Abstract
Objectives:
1) Characterize changes in the surgical treatment of anorectal melanoma over time. 2) Determine if the extent of surgical resection is associated with outcome. 3) Identify prognostic factors correlating with survival.
Summary Background Data:
Although early data suggested improved survival in patients undergoing abdominoperineal resection (APR) for primary anorectal melanoma, such an aggressive approach may be unwarranted as distant relapse rates are high. We have seen a trend toward less aggressive surgical treatment of the local disease over the past 20 years.
Methods:
A retrospective review was performed of all patients with anorectal melanoma treated at our institution between 1984 and 2003. Extent of primary resection and pathologic factors were studied.
Results:
Forty-six patients underwent a curative resection with a median follow-up of 29 months, and 5-year disease-specific survival (DSS) rate of 35%. While patient and tumor characteristics remained similar, there was a dramatic shift in surgical treatment toward less radical procedures. Prior to 1997, the majority of patients (15 of 21, 71%) underwent APR. After 1997, the majority of patients (21 of 25, 84%) underwent local excision (LE) (P < 0.0001). Local recurrence was noted in 11 of 46 (24%) patients: 4 of 19 (21%) who underwent APR and 7 of 27 (26%) who underwent LE (P = not significant). Five-year DSS was similar: 34% following APR and 35% following LE. Tumor perineural invasion (PNI) was the only factor identified as an independent predictor of worse outcome (P = 0.01).
Conclusion:
The extent of surgical treatment is not associated with outcome in primary anorectal melanoma. Therefore, LE of the primary tumor is recommended when technically feasible. The presence of PNI is an important prognostic factor and should be considered in future clinical trials.
Anorectal melanoma is a lethal disease with limited effective treatment options. Improved survival has been suggested with abdominoperineal resection, but such an aggressive approach may be unwarranted as distant relapse rates are uniformly high. Our aims were to examine the evolution of surgical treatment for anorectal melanoma at our institution and identify prognostic factors correlating with survival.
Anorectal melanoma is rare, accounting for 24% of mucosal melanomas, 4% of anal canal tumors, and less than 1% of all melanomas.1,2 Lesions are difficult to diagnose because many are amelanotic and patients present with nonspecific complaints such as anal discomfort or rectal bleeding. Although more than 70% of patients will present with localized and apparently curable primary tumor, mean survival is only 2 years despite optimal surgical therapy.3–8 The majority of patients die of distant metastases. Overall 5-year survival is less than 20%, in sharp contrast to 80% survival for primary cutaneous melanoma.2
There has been a debate in the literature regarding the extent of surgery necessary for treatment of primary disease. Early studies have suggested that aggressive treatment of the primary anorectal lesion with abdominoperineal resection (APR) was associated with improved outcome, possibly due to regional lymphadenectomy.8,9 However, other studies, describing local excision (LE) of the primary anorectal lesion, without regional lymphadenectomy, have reported similar patterns of recurrence and survival with no significant increase in local failure.4,7,10–12 All studies are concordant with the fact that relapse is usually distant and lethal.
Concurrently with these recent studies, we have noted a trend in our own institution toward less radical resection of the primary lesion. The purpose of this study was to characterize changes in the surgical approach to anorectal melanoma patients over a 20-year period at Memorial Sloan-Kettering Cancer Center (MSKCC), determine the relationship between extent of operation and outcome and attempt to define prognostic factors by examining clinicopathologic data.
PATIENTS AND METHODS
A retrospective review identified 62 consecutive patients with anorectal melanoma treated at MSKCC between 1984 and 2003. Thirteen patients presented with metastatic disease and 3 patients were lost to follow-up. Forty-six patients with primary anorectal melanoma underwent curative resection. The date of recurrence could not be determined for 1 patient; however, date and cause of death were discerned. Ten patients with localized disease presented prior to 1993 and may have been included in a previous report by our institution.8 However, because a systematic analysis of clinicopathologic factors had not been examined previously, these patients were included in the current study.
Tumor specimens from 38 patients were available for review and were examined by a single pathologist (J.S.). Clinical characteristics, extent of resection, and histopathology were examined. Histopathologic features, including nodal status, thickness, maximum diameter, number of mitoses per mm2, presence or absence of melanin pigment, extent of mural involvement, histologic type (spindle cell vs. epithelioid), lymphovascular invasion, perineural invasion (PNI), ulceration, in situ melanoma, and necrosis were examined.
Disease-specific (DSS) and recurrence-free survival (RFS) were analyzed using the Kaplan-Meier product-limit method and the significance of clinicopathologic variables were measured by the log-rank test. Continuous variables such as age, diameter, and thickness were examined using the Cox proportional hazards regression analysis. The Fisher exact test was used to analyze associations between 2 variables, the Pearson χ2 test was used to analyze associations between more than 2 variables, and the Mann-Whitney U test was used to compare nonparametric groups. Multivariate analysis was performed using the Cox proportional hazards regression method.
RESULTS
Demographics
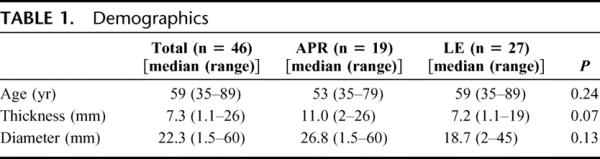
The median age of the 46 patients comprising the study cohort was 59 years (range, 35–89 years). Twenty-eight patients were female and 18 were male. Nineteen patients underwent an APR, and 27 patients underwent a LE. Four patients in the LE group underwent either a sentinel lymph node biopsy (2) or lymph node dissection (2) of the inguinal lymph node basin. One patient in the APR group underwent a therapeutic inguinal lymphadenectomy. The median tumor diameter was 22 mm (range, 1.5–60 mm) and median thickness was 7.3 mm (range, 1.1–26 mm) (Table 1). Nine patients in the APR group and 14 patients in the WLE group received postoperative adjuvant therapy, including interferon, temozolamide, dacarbazine, and immunotherapy based regimens. No patient received adjuvant radiation therapy.
TABLE 1. Demographics
Treatment Changes Over Time
During the 20-year period under consideration, a generalized shift in treatment paradigm was noted in 1997, correlating with the publication of two seminal studies.7,8
The change in treatment approach is illustrated in Table 2. From 1984 to 1996, 21 patients were treated for primary anorectal melanoma and 15 of these (71%) underwent APR. In contrast, from 1997 to 2003, 25 patients were treated and 21 of these (84%) underwent LE (P < 0.001) (Table 2). Thickness and diameter of the tumor were unchanged during this period. In addition, pattern of relapse, time to relapse, and disease-specific mortality remained similar in these two cohorts (Table 2; Fig. 1).
TABLE 2. Primary Tumor, Treatment, and Outcome in Patients Treated Before and After 1997
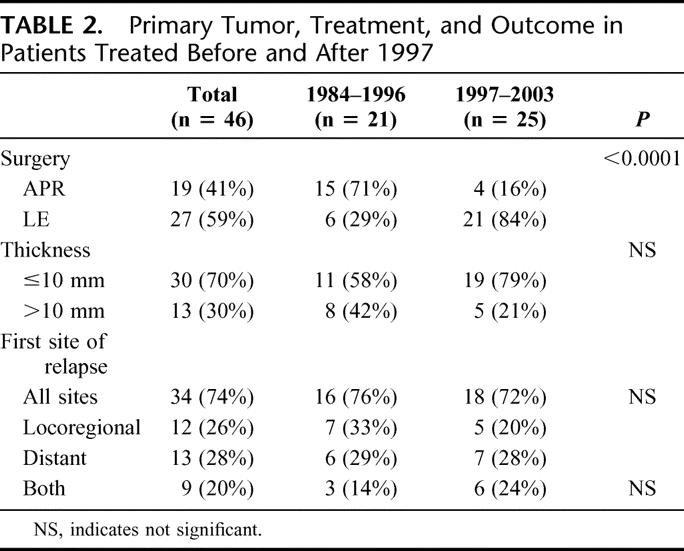
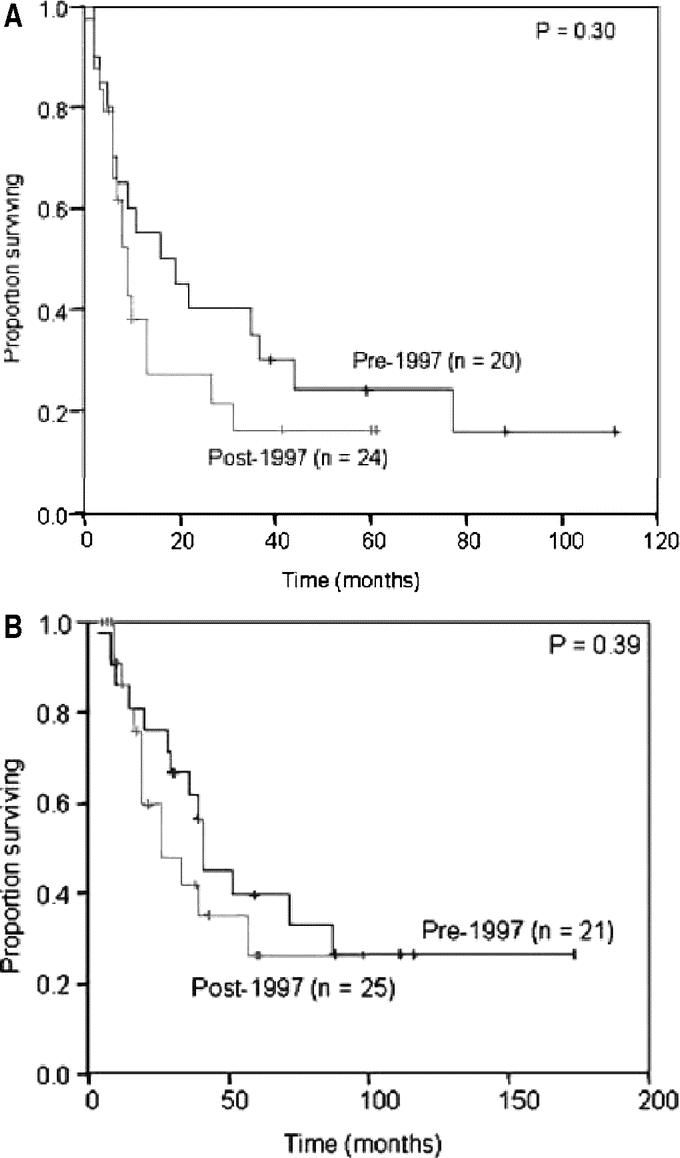
FIGURE 1. Changes in practice at MSKCC. A, Recurrence-free survival before and after 1997. B, Disease-specific survival before and after 1997.
Patients Undergoing APR Versus LE
Patients undergoing APR tended to have lesions of greater thickness compared with those undergoing LE. The median thickness in the APR cohort was 11.0 mm (range, 1.1–26 mm) compared with 7.2 mm (range, 1.1–19 mm) in the LE cohort, but was not significantly different (Table 1). The location and diameter of the lesion, age and sex of the patient, incidence of PNI, ulceration, necrosis, and other histologic characteristics did not correlate with the type of operation performed or the time period during which it was performed.
Outcome
With a median follow-up of 39 months for survivors, the median DSS was 39 months and 5-year DSS was 34%. Of the 46 patients that underwent curative resection, 34 (74%) relapsed with a median RFS of 10 months and a recurrence rate of 53% at 1 year. The majority of patients failed at distant sites with or without local recurrence: 13 with distant, 9 with both distant and locoregional, and 12 with locoregional sites (Table 3). No difference in relapse pattern was seen between those patients treated with LE or APR. Five of 19 (26%) patients treated with APR and 7 of 27 (26%) patients treated with LE were noted to have local recurrence as the first site of relapse. Ten of these patients underwent a salvage operation for localized disease. Two patients were treated with systemic therapy. DSS was similar between groups with a 5-year survival of 32% for the APR compared with 35% for the LE cohort (Fig. 2). Factors associated with RFS and DSS are shown in Table 4. The strongest predictor of outcome was the presence of PNI in the primary tumor. Eight of 35 tumors (23%) had tumor PNI: 3 were treated with APR and 5 with LE (P = not significant). All patients with tumor PNI recurred compared with 18 of 27 (67%) without tumor PNI. The median survival for the cohort with tumor PNI was 19 months compared with 39 months for the cohort without tumor PNI (hazard ratio 3.3 [1.3, 8.6], Fig. 3). Of the 8 patients (22%) with tumor PNI, only 1 patient was alive at 12 months with lung, liver, and perirectal lymph node metastases. This patient had undergone a LE. Of the 29 (78%) patients without tumor PNI, 12 underwent APR and 17 underwent LE (P = not significant). Ten (4 APR, 6 LE) were alive with no evidence of disease at 5, 7, 38, 59, 60, 61, 88, 111, 116, and 173 months.
TABLE 3. Characteristics of Patients Undergoing APR Versus LE
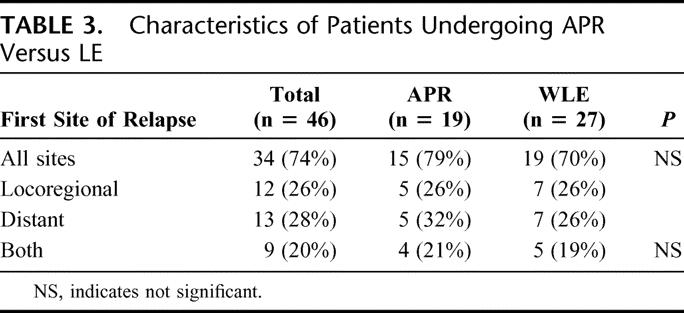
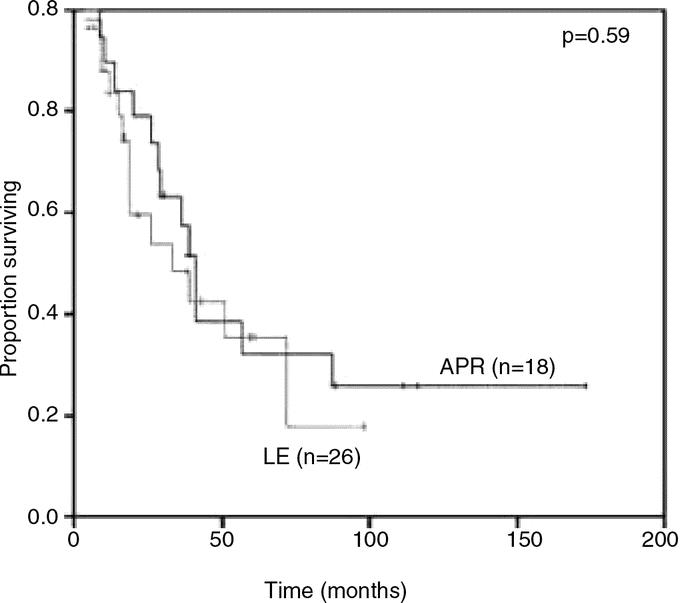
FIGURE 2. Disease-specific survival in patients who underwent APR compared with LE.
TABLE 4. Univariate Analysis of Prognostic Factors Associated With Recurrence and Survival
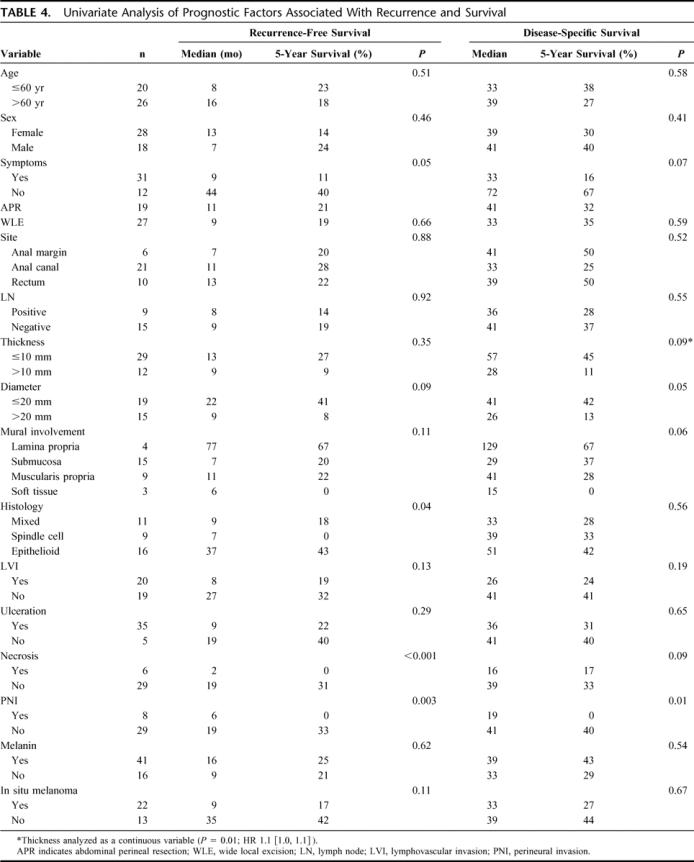
FIGURE 3. Disease-specific survival in patients with perineural invasion.
The presence of tumor necrosis was a poor histologic feature associated with disease recurrence. All 6 patients with evidence of tumor necrosis recurred, whereas 19 of 26 (73%) patients without tumor necrosis recurred. Of those with tumor necrosis, 3 patients underwent APR and 3 underwent LE. Median RFS was significantly less in those patients with tumor necrosis, and there was a trend toward worse DSS in this cohort as well. Interestingly, only 2 patients had both tumor necrosis and PNI.
Tumor size was also associated with outcome. Patients with tumors measuring more than 2 cm in diameter had shorter DSS. Tumor thickness, when analyzed as a continuous variable, predicted worse DSS with a hazard ratio of 1.1 [1.0, 1.1]. Tumor histologic type was associated with recurrence, but not with survival. Tumors with pure epithelioid histology were less likely to recur compared with tumors showing pure spindle cell or mixed histology. The presence of regional nodal metastasis was not associated with disease recurrence or survival in patients who underwent APR or LE. Other pathologic features such as ulceration, lymphovascular invasion, presence of melanin, and in situ melanoma had no association with outcome.
Patients who presented with symptoms had a trend toward a decreased RFS and DSS. Clinical features such as patient age, sex, site of disease, and type of resection were not significant prognostic variables.
Multivariate Analysis of Disease-Specific Mortality
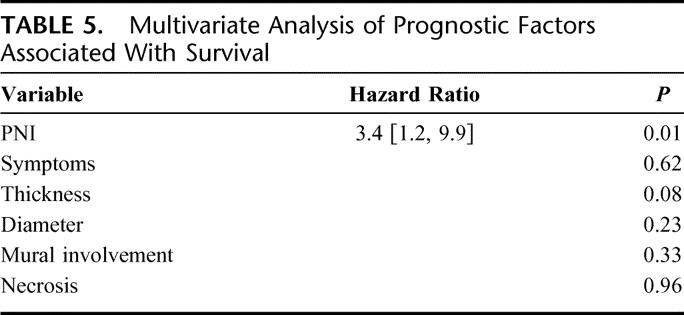
Twenty-seven patients had complete data available for multivariate analysis. Tumor PNI was the only independent predictor of DSS with a HR 3.4 [1.2, 9.9] (P = 0.02; Table 5). Tumor thickness and diameter were not significant factors by multivariate analysis.
TABLE 5. Multivariate Analysis of Prognostic Factors Associated With Survival
DISCUSSION
There has long been a debate regarding the extent of resection necessary to optimally treat anorectal melanoma.9,13,14 Because of the rarity of this disease, small retrospective studies provide the only guidance for treatment planning. The benefits of LE are clear and include quicker recovery from a less invasive procedure, minimal impact on bowel function, and no need for a stoma. However, this approach does not address the importance of regional lymph nodes, which are one of the most significant predictors of outcome in primary cutaneous melanoma.15
A previous series of 56 patients with localized anorectal melanoma treated with either APR or LE treated at our institution between 1929 and 1993 favored regional lymphadenectomy with 9 of 10 long-term survivors in the APR group. Two of the long-term survivors had positive mesenteric lymph nodes, 1 in the APR group and 1 in the LE group, who subsequently underwent therapeutic pelvic lymphadenectomy.8 However, it is noteworthy that the rate of isolated local recurrence was comparable in patients undergoing LE and APR in that series. Additional reported series of LE for anorectal melanoma have not observed high rates of isolated regional relapse leading to the hypothesis that regional relapse is not the basis of patient demise.4,7,10–12
Our own institutional practice has evolved toward LE, and the current study represents an evaluation of practice patterns and outcomes for patients with anorectal melanoma treated at MSKCC over the past 20 years. Our data confirm that the majority of resections currently performed for anorectal melanoma at MSKCC are LE when feasible (Table 2). Despite a clear change in practice patterns, we observed no significant difference in outcome (Fig. 1), with 75% of patients recurring regardless of the extent of resection. This suggests that the extent of surgical therapy is not associated with the rate of local recurrence or survival in anorectal melanoma. We hypothesize that systemic dissemination is an early event in tumorigenesis and by the time the lesion is clinically apparent, micrometastases are well established. It is clear, therefore, that efforts should be focused on multimodality therapy to improve outcome in this lethal disease.16,17
Although outcome was generally poor for the entire cohort, we were able to identify risk factors associated with survival. Factors such as gender, presence of melanin, depth of invasion, and lymph node metastases have been previously studied with varying results.3,6–8 Brady et al8 found a trend toward better survival for female gender; however, we were not able to confirm this finding in the current series. No survival benefit was seen in either gender group. Thibault et al were unable to correlate depth of tumor invasion with recurrence.7 In our series, patients with thin tumors tended to have a better DSS, but this was not statistically significant on multivariate analysis.
The need for regional lymphadenectomy has been at the center of the debate regarding extent of resection for anorectal melanoma. Inguinal, pelvic sidewall, and mesorectal lymph nodes are at risk for metastases from anorectal lesions. During APR, mesorectal lymph nodes are resected en bloc with the primary tumor. However, as alluded to previously, the significance of regional lymphadenectomy is unclear.7,8 In the current study, lymph node metastases did not predict outcome in patients who underwent APR. Additionally, no survival difference was seen in the 5 patients who underwent inguinal sentinel lymph node biopsy. Since lymph node disease at any basin did not predict outcome in the current study, we conclude that nodal disease may not carry the same biologic significance as in cutaneous melanoma.
In the present series, histologic features were associated with relapse and survival. Tumors with necrosis, PNI, and, less significantly, spindle cell histology and larger diameter were associated with poor outcome. Regardless of surgical approach, all patients who had tumors with either necrosis or PNI recurred with a median RFS of 6 months or less. The presence of tumor necrosis appears to be an important histologic feature, representing a biologically more aggressive tumor. Five of 6 patients with this finding died within 26 months, compared with 7 of 29 patients without tumor necrosis during the same time period, although this was not statistically significant.
The only significant prognostic factor associated with long-term survival was tumor PNI. Interestingly, tumor PNI has also been identified as an independent predictor of disease recurrence and survival in other intestinal cancers such as rectal cancer.18–20 In contrast to cutaneous melanoma, factors such as lymphovascular invasion, ulceration, and nodal status were not associated with outcome in patients with anorectal melanoma. Recent genetic analyses demonstrate molecular differences between cutaneous and mucosal melanomas. Mutations in exon 15 of B-raf are found in up to 69% of primary cutaneous melanomas, but no mutations have been found in any of the 13 mucosal melanomas examined.21,22 Given the clinical, biologic, and molecular differences, mucosal and cutaneous melanomas may be distinct disease entities.
CONCLUSION
There is no convincing evidence to indicate that radical resection of primary anorectal melanoma is associated with improvement in local control or survival. Patients with localized disease should undergo LE whenever technically feasible. This approach aims to minimize morbidity and maximize quality of life in a disease that, even when localized, is rarely curable. Because anorectal melanomas are rare, staging of the disease has previously been limited to local, regional, and distant disease. The presence of PNI is an important prognostic factor and should be considered in future clinical trials. Further study of the molecular mechanisms of anorectal melanoma oncogenesis and tumor progression is needed to develop innovative treatment paradigms that may ultimately impact outcome.
Footnotes
Reprints: Martin R. Weiser, MD, Memorial-Sloan Kettering Cancer Center, 1275 York Avenue, Room C-1075, New York, NY 10021. E-mail: weiser1@mskcc.org.
REFERENCES
- 1.Klas JV, Rothenberger DA, Wong WD, et al. Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer. 1999;85:1686–1693. [DOI] [PubMed] [Google Scholar]
- 2.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. [DOI] [PubMed] [Google Scholar]
- 3.Pessaux P, Pocard M, Elias D, et al. Surgical management of primary anorectal melanoma. Br J Surg. 2004;91:1183–1187. [DOI] [PubMed] [Google Scholar]
- 4.Ross M, Pezzi C, Pezzi T, et al. Patterns of failure in anorectal melanoma: a guide to surgical therapy. Arch Surg. 1990;125:313–316. [DOI] [PubMed] [Google Scholar]
- 5.Weyandt GH, Eggert AO, Houf M, et al. Anorectal melanoma: surgical management guidelines according to tumour thickness. Br J Cancer. 2003;89:2019–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slingluff CL Jr, Vollmer RT, Seigler HF. Anorectal melanoma: clinical characteristics and results of surgical management in twenty-four patients. Surgery. 1990;107:1–9. [PubMed] [Google Scholar]
- 7.Thibault C, Sagar P, Nivatvongs S, et al. Anorectal melanoma: an incurable disease? Dis Colon Rectum. 1997;40:661–668. [DOI] [PubMed] [Google Scholar]
- 8.Brady MS, Kavolius JP, Quan SH. Anorectal melanoma: a 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum. 1995;38:146–151. [DOI] [PubMed] [Google Scholar]
- 9.Wanebo HJ, Woodruff JM, Farr GH, et al. Anorectal melanoma. Cancer. 1981;47:1891–1900. [DOI] [PubMed] [Google Scholar]
- 10.Malik A, Hull TL, Floruta C. What is the best surgical treatment for anorectal melanoma? Int J Colorectal Dis. 2004;19:121–123. [DOI] [PubMed] [Google Scholar]
- 11.Yap LB, Neary P. A comparison of wide local excision with abdominoperineal resection in anorectal melanoma. Melanoma Res. 2004;14:147–150. [DOI] [PubMed] [Google Scholar]
- 12.Bullard KM, Tuttle TM, Rothenberger DA, et al. Surgical therapy for anorectal melanoma. J Am Coll Surg. 2003;196:206–211. [DOI] [PubMed] [Google Scholar]
- 13.Cooper PH, Mills SE, Allen MS Jr. Malignant melanoma of the anus: report of 12 patients and analysis of 255 additional cases. Dis Colon Rectum. 1982;25:693–703. [DOI] [PubMed] [Google Scholar]
- 14.Ward MW, Romano G, Nicholls RJ. The surgical treatment of anorectal malignant melanoma. Br J Surg. 1986;73:68–69. [DOI] [PubMed] [Google Scholar]
- 15.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. [DOI] [PubMed] [Google Scholar]
- 16.Kim KB, Sanguino AM, Hodges C, et al. Biochemotherapy in patients with metastatic anorectal mucosal melanoma. Cancer. 2004;100:1478–1483. [DOI] [PubMed] [Google Scholar]
- 17.Yeh JJ, Weiser MR, Shia J, et al. Response of Stage IV anal mucosal melanoma to chemotherapy. Lancet Oncol. 2005;6:438–439. [DOI] [PubMed] [Google Scholar]
- 18.Shirouzu K, Isomoto H, Morodomi T, et al. Clinicopathologic study of perineural invasion in rectal cancer. Kurume Med J. 1992;39:41–49. [DOI] [PubMed] [Google Scholar]
- 19.Ueno H, Hase K, Mochizuki H. Criteria for extramural perineural invasion as a prognostic factor in rectal cancer. Br J Surg. 2001;88:994–1000. [DOI] [PubMed] [Google Scholar]
- 20.Shirouzu K, Isomoto H, Kakegawa T. Prognostic evaluation of perineural invasion in rectal cancer. Am J Surg. 1993;165:233–237. [DOI] [PubMed] [Google Scholar]
- 21.Pavey S, Johansson P, Packer L, et al. Microarray expression profiling in melanoma reveals a BRAF mutation signature. Oncogene. 2004;23:4060–4067. [DOI] [PubMed] [Google Scholar]
- 22.Helmke BM, Mollenhauer J, Herold-Mende C, et al. BRAF mutations distinguish anorectal from cutaneous melanoma at the molecular level. Gastroenterology. 2004;127:815–820. [DOI] [PubMed] [Google Scholar]


