar (Nissui Pharmaceutical) for Pseudomonas; and GS agar (Nissui Pharmaceutical) for Candida. TOS agar35 supplemented with 6.25 mg/mL streptomycin sulfate (Sigma Chemical, St. Louis, MO) and 1 ìg/mL carbenicillin disodium salt (Sigma, T-CBPC agar) was used for quantitation of Bifidobacterium breve strain Yakult. LLV agar36 was used for quantitation of Lactobacillus casei strain Shirota. CW agar, LBS agar, and T-CBPC agar media were cultured anaerobically at 37°C for 72 hours. After incubation, colonies on plates were counted and Gram stained. Numbers of viable bacteria per gram of feces (wet weight) were calculated. Bifidobacterium breve strain Yakult and Lactobacillus casei strain Shirota were identified by ELISA using strain-specific monoclonal antibodies.36 All bacterial counts [colony-forming units (CFU)/g of wet feces] were transformed to logarithms (log10CFU) for ease of statistical analysis. The lower limit of bacterial detection with this procedure was 1000 CFU per gram of feces for the obligate anaerobes, Bacteroidaceae and Bifidobacterium, and 100 CFU per gram of feces for other bacteria.
Determination of Fecal Organic Acid Concentrations
Feces were homogenized in 1 mL of distilled water. The homogenate was placed in an Eppendorf tube and centrifuged at 10,000 rpm at 4°C for 10 minutes. A mixture of 0.9 mL of the resulting supernatant and 0.1 mL of 1.5 mol/L perchloric acid were mixed well in a glass tube and allowed to stand at 4°C for 12 hours. The suspension then was passed through a filter with a pore size of 0.45 μm (Millipore Japan, Tokyo). The sample was analyzed for organic acids by high-performance liquid chromatography as previously described,37 using a Waters system (432 Conductivity Detector; Waters, Milford, MA) equipped with 2 columns (Shodex Rspack KC-811; Showa Denko, Tokyo). Concentrations of organic acids were calculated using external standards, and were expressed as micromoles per gram of wet feces.
Recording of Infectious Complications
Detailed daily records of patients’ postoperative courses were kept, and infectious complications were recorded for up to 30 days after surgery. Wound infection was defined as spontaneous or surgically released purulent discharge with positive cultures. Intra-abdominal abscess was defined as purulent discharge with positive cultures from abdominal drains placed at surgery, or as fluid collection requiring a drainage procedure. Pneumonia was defined as a characteristic pulmonary infiltrate on a chest radiograph accompanied by leukocytosis.
Blood was obtained for cultures if a patient developed a fever exceeding 38.5°C at any time after hepatectomy, irrespective of the presence or absence of other infectious sources. For each set of blood cultures, 10 mL of blood was drawn under sterile conditions and then immediately inoculated into separate culture bottles (Organon Teknika, Durham, NC) for aerobic and anaerobic cultures. Blood was incubated until bacterial growth was detected, or for 7 days. Bacteremia was diagnosed when a single blood culture grew an isolate of organisms unless the isolate was Staphylococcus epidermidis or coagulase-negative Staphylococcus species. In these instances, diagnosis required isolation from 2 or more blood cultures.38
Statistics
Results are expressed as the mean ± SD. Statistical analysis was performed using the Fisher exact test probability test and paired and unpaired Student t tests, as appropriate. A value of P below 0.05 was considered statistically significant.
RESULTS
Demographics of Patients Studied
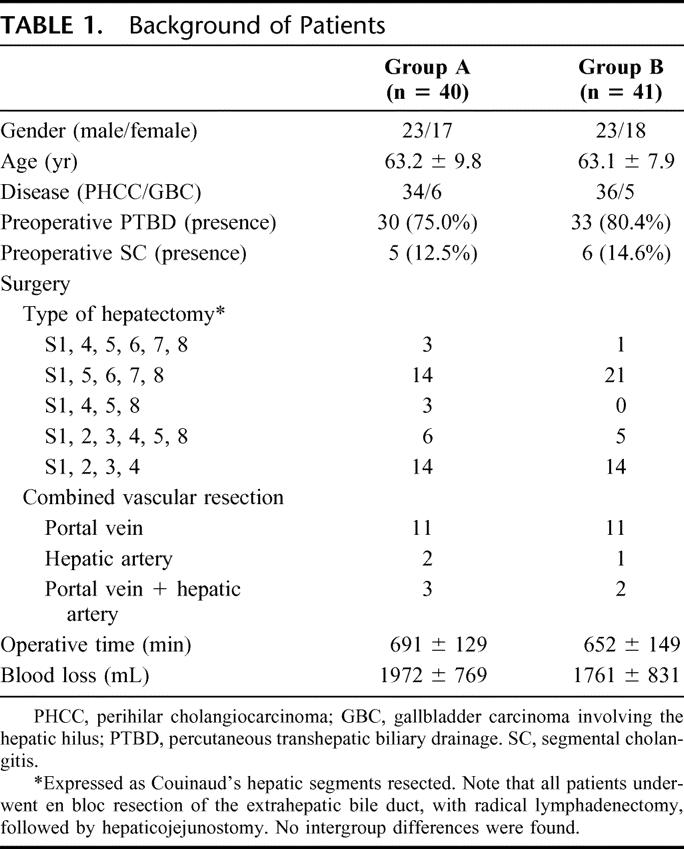
Of the 101 patients, 20 were excluded from analysis because they ultimately underwent laparotomy without resection because of intraoperative findings of peritoneal dissemination, liver metastasis, and/or locally advanced unresectable tumor. The remaining 81 patients who underwent curative resection completed the trial; 40 had been assigned to group A (postoperative synbiotic treatment only), and 41 had been assigned to group B (both preoperative and postoperative synbiotic treatment). No patient had problems related to synbiotic treatment. In the preoperative treatment period, there were no intergroup differences as concerning as the presence of preoperative infectious complications such as segmental cholangitis.39 All patients underwent hemihepatectomy or more extensive resection with en bloc resection of the caudate lobe, the extrahepatic bile duct, and with radical lymphadenectomy. Baseline characteristics were well matched between the 2 groups (Table 1).
TABLE 1. Background of Patients
Changes in L/M Ratio and Serum DAO Activity
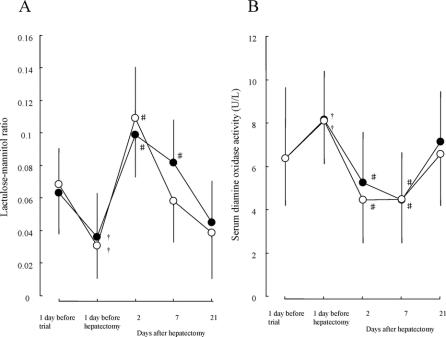
In both groups, L/M ratios decreased significantly (P < 0.05) during the preoperative period, reflecting bile replacement.27 After hepatectomy, ratios increased significantly (P < 0.05) on postoperative day 2 and then gradually decreased, returning to the value 1 day before hepatectomy by day 21. Perioperative changes in L/M ratio showed no intergroup differences (Fig. 1A).
FIGURE 1. Lactulose-mannitol ratio (A) and serum diamine oxidase activity (B) before and after hepatectomy. •Patients with postoperative synbiotics (group A). ○Patients with preand postoperative synbiotics (group B). †P < 0.05 versus 1 day before trial. #P < 0.05 versus 1 day before hepatectomy. Results are mean ± SD.
In both groups, serum DAO activities increased significantly (P < 0.05) during the preoperative period, reflecting bile replacement.27 After hepatectomy, activities decreased significantly (P < 0.05) on postoperative day 2 and remained low on day 7, then returning to the value 1 day before hepatectomy by day 21. Perioperative changes in serum DAO activity showed no intergroup differences (Fig. 1B).
Changes in NK Cell Activity and Lymphocyte Counts
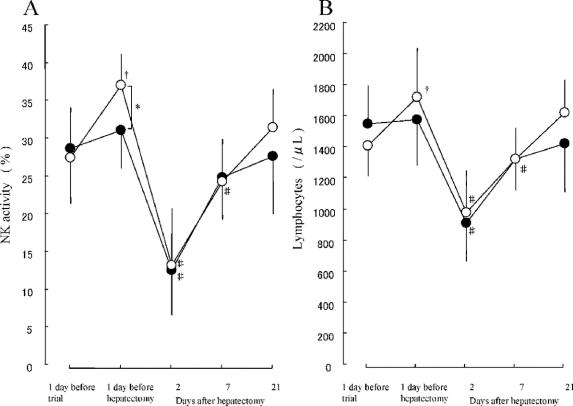
NK cell activity in group B patients increased significantly (P < 0.05) after preoperative synbiotic treatment; in contrast, NK activity in group A patients was unchanged during the preoperative period. NK activity 1 day before hepatectomy was higher in group B than in group A (37.1% vs. 31.2%, P < 0.05). Postoperative changes in NK cell activity were similar between the 2 groups; activities decreased significantly on day 2 and then gradually increased, returning to the preoperative value by day 21 (Fig. 2A).
FIGURE 2. Natural killer cell activity (A) and lymphocyte counts (B) before and after hepatectomy. •Patients with postoperative synbiotics (group A). ○Patients with preand postoperative synbiotics (group B). *P < 0.05 between the 2 groups. †P < 0.05 versus 1 day before trial. #P < 0.05 versus 1 day before hepatectomy. Results are mean ± SD.
Lymphocyte counts in group B patients increased significantly (P < 0.05) from 1404 ± 226/ìL before preoperative synbiotic treatment to 1721 ± 285/μL after treatment, while lymphocyte counts in group A patients were unchanged during the preoperative period. Postoperative changes in lymphocyte counts were similar between the 2 groups with a significant decrease (P < 0.05) on day 2 and then a gradual increase, returning to the preoperative value by day 21 (Fig. 2B).
Changes in Serum IL-6, White Blood Cell Counts, and C-Reactive Protein
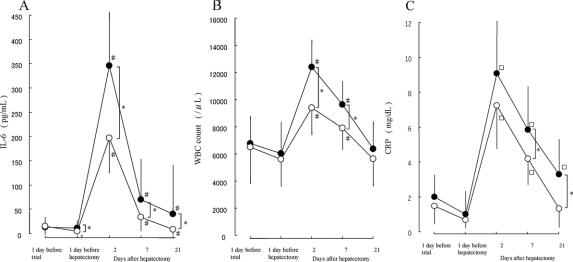
Serum IL-6 concentrations in group B patients decreased significantly (P < 0.05) after preoperative synbiotic treatment, while IL-6 in group A patients was unchanged during the preoperative period. IL-6 1 day before hepatectomy was lower in group B than in group A (5.1 vs. 11.0 pg/dL, P < 0.05). Postoperatively, serum IL-6 concentrations increased significantly (P < 0.05) on day 2 and then gradually decreased in both groups. However, IL-6 was significantly (P < 0.05) lower in group B than in group A at each time point (Fig. 3A). Similarly, white blood cell counts and serum C-reactive protein (CRP) concentrations were lower in group B than in group A after hepatectomy (Fig. 3B, C).
FIGURE 3. Serum IL-6 (A), white blood cell counts (B), and C-reactive protein (C) before and after hepatectomy. •Patients with postoperative synbiotics (group A). ○Patients with preand postoperative synbiotics (group B). *P < 0.05 between the 2 groups. †P < 0.05 versus 1 day before trial. #P < 0.05 versus 1 day before hepatectomy. Results are mean ± SD.
Preoperative and postoperative values for hemoglobin, serum total protein, and serum total bilirubin were distributed essentially equally between the 2 groups (data not shown).
Changes in Fecal Microflora
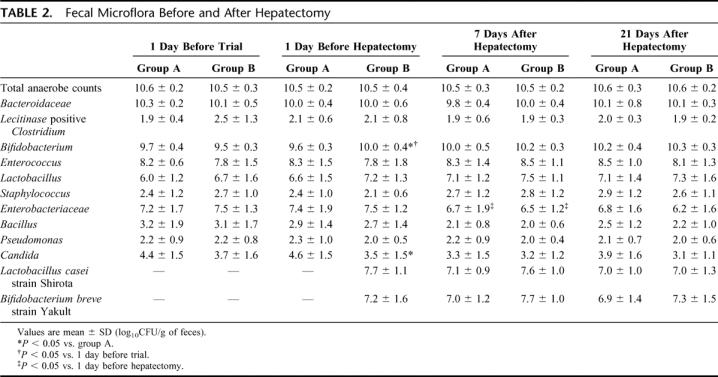
Total numbers of anaerobic bacteria and numbers of Bacteroidaceae, the dominant anaerobic species, were unchanged before and after surgery in the 2 groups. Numbers of Bifidobacterium increased significantly in group B patients (P < 0.05) after preoperative synbiotic treatment; although numbers of Lactobacillus also increased, the change was not statistically significant. Numbers of Bifidobacterium 1 day before hepatectomy were higher in group B than group A, while numbers of Candida 1 day before hepatectomy were lower in group B than group A (P < 0.05). Postoperative changes in fecal microflora were similar between the 2 groups, without intergroup differences. Lactobacillus casei strain Shirota and Bifidobacterium breve strain Yakult, which were administered as probiotics, were confirmed to be isolated from feces, indicating that these 2 beneficial bacteria had colonized the intestine (Table 2).
TABLE 2. Fecal Microflora Before and After Hepatectomy
Changes in Fecal Organic Acid Concentrations
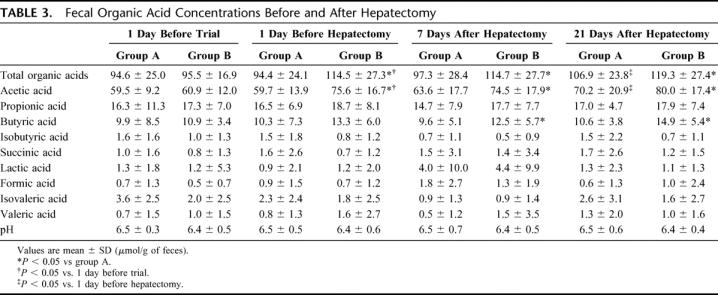
Total organic acid and acetic acid concentrations in group B patients increased significantly (P < 0.05) after preoperative synbiotic treatment, while those in group A patients were unchanged during the preoperative period. Concentrations of these acids 1 day before hepatectomy were significantly (P < 0.05) higher in group B than in group A. After hepatectomy, total organic acid and acetic acid concentrations in group A patients increased gradually but remained significantly (P < 0.05) lower than those in group B patients (Table 3).
TABLE 3. Fecal Organic Acid Concentrations Before and After Hepatectomy
Surgical Outcome
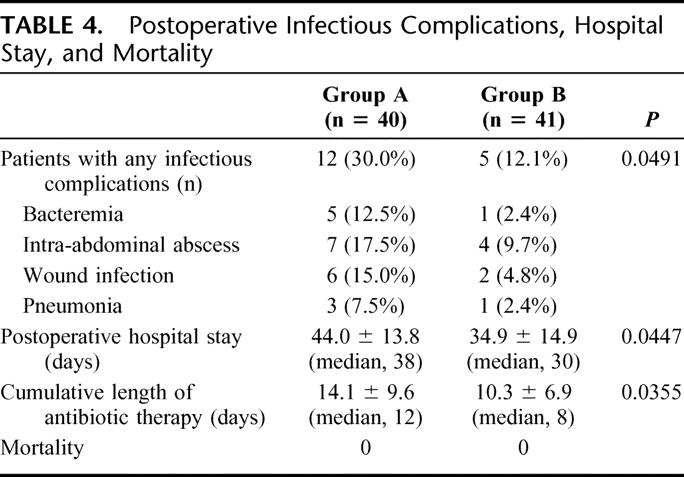
Infectious complications of various sorts occurred after surgery, including bacteremia, intra-abdominal abscess, wound infection, and pneumonia. Urinary tract infection did not develop in any patient. Each of the complications occurring was less frequent in group B. Of the 40 group A patients, 12 (30.0%) had postoperative infectious complications, while only 5 (12.1%) of the 41 group B patients had infectious complications (P < 0.05). Consequently, postoperative hospital stay and cumulative duration of antibiotic therapy were significantly (P < 0.05) shorter in group B than in group A. All patients tolerated surgery and were discharged from the hospital in good condition (Table 4).
TABLE 4. Postoperative Infectious Complications, Hospital Stay, and Mortality
DISCUSSION
Increased intestinal permeability, intestinal microbial imbalance, and host immunodeficiency are important triggers for bacterial translocation.40 Synbiotics can improve the intestinal microbial environment1,26 and activate host immune function,41–43 leading to prevention of bacterial translocation. This beneficial effect of synbiotics may reduce infectious complications after surgery, especially high-risk hepatectomy or liver transplantation.25,26 In this context, we previously reported that postoperative use of synbiotics reduced infectious complications after hepatectomy, mainly by correcting intestinal microbial imbalances.26 In the present study, we demonstrated that additional preoperative use of synbiotics enhanced the beneficial effect offered by postoperative use.
We determined the L/M ratio to evaluate intestinal permeability and serum DAO activity to assess intestinal integrity.6,44 Previously, we demonstrated that patients with obstructive jaundice who underwent external biliary drainage had decreased L/M ratios and increased DAO activity as a result of bile replacement therapy,27 concluding that bile replacement during external biliary drainage can restore intestinal barrier function, primarily by promoting repair of physical damage to the intestinal mucosa. In the present study, the L/M ratio decreased and serum DAO activity increased during the preoperative period in association with bile replacement therapy, irrespective of use of synbiotics. On the other hand, the L/M ratio increased and DAO activity decreased after hepatectomy despite use of synbiotics. These findings indicate that jaundice-related physical damage to the intestinal mucosa can be reversed with bile replacement rather than preoperative use of synbiotics, while surgery-related physical damage is not preventable, even by perioperative use of synbiotics. McNaught et al also studied effect of probiotics on gut barrier function in critically ill patients, finding that probiotics has no identifiable effect on intestinal permeability.45
An important finding arising from the present study is that preoperative synbiotics enhanced NK cell activity during treatment. Sheih et al42 studied the effect of probiotic lactic acid bacteria on natural immunity in healthy volunteers, noting an increase in phagocytic capacity of peripheral blood polymorphonuclear leukocytes and tumoricidal activity of NK cells. Nagao et al41 also reported that enhancement of NK cell activity by oral intake of fermented milk containing Lactobacillus. NK cells, which act as cytolytic effector cells, plays a pivotal role in the innate immune system.43 Lactobacillus has been found to induce IL-12 production by macrophages; in turn, IL-12 stimulates T cells to secrete interferon-γ (IFN-γ),46–48 while both IL-12 and IFN-γ augment NK cell activity.41 Thus, probiotics can enhance systemic cellular immune responses and may be useful as a dietary supplement to promote natural immunity, although the mechanism of macrophage activation remains to be identified.
In the present study, extent of systemic inflammation was evaluated using serum IL-6 and CRP concentrations as well as WBC counts. IL-6 is a good marker of the activated cytokine cascade that can predict infectious conditions and organ dysfunction.49 CRP is an acute phase reactant that increases in response to cytokine stimulation, serving as a marker of magnitude of inflammation.50 During preoperative treatment with synbiotics, IL-6 concentrations were significantly decreased. As most subjects had obstructive jaundice and underwent biliary drainage, some of the jaundiced patients presumably had cholangitis preoperatively. Administration of synbiotics may have reduced inflammation related to cholangitis. After hepatectomy, IL-6 and CRP concentrations and WBC counts were significantly lower in group B than in group A. Preoperative administration of synbiotics enhance host immune function, as suggested above, presumably aiding in resistance to infection, attenuation of postoperative inflammatory responses, and reduction of postoperative infectious complications, decreasing hospital stay and duration of antibiotic therapy.51 Our results suggest that systemic inflammatory responses can be favorably modified by synbiotics.
Intestinal microflora importantly influence the host immune system and ideally provide a natural defense against invading pathogens.52 Microflora include beneficial, opportunistic, and harmful bacteria. Beneficial intestinal flora protect the intestinal tract from proliferation of harmful bacteria, while harmful bacteria manifest pathogenicity when host resistance is decreased.53 Previously, we reported that after hepatectomy beneficial bacteria including Lactobacillus and Bifidobacterium decrease while harmful microorganisms including Enterobacteriaceae, Pseudomonas, and Candida increase, and that such microbial imbalance is improved by postoperative use of synbiotics.26 In the present study, beneficial bacteria increased and harmful bacteria decreased with preoperative administration of synbiotics for 2 weeks. After hepatectomy, balance of beneficial and harmful bacteria was well maintained in both groups without significant intergroup differences, most likely because both groups received postoperative synbiotic treatment. Considering our previous and present studies, microbial imbalance induced by surgical stress may be improved rapidly by postoperative synbiotic treatment irrespective of preoperative treatment. In any case, increasing beneficial bacteria and decreasing harmful bacteria are important for maintaining host defenses, especially during recovery from major surgery.
Beneficial bacteria such as Lactobacillus and Bifidobacterium ferment carbohydrate to produce organic acids. Among the latter, acetic, propionic, and butyric acids are considered short chain fatty acids (SCFAs). SCFAs play various important roles in the colon, including maintenance of intestinal environment acidity, stimulation of epithelial proliferation,54 stimulation of intestinal motility,55 and enhancement of epithelial mucin secretion,56 as well as serving as metabolites for energy metabolism in epithelial cells. Colonic SCFAs, thus, may have beneficial effects on epithelial cell integrity and participate in local defenses of the colon. In the present study, fecal organic acids and acetic acid concentrations remained higher in group B than in group A (P < 0.05) during the preoperative and postoperative periods. Evidently, this resulted from an increase in beneficial bacteria from preoperative synbiotic treatment. These observations suggest that preoperative synbiotic treatment contributes to maintaining a favorable intestinal environment before and after hepatectomy.
CONCLUSION
Consecutive preoperative and postoperative synbiotic treatment can reduce infectious complications more effectively than postoperative synbiotic treatment alone. This “booster effect” probably involves preoperative enhancement of immune function by synbiotics. Administration of synbiotics as a food supplement is safe, simple, and convenient. We recommend preoperative oral intake of synbiotics combined with postoperative treatment in patients scheduled to undergo high-risk gastrointestinal surgery such as hepatobiliary resection.
Footnotes
Reprints: Yuji Nimura, MD, PhD, Department of Surgery, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan. E-mail: ynimura@med.nagoya-u.ac.jp.
REFERENCES
- 1.Isolauri E, Sutas Y, Kankaanpaa P, et al. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73(suppl):444–450. [DOI] [PubMed] [Google Scholar]
- 2.Anderson AS. Lifestyle interventions: how joined up are we? J Hum Nutr Diet. 2002;15:241–242. [DOI] [PubMed] [Google Scholar]
- 3.Yasui H, Kiyoshima J, Hori T, et al. Protection against influenza virus infection of mice fed Bifidobacterium breve YIT4064. Clin Diagn Lab Immunol. 1999;6:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa H, Saito H, Suzuki K. New gut associated lymphoid tissue: cryptopatches breed murine intestinal intraepitherial T cell precursors. Immunol. Res. 1999;20:243–250. [DOI] [PubMed] [Google Scholar]
- 5.Parks RW, Clements WD, Pope C, et al. Bacterial translocation and gut microflora in obstructive jaundice. J Anat. 1996;189(Pt 3):561–565. [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh FK, Ramsden CW, MacLennan K, et al. Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg. 1998;227:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouma DJ, Coelho JC, Fisher JD, et al. Endotoxemia after relief of biliary obstruction by internal and external drainage in rats. Am J Surg. 1986;151:476–479. [DOI] [PubMed] [Google Scholar]
- 8.Nimura Y, Hayakawa N, Kamiya J, et al. Combined portal vein and liver resection for carcinoma of the biliary tract. Br J Surg. 1991;78:727–731. [DOI] [PubMed] [Google Scholar]
- 9.Nagino M, Nimura Y, Hayakawa N, et al. Logistic regression and discriminant analyses of hepatic failure after liver resection for carcinoma of the biliary tract. World J Surg. 1993;17:250–255. [DOI] [PubMed] [Google Scholar]
- 10.Nimura Y, Kamiya J, Nagino M, et al. Aggressive surgical treatment of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 1998;5:52–61. [DOI] [PubMed] [Google Scholar]
- 11.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818; discussion 819. [DOI] [PMC free article] [PubMed]
- 12.Nagino M, Kamiya J, Uesaka K, et al. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg. 2001;25:1277–1283. [DOI] [PubMed] [Google Scholar]
- 13.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–511; discussion 511–513. [DOI] [PMC free article] [PubMed]
- 14.Shirabe K, Matsumata T, Shimada M, et al. A comparison of parenteral hyperalimentation and early enteral feeding regarding systemic immunity after major hepatic resection: the results of a randomized prospective study. Hepatogastroenterology. 1997;44:205–209. [PubMed] [Google Scholar]
- 15.Zulfikaroglu B, Zulfikaroglu E, Ozmen MM, et al. The effect of immunonutrition on bacterial translocation, and intestinal villus atrophy in experimental obstructive jaundice. Clin Nutr. 2003;22:277–281. [DOI] [PubMed] [Google Scholar]
- 16.Anderson AD, McNaught CE, Jain PK, et al. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut. 2004;53:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu Q, Gill HS. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol. 2002;34:59–64. [DOI] [PubMed] [Google Scholar]
- 18.Eizaguirre I, Urkia NG, Asensio AB, et al. Probiotic supplementation reduces the risk of bacterial translocation in experimental short bowel syndrome. J Pediatr Surg. 2002;37:699–702. [DOI] [PubMed] [Google Scholar]
- 19.Gill HS, Shu Q, Lin H, et al. Protection against translocating Salmonella typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med Microbiol Immunol (Berl). 2001;190:97–104. [DOI] [PubMed] [Google Scholar]
- 20.Fuller R. Probiotics in human medicine. Gut. 1991;32:439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson GR, Beatty ER, Wang X, et al. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. [DOI] [PubMed] [Google Scholar]
- 22.Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69(suppl):1052–1057. [DOI] [PubMed] [Google Scholar]
- 23.McNaught CE, Woodcock NP, MacFie J, et al. A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut. 2002;51:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayes N, Hansen S, Seehofer D, et al. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition. 2002;18:609–615. [DOI] [PubMed] [Google Scholar]
- 25.Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74:123–127. [DOI] [PubMed] [Google Scholar]
- 26.Kanazawa H, Nagino M, Kamiya S, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390:104–113. [DOI] [PubMed] [Google Scholar]
- 27.Kamiya S, Nagino M, Kanazawa H, et al. The value of bile replacement during external biliary drainage: an analysis of intestinal permeability, integrity, and microflora. Ann Surg. 2004;239:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen G, Muskiet FA, Schierbeek H, et al. Capillary gas chromatographic profiling of urinary, plasma and erythrocyte sugars and polyols as their trimethylsilyl derivatives, preceded by a simple and rapid prepurification method. Clin Chim Acta. 1986;157:277–293. [DOI] [PubMed] [Google Scholar]
- 29.Takagi K, Nakao M, Ogura Y, et al. Sensitive colorimetric assay of serum diamine oxidase. Clin Chim Acta. 1994;226:67–75. [DOI] [PubMed] [Google Scholar]
- 30.Nagao F, Yabe T, Xu M, et al. Application of non-radioactive europium (Eu3+) release assay to a measurement of human natural killer activity of healthy and patient populations. Immunol Invest. 1996;25:507–518. [DOI] [PubMed] [Google Scholar]
- 31.Gaines Das RE, Poole S. The international standard for interleukin-6. Evaluation in an international collaborative study. J Immunol Methods. 1993;160:147–153. [DOI] [PubMed] [Google Scholar]
- 32.Azuma R, Suto T. Validity of transfer of the taxonomical position of Corynebacterium pseudopyogenes from genus Corynebacterium to genus Actinomyces. In: Izuka H, Hasegawa T, eds. Proceeding of the First International Conference on Culture Collection. Tokyo: University of Tokyo, 1970:493–505. [Google Scholar]
- 33.Tanaka R, Mutai M. Improved medium for selective isolation and enumeration of Bifidobacterium. Appl Environ Microbiol. 1980;40:866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petts DN. Colistin-oxolinic acid-blood agar: a new selective medium for streptococci. J Clin Microbiol. 1984;19:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonoike K, Mada M, Mutai M. Selective agar medium for counting viable cells of bifidobacteria in fermented milk. J Food Hyg Soc Jpn. 1986;27:238–244. [Google Scholar]
- 36.Kitajima H, Sumia Y, Tanaka R, et al. Early administration of Biffidobacterium Breve to preterm infants: randomized controlled trial. Arch Dis Child. 1997;76:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikuchi H, Yajima T. Correlation between water-holding capacity of different types of cellulose in vitro and gastrointestinal retention time in vivo of rats. J Sci Food Agr. 1992;60:139–146. [Google Scholar]
- 38.Shigeta H, Nagino M, Kamiya J, et al. Bacteremia after hepatectomy: an analysis of a single-center, 10-year experience with 407 patients. Langenbecks Arch Surg. 2002;387:117–124. [DOI] [PubMed] [Google Scholar]
- 39.Kanai M, Nimura Y, Kamiya J, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498–504. [DOI] [PubMed] [Google Scholar]
- 40.Deitch EA. Nutrition and the gut mucosal barrier. Curr Opin Gen Surg. 1993;85–91. [PubMed] [Google Scholar]
- 41.Nagao F, Nakayama M, Muto T, et al. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci Biotechnol Biochem. 2000;64:2706–2708. [DOI] [PubMed] [Google Scholar]
- 42.Sheih YH, Chiang BL, Wang LH, et al. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr. 2001;20:149–156. [DOI] [PubMed] [Google Scholar]
- 43.Matsuzaki T, Chin J. Modulating immune responses with probiotic bacteria. Immunol Cell Biol. 2000;78:67–73. [DOI] [PubMed] [Google Scholar]
- 44.Iwata H, Matsushita M, Nishikimi N, et al. Intestinal permeability is increased in patients with intermittent claudication. J Vasc Surg. 2000;31:1003–1007. [DOI] [PubMed] [Google Scholar]
- 45.McNaught CE, Woodcock NP, Anderson AD, et al. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211–219. [DOI] [PubMed] [Google Scholar]
- 46.Shida K, Makino K, Morishita A, et al. Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol. 1998;115:278–287. [DOI] [PubMed] [Google Scholar]
- 47.Kato I, Tanaka K, Yokokura T. Lactic acid bacterium potently induces the production of interleukin-12 and interferon-gamma by mouse splenocytes. Int J Immunopharmacol. 1999;21:121–131. [DOI] [PubMed] [Google Scholar]
- 48.Takagi A, Matsuzaki T, Sato M, et al. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis. 2001;22:599–605. [DOI] [PubMed] [Google Scholar]
- 49.Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth. 1996;77:110–117. [DOI] [PubMed] [Google Scholar]
- 50.Sheeran P, Hall GM. Cytokines in anaesthesia. Br J Anaesth. 1997;78:201–219. [DOI] [PubMed] [Google Scholar]
- 51.Duc le H, Hong HA, Barbosa TM, et al. Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol. 2004;70:2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartosch S, Woodmansey EJ, Paterson JC, et al. Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin Infect Dis. 2005;40:28–37. [DOI] [PubMed] [Google Scholar]
- 53.Mitsuoka T. Significance of dietary modulation of intestinal flora and intestinal enviroment. Biosci Microflora. 2000;19:15–25. [Google Scholar]
- 54.Sakata T. Effect of short-chain fatty acids on the proliferation of gut epithelial cells in vivo. In: Cummings J, Rombeau J, Sakata T, eds. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge: Cambridge University Press, 1995:191–208. [Google Scholar]
- 55.Cherbut C. Effects of short-chain fatty acids on gastrointestinal motility. In: Cummings J, Rombeau J, Sakata T, eds. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge: Cambridge University Press, 1995:191–208. [Google Scholar]
- 56.Willemsen LE, Koetsier MA, van Deventer SJ, et al. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]