Abstract
Objective:
To clarify whether middle segmental pancreatic resection can be performed with comparable morbidity and mortality to classic pancreatic resections for lesions in the mid-portion of the pancreas.
Summary Background Data:
Pancreaticoduodenectomies or distal pancreatectomy, traditionally used to treat lesions of the pancreatic body, sacrifice a significant amount of normal pancreatic tissue. Middle segmental pancreatic resection has therefore been introduced to minimize loss of functioning pancreatic tissue.
Patients and Methods:
In a prospective 4-year single-center study, 40 consecutive patients with lesions of the neck or the body of the pancreas underwent a middle segmental pancreatic resection. A matched-pairs analysis comparing middle segmental pancreatic resection with pp-Whipple and distal pancreatectomy was included.
Results:
Seventeen patients had neoplastic lesions (4 solid malignancies, 9 cystic lesions, 4 neuroendocrine tumors) and 23 patients had focal chronic pancreatitis. Postoperative surgical morbidity was 27.5% and mortality 2.5%. The reoperation rate was 5.0%. Three patients (7.5%) developed pancreatic fistula. Median postoperative hospital stay was 11 days (range, 6–62 days). After a median follow-up of 29 months, 97.4% (38 patients) of the patients were satisfied with the operation. The mean quality of life status (EORTC QLQ-C30) was comparable to a normal control population. Matched-pairs analysis revealed no differences of perioperative parameters (except operation time), morbidity, and mortality. However, endocrine pancreatic function was better preserved (P < 0.05) in patients with middle segmental pancreatic resection.
Conclusions:
Middle segmental pancreatic resection is an appropriate procedure for selected patients with tumorous lesions in the mid-portion of the pancreas. It preserves pancreatic parenchyma and function and has a mortality and morbidity rate comparable to other pancreatic resection procedures.
In the past, lesions of the pancreatic body were treated by pancreaticoduodenectomy or distal pancreatectomy. To preserve normal pancreatic tissue, middle segmental pancreatic resection has been introduced. In a prospective single-center study of 40 consecutive patients, this procedure was as safe as classic pancreatic resections and preserved exocrine and endocrine function.
Pancreatic pathologies localized in the pancreatic neck or body are usually removed by partial pancreatoduodenectomy or distal pancreatectomy. However, these standard pancreatic resections result in a significant loss of normal pancreatic parenchyma with subsequent impairment of exocrine and endocrine pancreatic function. Tumor enucleation is often not feasible because of the risk of injury to the main pancreatic duct. By using a middle segmental pancreatic resection, the lesion is removed with preservation of most of the pancreatic parenchyma, extrahepatic bile duct, duodenum, and spleen. In 1957, Guillemin and Bessot1 first performed a central segmental pancreatic resection with an anastomosis to both pancreatic remnants with an omega-shaped jejunal loop in a patient with chronic pancreatitis. Two years later, Letton and Wilson2 performed, in 2 cases of severe traumatic injury of the pancreatic body, a Roux-en-Y jejunal loop anastomosis to the tail of the pancreas and blind closure of the pancreatic head remnant instead of a distal pancreatectomy with splenectomy. Other cases of segmental pancreatic resection, using either single or double pancreatic anastomosis, have since been reported using the following terms: segmental pancreatectomy,3–10 medial or median pancreatectomy,11–16 central pancreatectomy,17–20 intermediate resection,21 or middle segment pancreatectomy.22 These procedures were advocated for chronic pancreatitis, islet cell hyperplasia, small benign cystic tumors (ie, serous and mucinous cystic neoplasm), and low-grade malignancies (ie, endocrine tumors, nonfunctioning islet cell tumors, noninvasive intraductal papillary mucinous neoplasm), to preserve normal pancreatic parenchyma and adjacent organs.3,11,12,17,20,22 Middle segmental pancreatic resection, as opposed to standard pancreatic resection, requires handling of 2 (distal and proximal) pancreatic remnants and is therefore reported to be associated with a higher pancreatic fistula rate with subsequent increases in morbidity and hospital stay.12 In the present study, we report the outcomes from the largest single-center cohort for middle segmental pancreatic resection (SegRes), achieved within a circumscribed time period of 4 years, and discuss implications for operative technique and patient selection.
PATIENTS AND METHODS
Patient Characteristics
Data for patients undergoing middle segmental pancreatic resection (SegRes) between October 2001 and August 2005 were prospectively entered into a standardized electronic database and subsequently analyzed. The indication for surgery was a symptomatic or nonsymptomatic localized lesion in the pancreatic neck or body of unknown histology.
Preoperative staging included computed tomography (CT) or magnetic resonance imaging (MRI), with most patients undergoing additional evaluation with endoscopic ultrasound. Preoperative imaging determined the potential suitability for segmental pancreatic resection in all cases. Exocrine and endocrine evaluation was performed preoperatively in all patients.
To compare in the long-term follow-up exocrine and endocrine function as well as quality of life (QoL), a matched-pairs analysis between SegRes patients and patients with pp-Whipple and distal pancreatectomy was performed. Patients were matched in regard to age, gender, and histopathology.
Surgical Procedure
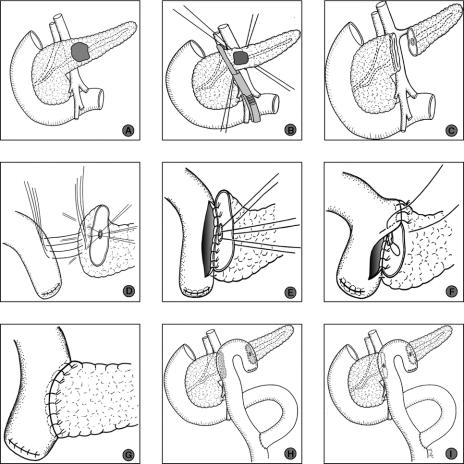
After midline or transverse upper abdominal laparotomy, the lesser sac was opened by division of the gastrocolic ligament preserving the gastroepiploic vessels. The anterior aspect of the pancreas was exposed by dividing the adhesions between the posterior surface of the stomach and the pancreas (Fig. 1A). Intraoperative ultrasonography of the pancreas was carried out to detect the tumor, exclude additional pancreatic lesions, and determine the relationship of the tumor to vascular structures and the main pancreatic duct.
FIGURE 1. Operative steps of middle segmental pancreatic resection. A, Tumor in the pancreatic body. B, Dissection of the pancreas followed by its proximal division using a Linear cutter stapling device. C, Tumor in pancreatic body resected following distal division of the pancreas with scalpel between stay sutures. D, Posterior outer layer of pancreatico-jejunostomy (PJ): end-to-side PJ of the left pancreas. E, Posterior inner layer of PJ. F, Anterior inner layer of PJ. G, Anterior outer layer of PJ. H, Completed reconstruction with seromuscular patch of jejunum covering the stapled surface of the right pancreas. I, Instead of a seromuscular patch of jejunum, the right pancreas is anastomosed in a fashion similar to the left pancreas (D–G).
After a Kocher maneuver of the duodenum, the superior mesenteric, portal, and splenic veins were dissected free from the posterior aspect of the pancreas with care taken to ligate multiple small side branches to the pancreas. The lesion, localized in the central part of the pancreas, was resected with a margin of at least 1 cm to both cut pancreatic ends. Pancreatic transection was carried out proximally with a stapler (Linear cutter, Ethicon Products, Norderstedt, Germany) (Fig. 1B), and distally with a scalpel (Fig. 1C). Arterial bleeding points in the cut edge were ligated by 5-0 monofilament sutures. The tumor and the 2 resection margins were submitted for intraoperative frozen section analysis in all cases. Subsequently, the distal stump of the pancreas was further mobilized from the splenic vein and artery, with ligation of small tributaries, for 2 cm lateral to the cut end. Reconstruction was accomplished with a 40- to 60-cm retrocolic Roux-en-Y limb of jejunum. An end-to-side pancreaticojejunostomy was constructed using a double layer of interrupted monofilament absorbable sutures (PDS 5-0, Ethicon Products) (Fig. 1D). This inner layer of this anastomosis included 3 ventral and 3 dorsal pancreatic duct to mucosa stitches (Fig. 1E–G). The stapler closed pancreatic head remnant was covered with the same jejunal loop using interrupted absorbable monofilament sutures (PDS 5-0) between the seromuscular layer of the jejunum and the capsule of the pancreas (Fig. 1H). In cases with suspicious alterations of the duct in the pancreatic head remnant or in whom stapler dissection was impossible because of the thickness of the pancreas at the dissection line, the proximal dissection was also performed by scalpel with subsequent anastomosis to the jejunal loop as performed for the distal pancreatic end (Fig. 1I). Reconstruction was completed by an end-to-side Roux-Y enteroenterostomy 20 to 25 cm distal to the ligament of Treitz (Fig. 1H, I).
In all cases, 200 μg of the somatostatin analogue octreotide (Sandostatin, Novartis Pharma GmbH, Nürnberg, Germany) was given every 8 hours subcutaneously for the next 7 days to prevent secretion related complications especially pancreatic fistula.23 One drain (Easy flow drain, Dahlhausen, Cologne, Germany) was placed close to the pancreatic anastomosis. Drains were removed routinely at the second postoperative day.
After a median follow-up of 29 months all patients were evaluated. QoL in the follow-up was assessed using the EORTC QLQ-C30 QoL questionnaire, version 3.0.24,25 A linear transformation was used to standardize the raw score, revealing a score ranging from 0 to 100. A high score for a functional scale represents a high/healthy level of functioning. A high score for the global health status/QoL represents a high QoL. In contrast, a high score in a symptom scale represents a high level of symptomatology/problems. Reference data obtained from a healthy control population revealed an average functional score of 89 and a global health status score of 71.25–27
Statistical Analysis
SAS software (Release 9.1, SAS Institute, Inc., Cary, NC) was used for statistical analysis. QoL parameters using the QLQ-C30 questionnaire as well as age, follow-up time, operation time, and blood loss are presented as mean with standard deviation and as median with range. Correlation analysis between age, follow-up time, symptoms scale, and functioning scale were performed using the Spearman correlation coefficient. The Mann-Whitney U test was used to analyze the influence of the follow-up time interval (<2 years vs. ≥2 years) on the QoL parameters. The surgical and medical complication rates were compared with respect to the diagnosis (chronic pancreatitis vs. tumor) using the Fisher's exact test. Two-sided P values were always computed, and an effect was considered statistically significant at P < 0.05.
RESULTS
Perioperative Data
Forty patients (20 female, 20 male) underwent a SegRes. Median age was 53 years (range, 10–80 years). Preoperatively, 23 patients (57.5%) reported pain, with 11 (27.5%) requiring pain medication. Nicotine abuse was noted in 14 patients (35.0%) and alcohol consumption in 23 patients (57.5%). Weight loss was recognized in 15 patients (37.5%) with a mean decrease of 6.7 ± 4.2 kg. Twelve patients (30.0%) had pancreatic enzyme replacement preoperatively. Patient characteristics are shown in Table 1.
TABLE 1. Demographics and Clinicopathologic Findings of the 40 Patients Undergoing Middle Segmental Pancreatic Resection
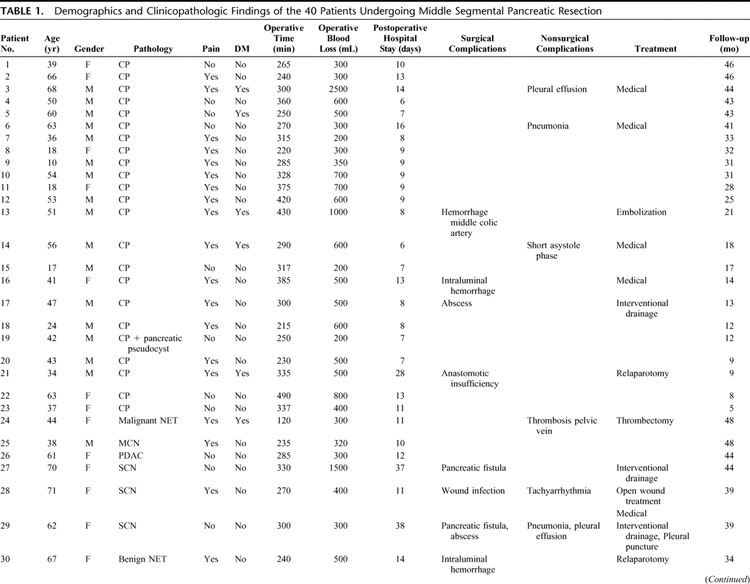
TABLE 1. (Continued)
All tumors were resected with clear surgical margins, as shown by intraoperative frozen sections and confirmed by definitive histopathological examination. Definitive histology of the resected lesions revealed a cystic tumor in 9 patients (7 serous cystadenomas, 2 mucinous cystadenomas), an endocrine tumor in 4 (3 adenomas, 1 malignant tumor), a solid malignancy in 4 (1 ductal adenocarcinoma, 1 circumscribed infiltration of a gastrointestinal stromal tumor, 2 pancreatic metastasis from renal cell carcinoma), and 23 cases of focal chronic pancreatitis (CP). In CP patients, preoperative diagnostics revealed a circumscribed pancreatic tumor in the mid-portion of the pancreas. Final histopathologic analysis revealed focal chronic pancreatitis, which was not suspected preoperatively. In the case of the ductal adenocarcinoma, the diagnosis of malignancy was proven by final pathology on the paraffin-embedded specimen.
The mean operation time ± SD was 304 ± 73 minutes (median, 295 minutes; range, 120–490 minutes). The mean intraoperative blood loss was 564 ± 433 mL (median, 500 mL; range, 200–2500 mL). Three patients (7.5%) received blood transfusions (2, 2, and 5 units). The splenic artery and vein, and spleen were preserved in all cases. In 23 patients (57.5%), a cholecystectomy was performed. The indication for cholecystectomy was based on individual decision of the surgeon or by the presence of gallbladder stones. The pancreatic head remnant was anastomosed in 11 patients and blindly closed in 29 patients. The median intensive care stay was one day (range, 0–28 days; mean ± SD, 2.1 ± 4.8 days) and the median hospital stay was 11 days (range, 6–62 days).
Surgical morbidity occurred in 27.5% (11 patients). Reoperation rate was 5% (2 patients).
Five patients (12.5%) had postoperative bleeding: 1 patient was managed conservatively, 1 patient underwent reoperation, and 2 (1 gastroduodenal artery and 1 middle colic artery) had successful radiologically guided embolization. One patient, who was discharged on the 11th postoperative day and readmitted in a regional hospital 10 days later, died of delayed hemorrhage and was the only postoperative death in the cohort (2.5%).
One patient had pancreatic anastomotic insufficiency requiring reoperation and removal of the residual left pancreas. Three (2 requiring readmission) patients developed a perianastomotic fluid collection due to a pancreatic fistula with radiologic guided external drainage and subsequent spontaneous resolution occurring in all 3 patients. A peri-pancreatic abscess occurred in 1 patient (2.5%) and 1 patient developed a wound infection.
Medical complications were observed in 10 patients (25%), 4 of whom had concomitant surgical complications. Seven of these patients had pulmonary complications, including pleural effusions in 5 (4 required drainage) and pneumonia in 3 patients. Two patients developed pulmonary insufficiency, requiring reintubation and ventilation for 2 and 5 days, respectively. There was 1 case of tachyarrhythmia due to atrial fibrillation, and 1 patient developed a short phase of asystole postoperatively.
Twenty-three patients (57.5%) had an uneventful postoperative course.
Since an anastomosis with a soft pancreas has a higher risk for complications than an anastomosis with a more fibrotic pancreas,28,29 the postoperative complication rate in patients with focal chronic pancreatitis versus patients with other histologic findings was compared. There was no difference in surgical complications in patients with focal CP compared with neoplastic lesions; 4 of 23 (17.4%) versus 7 of 17 (41.2%) (P = 0.153), as well as for pancreatic anastomotic insufficiency/pancreatic fistula; 1 of 23 (4.3%) patients versus 3 of 17 (17.6%) (P = 0.294). Furthermore, there was no difference in medical complications between the 2 groups (P = 0.140).
Postoperative Long-term Follow-up
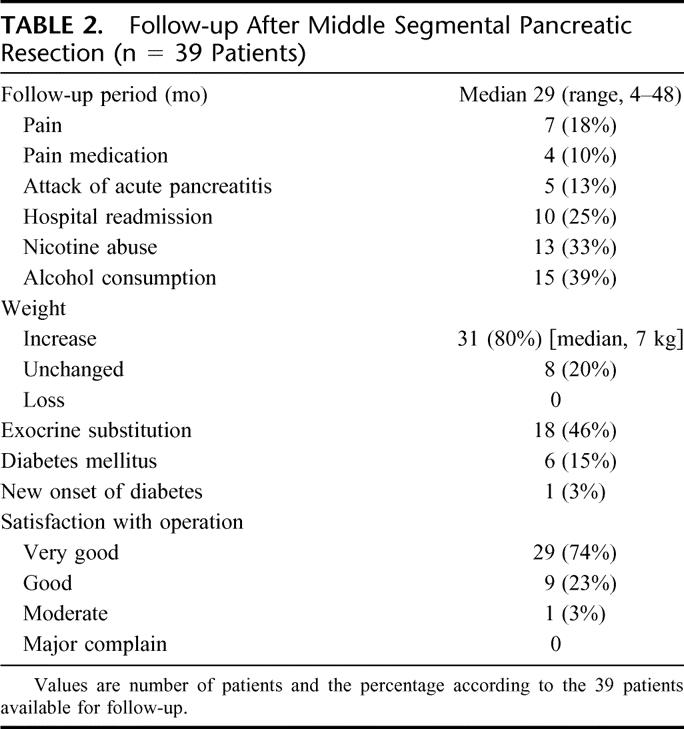
Long term follow-up was performed using a standardized protocol. The median follow-up time was 29 months (range, 4–48 moths). The findings of the postoperative follow-up are shown in Table 2. Hospital readmission occurred in 10 patients during the follow-up. One CP patient required hospital treatment because of alcohol abuse, 3 patients required incisional hernia repair, and 2 patients had an attack of acute pancreatitis. Four patients, previously mentioned under postoperative complications, were readmitted to hospital within 30 days of their operation with postoperative complications (peripancreatic fluid collection, n = 2; and bleeding, n = 2).
TABLE 2. Follow-up After Middle Segmental Pancreatic Resection (n = 39 Patients)
Ninety-seven percent of the patients were satisfied with the operation.
Mean global health status, measured by the EORTC QLQ-C30 questionnaire, was 75.2 ± 16.8 (median, 83.3; range, 16.7–100.0) and was therefore comparable to a normal adult control population.25 The results for the functional scale and the symptom scale showed also comparable results to a normal control population and are shown in Table 3.
TABLE 3. Quality of Life Evaluation According to the EORTC QLQ-30 Questionnaire in the Follow-up After Middle Segmental Pancreatic Resection (n = 39 Patients)
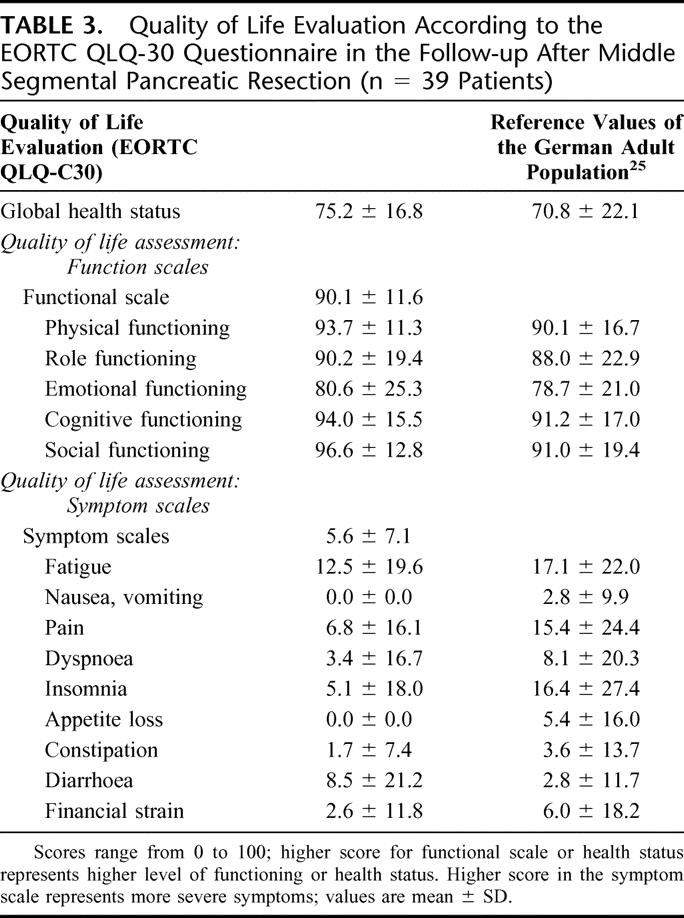
No differences regarding the QoL scales were found when comparing the group of CP patients (n = 23) with patients with neoplastic lesions. No difference was found when comparing the patients with postoperative complications (n = 16) with the group of patients who had an uneventful postoperative course (n = 23). There was a positive correlation between the global health status and the functional and symptom scale in all patients (P < 0.0001). Furthermore, there was a positive correlation between the time of follow-up and the functional scale (P = 0.019). No correlation was found for the global health and symptom scale with the time of follow-up or for age with the different QoL scales.
Comparison of the QoL scores in patients with less than 2 years postoperative follow-up (15 patients) with patients with a follow-up of more than 2 years (24 patients), revealed no statistical difference for the global health status (72.2 ± 18.8 vs. 77.1 ± 15.6) and for the symptom scale (7.9 ± 7.9 vs. 4.2 ± 6.3). Comparison of the functional scale in the group with the shorter follow-up with the group with the longer follow-up time (84.2 ± 13.9 vs. 93.7 ± 8.3) revealed a statistically significant difference (P = 0.027).
Pancreatic Function
Pancreatic endocrine function, measured by fasting blood glucose levels and HbA1 levels, was unchanged in 97.5% of the patients (38/39).
Normal endocrine function was present in 33 patients before SegRes and in 32 patients in the follow-up. One patient with an endocrine tumor and 4 CP patients had insulin-dependent diabetes mellitus preoperatively, which was unchanged postoperatively. One CP patient with diabetes mellitus before surgery had normal endocrine function postoperatively, whereas 1 patient with a ductal adenocarcinoma and normal endocrine function preoperatively developed diabetes mellitus postoperatively. Twenty-one (54%) patients were no longer taking enzyme substitution at last follow-up.
Pain
Preoperatively, 23 patients (57.5%) reported pain as opposed to 7 patients (17.9%) at follow-up. Six of these 7 patients had surgery for focal CP. One patient underwent surgery for a benign endocrine tumor. Preoperatively analgesics were used by 11 patients (27.5%), whereas only 4 (10.3%) patients required ongoing pain medication in the follow-up period. Interestingly, only 1 of these 4 patients had a follow-up period of more than 18 months.
Matched-Pairs Analysis
In the matched-pairs analysis 40 patients with distal pancreatectomy and 40 patients with pp-Whipple were included and compared with the 40 SegRes patients. The groups were well matched with regard to age, gender, and histopathology (Table 4). The 3 procedures were comparable in regard to intraoperative blood loss, length of postoperative hospital stay, and the perioperative mortality and surgical morbidity. A statistically significant difference, however, was found for the operation time with a mean operation time for the pp-Whipple, distal pancreatectomy, and SegRes of 352 ± 77, 260 ± 125, and 304 ± 73 minutes, respectively (Table 4). Patients after SegRes showed a weight increase in the follow-up in 79% compared with 53% and 68% in the pp-Whipple and distal pancreatectomy group, respectively.
TABLE 4. Matched-Pairs Analysis Comparing Patients With SegRes, Pylorus-Preserving Whipple and Distal Pancreatectomy
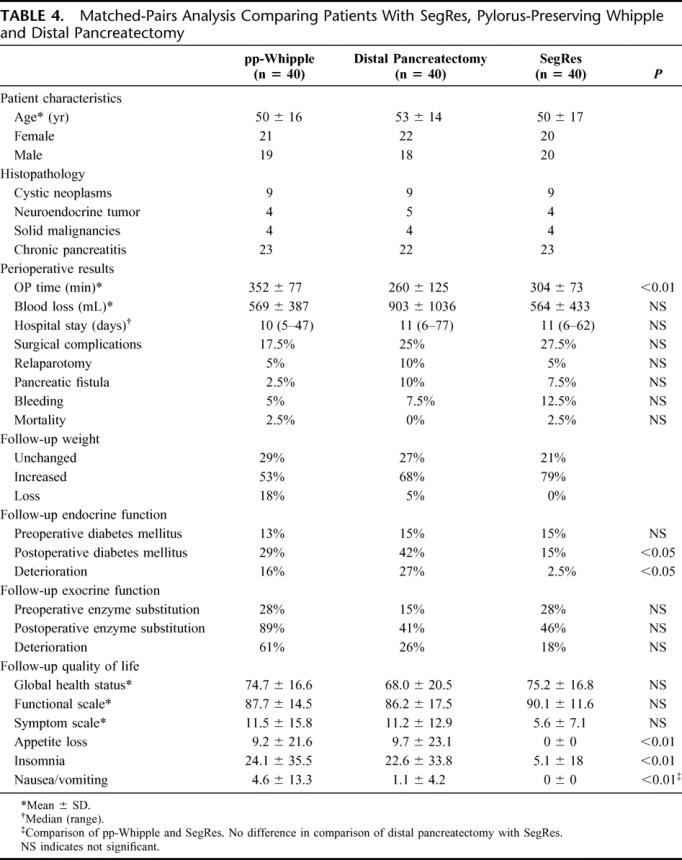
In the long-term follow-up 39 SegRes patients (1 patient died postoperative.), 38 patients with pp-Whipple (1 patient died postoperative and 1 patient in the follow-up) and 37 patients with distal pancreatectomy (3 patients died in the follow-up) were included. There was a statistically significant increase in the new onset of diabetes mellitus in the pp-Whipple group (16%) and in the distal pancreatectomy group (27%) compared with the SegRes group (0%).
Exocrine pancreatic function was deteriorated after pp-Whipple, distal pancreatectomy, and SegRes in 61%, 26%, and 18% of the patients, respectively (Table 4).
QoL analysis revealed no statistically significant difference between the 3 operation groups and a normal German control group in regard to the main parameters global health status, functional scale, and symptom scale (Table 4). However, testing of single parameters, such as appetite loss, insomnia, nausea, and vomiting, showed a significant benefit of SegRes compared with the pp-Whipple and the distal pancreatectomy group.
Tumor Recurrence
The only tumor recurrence (liver metastasis) occurred in the patient with ductal pancreatic adenocarcinoma, at 43 months.
DISCUSSION
Small circumscribed benign tumors or tumors of low malignant potential of the body or the neck of the pancreas may be locally removed. Fearing anastomotic-related problems following a more formal pancreatic resection, many surgeons perform instead a careful enucleation without compromising the main pancreatic duct. Seemingly technically more simple and potentially avoiding a pancreaticoenteric anastomosis, enucleation, however, has a high incidence of associated complications, including long-lasting pancreatic fistula (in up to 50%), pseudocysts, or acute pancreatitis.30,31 These aspects are underlined in a previous report in which 8 enucleations for serous cystadenomas resulted in 2 deaths and 4 major complications requiring reoperation.30
To avoid enucleation-related complications, formal resection of the right or the left pancreas has also been applied for small lesions of the body or neck. Although pancreaticoduodenectomy or a distal pancreatectomy can be performed in experienced centers with mortality rates between 0.5% and 3%32–38 and <1%,33,39 respectively, both procedures represent surgical overkill for benign and low-grade malignant tumors of the pancreatic neck and body. In both procedures, a significant amount of healthy pancreatic tissue is removed, leading to deterioration of exocrine and endocrine pancreatic function.40–43 In addition, loss of the duodenum alters the natural food passage leading to a disturbance of the duodenal-insulin axis, the regulated release of bile and pancreatic juice, and the mixture of oral nutrition with digestive enzymes. Furthermore, resection of the distal bile duct in patients undergoing a partial pancreaticoduodenectomy requires a bilio-digestive anastomosis, which increases the risk of ascending cholangitis and subsequently intrahepatic abscesses.28,44 Also, up to 30% of these patients develop intestinal mucosal ulceration when not receiving acid suppression.45,46
In patients with a tumor in the mid-portion of the pancreas, many surgeons prefer a distal pancreatectomy, being less demanding than a partial pancreaticoduodenectomy, which requires pancreatic, biliary (often with a small duct), and gastric anastomoses. Although splenic preservation is intended in these patients, it is technically demanding and time-consuming, and hence not often performed. Although it can be done safely, splenectomy carries not insignificant long-term risks, including postsplenectomy sepsis, reduced immune function, and even the risk of portal vein thrombosis is considerable.3,11,12,47–52
Segmental pancreatic resection was introduced for localized pancreatic tumors to avoid the extended loss of functional unaffected pancreatic parenchyma that is seen in a right- or left-sided pancreatectomy in which the pathologic lesion accounts for only a minor part of the resected specimen. Furthermore, a SegRes preserves gastroenteric and bilioenteric continuities, as well as the spleen, avoiding some of the potential morbidity of a partial pancreaticoduodenectomy, and distal pancreatectomy, respectively. On the other hand, SegRes appears to be associated with a higher incidence of pancreatic fistula compared with pancreaticoduodenectomy or distal pancreatectomy. This is not surprising since SegRes is often performed in small circumscribed pancreatic tumors in which an anastomosis has to be performed with a soft pancreas, and in which 2 pancreatic remnants have to be handled (either anastomosed or blindly closed).16 The data of our present study indicate that SegRes is a safe operation with morbidity and mortality rates comparable to pancreaticoduodenectomy or distal pancreatectomy.29,53 Anastomosis-related problems occurred in 4 of 40 patients (10%) and 1 patient died due to postoperative hemorrhage. In comparison to previous series of SegRes in which pancreatic fistula rates up to 40%7,9,11–14,16,18,22 have been reported, the fistula rate in our study was lower. This might be related to the inclusion of patients with focal CP, although the resection margins consisted of normal pancreatic parenchyma. Although there was a tendency for lower complications in CP patients compared with tumor patients, the difference was not statistically significant most likely due to the small number of patients in the 2 groups. Furthermore, complications following pancreatic resection have steadily decreased during the last 20 years; therefore, a contemporary fistula rate below 20% could reasonably be expected.29,33,36,38,39,54–56
The major advantages of SegRes are promising long-term results in terms of pancreatic function (exocrine and endocrine) and QoL. Following partial pancreaticoduodenectomy, the incidence of diabetes mellitus varies between 15% and 40%.40,57–59 This rate is even higher in distal or extended distal pancreatectomy in which diabetes mellitus rates in up to 72% have been reported.40,60 In our series of 40 patients, only 1 case of newly developed diabetes mellitus was observed and in 1 patient diabetes mellitus disappeared postoperatively. This confirms previous data showing unchanged endocrine pancreatic function following SegRes.3,4,11,12,20,22,61 The second major advantage of SegRes is preservation of exocrine pancreatic function. In previous series following partial pancreaticoduodenectomy and distal pancreatectomy, pathologic exocrine pancreatic function varied from 22% to 55% and more than 50%, respectively.40,60 In contrast, unchanged exocrine function is maintained following SegRes.11,12,22,61 Matched-pairs analysis comparing SegRes with pp-Whipple or distal pancreatectomy confirmed the superiority of this organ-preserving procedure. Exocrine and endocrine pancreatic function was statistically significant better preserved after SegRes. In our series, in addition to pancreatic function, QoL was evaluated for the first time. After a median follow-up time of 29 months, QoL status was equivalent between SegRes and a normal control population. Additional long-term follow-up is needed to draw a final conclusion whether SegRes really preserves exocrine and endocrine function as well as QoL in the long-term perspective.
Because of limited oncologic radicality, SegRes is only an adequate option in patients with benign and low-grade malignant tumors of the pancreas or with pancreatic metastases from other tumors. The lesion and the resection margins should therefore be examined by frozen section during the operation.62,63 The extent of resection in patients with benign cystic neoplasms, such as serous and mucinous cystadenoma, is dependent on the size of these lesions as only a small segment of tumor free margin is necessary to avoid recurrence.64,65 Of the 17 patients with neoplastic lesions in our series, only the patient with ductal pancreatic adenocarcinoma developed tumor recurrence at 43 months. However, in this patient intraoperative frozen section could not prove malignancy, and the final diagnosis of ductal adenocarcinoma was established on the paraffin-embedded tissue sections. The patient was not reoperated, although SegRes is not considered to be an adequate oncologic procedure in ductal adenocarcinoma. Previously, Takeyoshi et al63 reported 3 patients with cystadenocarcinoma, intraductal papillary adenocarcinoma, and a borderline cystadenoma, which were treated with SegRes and showed no tumor recurrence in a follow-up period of 33 to 77 months. Since the experience of SegRes in malignant tumors is very limited, this operation should not be considered as an adequate oncologic treatment.
CONCLUSION
Middle segmental pancreatic resection is a safe and reasonable technique appropriate for selected patients with benign tumors or lesions of low malignant potential in the neck and body of the pancreas. Although, even in high volume centers, the incidence of pancreatic fistula may still be higher compared with conventional partial pancreatectomies, the incidence of incurred exocrine and endocrine dysfunction is almost zero. SegRes should therefore be considered as an adequate operation for selected lesions in the neck or body of the pancreas.
ACKNOWLEDGMENTS
The authors thank Mrs. J. von Bergmann and Mr. D. Fischer for their graphical work and Dr. David Martin for his assistance in English correction of the manuscript text.
Discussions
Dr. Sergio Pedrazzoli: I congratulate the authors for the large number of segmental pancreatectomies, performed in only 4 years in their surgical department. I introduced this procedure to my department more than 20 years ago, but the number of resections is still only a little above 30. I have several questions for you.
This is deemed a procedure for benign or borderline malignant lesions. In the whole series, there are 5 malignant lesions and among them also a ductal adenocarcinoma. Furthermore, a malignant endocrine tumor was included in this series. How was the diagnosis of malignancy made? Do you consider that the procedure can be applied also to malignant lesions? I believe not.
You report a very low morbidity and mortality rate. However, you have shown that the patients without chronic pancreatitis have a high complication rate of 41% and a reoperation rate of 12%. Furthermore, you removed the abdominal drains on the second postoperative day, as I have seen in the paper. In my experience, the fistula usually appears more than a week after the procedure. If the drain is still in place, the fistula can be easily diagnosed, but if it is not left in place, a pseudocyst or a possibly infected fluid collection might develop. One of your patients died of delayed hemorrhage at least 21 days after surgery. Was this due to vascular erosion from an infected fluid collection? Did you verify the absence of a pseudocyst in the early follow-up period? That means during the first 2 or 3 months after surgery?
You have chosen the term “segmental pancreatectomy” for this procedure, but this is in some way misleading because you can remove different segments of pancreas, for example, part of the tail or of the head. I believe that the term “central” or “median” pancreatectomy is better.
All the 23 chronic pancreatitis patients underwent cholecystectomy. This is unusual, at least in my experience, for a central pancreatectomy. None of your nonchronic pancreatitis patients underwent cholecystectomy. How do you explain the need to remove the gallbladder in a chronic pancreatitis patient, and where is your limit on the right side of a central pancreatectomy?
Many thanks to the scientific committee for giving me the opportunity to comment on this paper.
Dr. Michael W. Müller: Thank you very much for your comments and for many important questions. I will try to answer all of them. You are right—we also performed this procedure in malignant disease. Intraoperative frozen section was performed in all patients to rule out malignancy. There is no question that this operation should primarily be done in patients with a benign lesion. However, we had 1 patient with an adenocarcinoma in our series. The patient is still alive after 43 months of follow-up. This is the only case. If you have a malignant endocrine tumor that is located in the mid-portion of the pancreas, you might think about performing a segmental resection. There are published data, which have shown good outcome, but they relate to a very, very small number of patients. Therefore, we should focus on benign indications for this procedure. You might consider this procedure for metastasis of other tumors, like renal cell carcinoma in the pancreas, as well, since there are no lymph node metastases in these cases.
Regarding the fistula rate, we removed the drain after 2 days. Pancreatic fistula was diagnosed according to the clinical status in these 3 patients, when they developed fever. Two of them had been discharged from hospital, and they were readmitted. We performed a CT scan, and in the presence of a fluid collection a CT-guided drainage was done. We obtained pancreatic enzyme-rich fluid and had to drain them for about 20 to 30 days.
The one death occurred in a patient who was discharged 10 days after operation following an absolutely uneventful postoperative course. He was readmitted 10 days later to a small hospital with intra-abdominal bleeding, and died. He was brought to the operating theater and a major hemorrhage was found. A bleeding from a splenic vessel was suspected, but it is not clear if this was an erosion, or what happened exactly.
With regard to the terminology, you are right. If you undertake a “segmental pancreatic resection,” you do not know if this is in the middle, and it might be better to name it: “central” or “middle” segmental pancreatic resection, so this is something we can discuss.
The other question was why we did a cholecystectomy. Some patients had gallstones. However, the decision was made by each single surgeon whether to perform a cholecystectomy or not. So there is no general recommendation.
Dr. James Garden: Yes, I just have 2 quick questions. One of these has been partially answered in the previous discussion. The first is regarding the radicality of the operation. Clearly, you have indicated that you would not wish to undertake this operation if you knew that there was malignant disease, and yet there is always the possibility that you may find that there is an underlying malignancy once you have resected the specimen. You have, in all patients, been able to preserve the spleen. Does that mean that you have always been able to preserve the vasculature or have there been occasions when you have had to sacrifice artery or vein and yet still been able to preserve the spleen?
The second point is about the quality of life. I am impressed that the quality of life is better after this operation than in the normal German population¡ In Scotland, if we resect pancreas in patients with chronic pancreatitis, the quality of life is certainly not good after the operation. It has usually not been good before the operation, and it is usually only better if the patient is still drinking alcohol following surgery¡ Could you just reassure us that the quality of life has been assessed before and after the surgical intervention? Thank you.
Dr. Michael W. Müller: Thank you very much for your questions and comments.
We preserved, in all of the patients, the splenic vessels. However, I think this is a problem in malignant disease. You cannot do a radical lymphadenectomy. Therefore, a Whipple or a distal pancreatectomy should be performed.
You are right—unfortunately, we don't have preoperative assessment of the quality of life (QoL) in the same patients. We have a specific questionnaire to evaluate different patients’ parameters preoperatively, but we did not have the QoL questionnaire preoperatively. However, our intention in measuring QoL was not to show that preoperative reduction of is removed by middle segmental pancreatic resection, but rather to evaluate whether after this operation QoL is as good as in a normal control population, which means that QoL is not affected by a limited resection.
Dr. Laureano Fernandez-Cruz: I enjoyed your presentation. I think, after listening to your talk, that this nonanatomic resection is questionable. Most of the time, it is associated with mortality and high morbidity. The mean hospital stay was 11 days. I think that this is higher than we could expect in patients undergoing a distal pancreatectomy or middle pancreatic resection. My question to you is that you didn't tell us the size of the tumor in your series. You have included various tumors and endocrine tumors and I think, in some of them because of the size, enucleation may be the operation of choice to avoid the morbidity that you have presented to us. What was the size of the tumors—the various tumors and the endocrine tumors—to know whether middle pancreatic resection was the operation of choice in your group of patients?
Dr. Michael W. Müller: The diameter of the tumor was a median of approximately 3 cm, and we did not perform enucleation because it was just not possible without the risk of injuring the main pancreatic duct. We normally perform an intraoperative ultrasound in all patients to assess the pancreatic duct and the vessels. Furthermore, enucleation is associated with considerable morbidity and fistula rates, which has been shown in the literature.
Dr. Helmut Friess: Laureano, you are completely right. In the case of a very small benign lesion, of course, we perform an enucleation. However, the patients in our series had larger tumors and enucleation was not possible.
Regarding the discussion on malignant tumors, it is sometimes difficult for the pathologist to judge intraoperatively whether a tumor is malignant or benign. In our case, intraoperative frozen section revealed a benign lesion. Postoperative histopathological evaluation on paraffin sections revealed adenocarcinoma. Clearly, if you have a malignant tumor in the pancreas, such as an adenocarcinoma, you should not do this operation because it does not fulfill the criteria of an adequate oncological operation.
Dr. John Howard: I congratulate you on a lovely series. I rise to make one point. This is technically a difficult procedure and one problem can be the lack of exposure in identifying very small ducts. You can identify the distal duct by administering secretin when you cannot find it easily. I have had the experience of difficulty in identifying the duct on the ampullary side of the transection. Intraoperative ERCP by an associate provided a very easy solution. Thank you.
Dr. Michael W. Müller: Thank you very much for your comments.
Dr. Christian Partensky: Thank you very much for your nice presentation. I am also convinced it is a good operation. My personal experience is of 56 patients, 53 for neoplastic lesions. My questions are:
First: don't you think we have to give a definitive name to this operation? “Segmental,” “medial,” “central,” and “middle.” “Middle” may be the best name.
The second question is: what is your extension of the resection to the right and to the left? Is the gastroduodenal artery or the portal vein the limit to the right and what about the left? Do you sometimes hesitate to do a distal resection or a middle pancreatectomy? When do you decide to keep the tail of the pancreas? Is 6 cm the limit or is it more: 7 to 8 cm?
Third question: why do you use a Roux-en-Y anastomosis when it is easier to do a pancreatogastrostomy because the operation remains at the upper abdomen?
Regarding the indications, I agree with Dr. Friess. Sometimes you presume it is a benign lesion, but at the end it is malignant. I think there are some indications for slow-growing malignancies. We had a patient with a malignant VIPoma. She was operated on in 1993, with multiple liver metastases. The middle pancreatectomy was combined with a liver cytoreduction and, 8 years later, in 2001, she received an orthotopic liver transplant. She is perfectly well now.
Finally, we used to perform routine postoperative MRCP, and we observed that the pancreatic duct of the distal remnant increases in size progressively with atrophy of the pancreatic parenchyma. Do you have this experience?
Dr. Michael W. Müller: I believe that the name is under debate and that “middle segmental pancreatic resection” might be more appropriate. We did not measure the length of the pancreatic tail, but often the left-sided pancreatic remnant is long enough to preserve it. If you have a tumor in the mid-portion of the pancreas, you really should consider this operation.
We perform routinely a pancreaticojejunostomy. We do not perform a pancreaticogastrostomy. The outcome of the pancreaticojejunostomy is satisfactory, and this is the reason we do not perform a pancreaticogastrostomy.
No, we haven't done any follow-up to look if there is an increase in size of the pancreatic duct.
Dr. Pierre Clavien: Thank you for reporting your excellent results with this sometimes challenging procedure. I have one question. In the follow-up of these patients, did you conclusively document that there was no atrophy of the distal pancreas, in other words, that the distal pancreas was indeed functional? Did you perform follow-up MRCP or CT in all your patients?
Dr. Michael W. Müller: Thank you very much for your comments and questions. No, we did not perform CT or MRI routinely in the postoperative follow-up in all of the patients. The functional parameters such as exocrine and endocrine function were measured, and we believe that these are of more clinical relevance.
Footnotes
Results: Markus W. Büchler, MD, Department of General Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany. E-mail: markus_buechler@med.uni-heidelberg.de.
REFERENCES
- 1.Guillemin P, Bessot M. Chronic calcifying pancreatitis in renal tuberculosis: pancreatojejunostomy using an original technic. Mem Acad Chir (Paris). 1957;83:869–871. [PubMed] [Google Scholar]
- 2.Letton AH, Wilson JP. Traumatic severance of pancreas treated by Roux-Y anastomosis. Surg Gynecol Obstet. 1959;109:473–478. [PubMed] [Google Scholar]
- 3.Ikeda S, Matsumoto S, Maeshiro K, et al. Segmental pancreatectomy for the diagnosis and treatment of small lesions in the neck or body of the pancreas. Hepatogastroenterology. 1995;42:730–733. [PubMed] [Google Scholar]
- 4.Asanuma Y, Koyama K, Saito K, et al. An appraisal of segmental pancreatectomy for benign tumors of the pancreatic body: a report of two cases. Surg Today. 1993;23:733–736. [DOI] [PubMed] [Google Scholar]
- 5.Dobrowolski F, Saeger HD. The therapy of benign pancreatic tumors. Zentralbl Chir. 2003;128:424–428. [DOI] [PubMed] [Google Scholar]
- 6.Kanazumi N, Nakao A, Kaneko T, et al. Surgical treatment of intraductal papillary-mucinous tumors of the pancreas. Hepatogastroenterology. 2001;48:967–971. [PubMed] [Google Scholar]
- 7.Su CH, Shyr YM, Lui WY, et al. Segmental pancreatectomy for benign tumor of the pancreas. Zhonghua Yi Xue Za Zhi (Taipei). 2002;65:608–613. [PubMed] [Google Scholar]
- 8.Sugiura H, Kondo S, Islam HK, et al. Clinicopathologic features and outcomes of intraductal papillary-mucinous tumors of the pancreas. Hepatogastroenterology. 2002;49:263–267. [PubMed] [Google Scholar]
- 9.Yamaguchi K, Yokohata K, Ohkido M, et al. Which is less invasive: distal pancreatectomy or segmental resection? Int Surg. 2000;85:297–302. [PubMed] [Google Scholar]
- 10.Zamora C, Sahel J, Cantu DG, et al. Intraductal papillary or mucinous tumors (IPMT) of the pancreas: report of a case series and review of the literature. Am J Gastroenterol. 2001;96:1441–1447. [DOI] [PubMed] [Google Scholar]
- 11.Rotman N, Sastre B, Fagniez PL. Medial pancreatectomy for tumors of the neck of the pancreas. Surgery. 1993;113:532–535. [PubMed] [Google Scholar]
- 12.Sperti C, Pasquali C, Ferronato A, et al. Median pancreatectomy for tumors of the neck and body of the pancreas. J Am Coll Surg. 2000;190:711–716. [DOI] [PubMed] [Google Scholar]
- 13.Balzano G, Zerbi A, Veronesi P, et al. Surgical treatment of benign and borderline neoplasms of the pancreatic body. Dig Surg. 2003;20:506–510. [DOI] [PubMed] [Google Scholar]
- 14.Rotman N, Fagniez PL. Medial pancreatectomy. J Hepatobiliary Pancreat Surg. 2000;7:453–455. [DOI] [PubMed] [Google Scholar]
- 15.Partensky C, Apa D, Marchal F, et al. Medial pancreatectomy with pancreatogastric anastomosis in pancreatic neoplasms. Chirurgie. 1998;123:363–367. [DOI] [PubMed] [Google Scholar]
- 16.Sauvanet A, Partensky C, Sastre B, et al. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery. 2002;132:836–843. [DOI] [PubMed] [Google Scholar]
- 17.Aranha GV. Central (middle segment) pancreatectomy: a suitable operation for small lesions of the neck of the pancreas. Hepatogastroenterology. 2002;49:1713–1715. [PubMed] [Google Scholar]
- 18.Efron DT, Lillemoe KD, Cameron JL, et al. Central pancreatectomy with pancreaticogastrostomy for benign pancreatic pathology. J Gastrointest Surg. 2004;8:532–538. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein MJ, Toman J, Chabot JA. Pancreaticogastrostomy: a novel application after central pancreatectomy. J Am Coll Surg. 2004;198:871–876. [DOI] [PubMed] [Google Scholar]
- 20.Iacono C, Bortolasi L, Serio G. Is there a place for central pancreatectomy in pancreatic surgery? J Gastrointest Surg. 1998;2:509–516. [DOI] [PubMed] [Google Scholar]
- 21.Bassi C, Salvia R, Molinari E, et al. Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg. 2003;27:319–323. [DOI] [PubMed] [Google Scholar]
- 22.Warshaw AL, Rattner DW, Fernandez-del CC, et al. Middle segment pancreatectomy: a novel technique for conserving pancreatic tissue. Arch Surg. 1998;133:327–331. [DOI] [PubMed] [Google Scholar]
- 23.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37:1345–1351. [DOI] [PubMed] [Google Scholar]
- 26.Hjermstad MJ, Fayers PM, Bjordal K, et al. Using reference data on quality of life: the importance of adjusting for age and gender, exemplified by the EORTC QLQ-C30 (+3). Eur J Cancer. 1998;34:1381–1389. [DOI] [PubMed] [Google Scholar]
- 27.Hjermstad MJ, Fayers PM, Bjordal K, et al. Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: the QLQ=C30 (+ 3). J Clin Oncol. 1998;16:1188–1196. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Tanaka M, Chijiiwa K, et al. Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepatobiliary Pancreat Surg. 1999;6:303–311. [DOI] [PubMed] [Google Scholar]
- 29.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pyke CM, van Heerden JA, Colby TV, et al. The spectrum of serous cystadenoma of the pancreas: clinical, pathologic, and surgical aspects. Ann Surg. 1992;215:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talamini MA, Moesinger R, Yeo CJ, et al. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg. 1998;227:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aranha GV, Hodul PJ, Creech S, et al. Zero mortality after 152 consecutive pancreaticoduodenectomies with pancreaticogastrostomy. J Am Coll Surg. 2003;197:223–231. [DOI] [PubMed] [Google Scholar]
- 33.Balcom JH, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. [DOI] [PubMed] [Google Scholar]
- 34.Beger HG, Krautzberger W, Bittner R, et al. Duodenum-preserving resection of the head of the pancreas in patients with severe chronic pancreatitis. Surgery. 1985;97:467–473. [PubMed] [Google Scholar]
- 35.Buchler MW, Friess H, Bittner R, et al. Duodenum-preserving pancreatic head resection: long-term results. J Gastrointest Surg. 1997;1:13–19. [DOI] [PubMed] [Google Scholar]
- 36.Buchler MW, Friess H, Wagner M, et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:883–889. [DOI] [PubMed] [Google Scholar]
- 37.Neoptolemos JP, Russell RC, Bramhall S, et al. Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg. 1997;84:1370–1376. [PubMed] [Google Scholar]
- 38.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frey CF, Child CG, Fry W. Pancreatectomy for chronic pancreatitis. Ann Surg. 1976;184:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakorafas GH, Farnell MB, Nagorney DM, et al. Pancreatoduodenectomy for chronic pancreatitis: long-term results in 105 patients. Arch Surg. 2000;135:517–523. [DOI] [PubMed] [Google Scholar]
- 42.Tran K, Van EC, Di C, V, et al. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg. 2002;236:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rault A, SaCunha A, Klopfenstein D, et al. Pancreaticojejunal anastomosis is preferable to pancreaticogastrostomy after pancreaticoduodenectomy for long-term outcomes of pancreatic exocrine function. J Am Coll Surg. 2005;201:239–244. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez RE, Fernandez-del CC, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott HW Jr, Dean RH, Parker T, et al. The role of vagotomy in pancreaticoduodenectomy. Ann Surg. 1980;191:688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaguchi T, Nakamura S, Suzuki S, et al. Marginal ulceration after pylorus-preserving pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2000;7:193–197. [DOI] [PubMed] [Google Scholar]
- 47.Aldridge MC, Williamson RC. Distal pancreatectomy with and without splenectomy. Br J Surg. 1991;78:976–979. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Cruz L, Saenz A, Astudillo E, et al. Outcome of laparoscopic pancreatic surgery: endocrine and nonendocrine tumors. World J Surg. 2002;26:1057–1065. [DOI] [PubMed] [Google Scholar]
- 49.Fujita F, Lyass S, Otsuka K, et al. Portal vein thrombosis following splenectomy: identification of risk factors. Am Surg. 2003;69:951–956. [PubMed] [Google Scholar]
- 50.Kimura W, Inoue T, Futakawa N, et al. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Surgery. 1996;120:885–890. [DOI] [PubMed] [Google Scholar]
- 51.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553. [DOI] [PubMed] [Google Scholar]
- 52.Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg. 2002;184:631–635. [DOI] [PubMed] [Google Scholar]
- 53.Knaebel HP, Diener MK, Wente MN, et al. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539–546. [DOI] [PubMed] [Google Scholar]
- 54.Andersen HB, Baden H, Brahe NE, et al. Pancreaticoduodenectomy for periampullary adenocarcinoma. J Am Coll Surg. 1994;179:545–552. [PubMed] [Google Scholar]
- 55.Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohwada S, Ogawa T, Tanahashi Y, et al. Fibrin glue sandwich prevents pancreatic fistula following distal pancreatectomy. World J Surg. 1998;22:494–498. [DOI] [PubMed] [Google Scholar]
- 57.Beger HG, Buchler M, Bittner RR, et al. Duodenum-preserving resection of the head of the pancreas in severe chronic pancreatitis: early and late results. Ann Surg. 1989;209:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin RF, Rossi RL, Leslie KA. Long-term results of pylorus-preserving pancreatoduodenectomy for chronic pancreatitis. Arch Surg. 1996;131:247–252. [DOI] [PubMed] [Google Scholar]
- 59.Warren KW, Veidenheimer MC, Pratt HS. Pancreatoduodenectomy for periampullary cancer. Surg Clin North Am. 1967;47:639–645. [DOI] [PubMed] [Google Scholar]
- 60.Morrow CE, Cohen JI, Sutherland DE, et al. Chronic pancreatitis: long-term surgical results of pancreatic duct drainage, pancreatic resection, and near-total pancreatectomy and islet autotransplantation. Surgery. 1984;96:608–616. [PubMed] [Google Scholar]
- 61.Christein JD, Kim AW, Golshan MA, et al. Central pancreatectomy for the resection of benign or low malignant potential neoplasms. World J Surg. 2003;27:595–598. [DOI] [PubMed] [Google Scholar]
- 62.Kimura W, Makuuchi M. Operative indications for cystic lesions of the pancreas with malignant potential: our experience. Hepatogastroenterology. 1999;46:483–491. [PubMed] [Google Scholar]
- 63.Takeyoshi I, Ohwada S, Nakamura S, et al. Segmental pancreatectomy for mucin-producing pancreatic tumors. Hepatogastroenterology. 1999;46:2585–2588. [PubMed] [Google Scholar]
- 64.Balcom JH IV, Fernandez-del CC, Warshaw AL. Cystic lesions in the pancreas: when to watch, when to resect. Curr Gastroenterol Rep. 2000;2:152–158. [DOI] [PubMed] [Google Scholar]
- 65.Siech M, Tripp K, Schmidt-Rohlfing B, et al. Cystic tumours of the pancreas: diagnostic accuracy, pathologic observations and surgical consequences. Langenbecks Arch Surg. 1998;383:56–61. [DOI] [PubMed] [Google Scholar]