Abstract
Objective:
The aim of this study was to evaluate expression of cancer risk-associated biomarkers in columnar epithelium at squamocolumnar junctions produced by an ablation procedure and proton pump inhibitors in incompletely ablated Barrett’s esophagus (BE) patients that were nondysplastic prior to ablation.
Summary Background Data:
Ablation of BE to squamous epithelium is achievable by combining a re-injury method with acid suppression. We previously reported that, when there is complete ablation, the neo-squamous epithelium is normal histologically and in biomarker expression. However, squamous islands observed after prolonged use of PPIs were associated with abnormalities in p53 expression and Ki-67 labeling.
Methods:
Twenty-one nondysplastic BE cases with incomplete ablation were evaluated for the expression of Ki-67 (proliferation), cyclooxygenase-2 (COX-2), and p53 by immunohistochemistry.
Results:
Pre-ablation biopsies showed the normal staining patterns in columnar epithelium, ie, normal Ki-67 labeling, rare positive COX-2 staining of interstitial cells, and negative or mild staining for p53 in the majority of patients’ biopsies. However, post-ablation biopsies demonstrated abnormal staining patterns in the glandular area at the new squamocolumnar junctions. In 13 of 21 post-ablation cases (62%), increased Ki-67 staining was seen in BE glands. In 8 of 21 patients (38%), increased COX-2 expression was seen in columnar epithelium. Similarly, in 8 of 21 post-ablation junctions (38%), there was increased p53 staining.
Conclusions:
Our findings of increased expression of cancer-associated biomarkers in incompletely ablated BE patients raise a cautionary note regarding this procedure. We hypothesize that newly formed junctions contain cells undergoing replication, differentiation, etc, and are thus more susceptible to genomic damage.
Twenty-one Barrett esophagus patients with incomplete ablation were evaluated for proliferation and the expression of cyclooxygenase-2 and p53 by immunohistochemistry. This study indicates that there is an increase in the expression of cancer risk-associated biomarkers in columnar epithelium at squamocolumnar junctions in the patients that were nondysplastic prior to ablation.
Barrett’s esophagus (BE) is a common premalignant lesion of the distal esophagus that arises as a consequence of duodenogastroesophageal reflux.1–3 Histologically, BE is characterized as a condition where squamous epithelial cells are replaced by columnar epithelium containing goblet cells.3 Epidemiologic studies have shown that BE is associated with increased risk for the development of esophageal adenocarcinoma.4,5 A long-standing clinical goal has been to reverse BE to squamous epithelium, but this proved elusive for decades until recently. Based on the hypothesis that physical injury and disruption of the BE glands followed by healing in an acid-suppressed environment would allow repair and differentiation, several investigators developed methods that use reinjury procedures and acid suppression with proton pump inhibitors (PPIs).6–8 Reinjury approaches that have been used include laser, photodynamic therapy, multipolar electrocoagulation (MPEC), and argon plasma coagulation (APC).4,9–13 Although squamous epithelium can be generated by these methods and complete ablation is possible, incomplete ablation, manifested as the presence of intestinal metaplasia (IM), is frequent with clinical series showing up to 30% to 50% of patients being incompletely ablated.10,12,14–16 Furthermore, there is no evidence that this procedure eliminates or reduces cancer risk in nondysplastic patients.17 Despite lack of evidence of efficacy, attempts to ablate BE are being performed with rapidly increasing frequency in clinical practice.18
Previously, we showed that, after complete reversal of BE, new squamous epithelium is biologically similar to normal squamous epithelium.19 For example, low activity of ornithine decarboxylase and low expression of p53 and Ki-67 were observed in new squamous epithelium from completely reversed patients. Indeed, we have been unable to distinguish IM completely reversed to squamous epithelium from normal squamous epithelium, obtained from controls by any method thus far. In contrast, the squamous islands observed in BE patients after prolonged use of PPIs were associated with marked abnormalities in Ki-67 staining and p53 expression.19 This was of immediate concern to us from a clinical standpoint, which prompted us to assess incompletely reversed cases arising after endoscopic ablative procedures using well-characterized biomarkers associated with cancer.
The major aim of the present study was to characterize the staining pattern of biomarkers, Ki-67, p53, and cyclooxygenase-2 (COX-2), in nondysplastic BE patients at the new squamocolumnar junctions produced by ablative interventions and PPIs. Patients with nondysplastic BE are at relatively low risk for cancer progression. However, the injury caused by ablation together with persistent reflux might create an area of mucosal instability at new squamocolumnar junctions. These junctions may or may not be evident by endoscopy and are detected in the esophageal tissue by histopathologic evaluation. The hypothesis on which this study is based is that new junctions contain cells that are genomically less stable than mature epithelium, especially in the setting of ongoing reflux containing bile acids. These areas, which are distinguished by abnormal expression of biomarkers, are likely to be more susceptible to malignant transformation.
METHODS
Patient Selection
Twenty-one BE patients were included in the present study. They all had incomplete ablation while enrolled in clinical trials assessing the safety and efficacy of MPEC and APC based ablation in nondysplastic BE. The patients were participants in a clinical trial involving acid suppression with PPI. Being participants in a clinical trial assured that the ablation procedure, acid suppression, and subsequent follow-up were uniform. Patients gave written informed consent approved by the IRB Human Subjects Committee of the University of Arizona.
Endoscopic Biopsy Protocol
BE was defined histologically as the presence of intestinal-like metaplastic epithelium containing goblet cells. Endoscopic biopsies of BE were taken from patients prior to and after the ablation procedure from the area of former BE and any endoscopically apparent residual BE. A therapeutic endoscope and large capacity biopsy forceps were used to take samples from successive areas 2 cm apart. In each area, biopsies were taken from four quadrants according to a standard protocol. All biopsies were stained with hematoxylin and eosin and Alcian blue (pH 2.5) for goblet cells. The neo-squamocolumnar junctions were defined after staining with hematoxylin and eosin as areas where the Barrett’s lesion was present prior to intervention and where squamous epithelium adjacent to columnar epithelium was found after ablation. Ablation of Barrett’s lesions was accomplished by using the PPI, in combination with MPEC or APC. MPEC ablation was performed with a 10-F gold probe (Microvasive, Boston, MA), using a 50-watt energy source at a setting of 3 and continuous power. Clinical details of this procedure have been previously described.11 Patients treated with APC were treated with a 10-F probe at 60 watts and a 1.2 L/min flow rate.20 Biomarkers were evaluated after a minimum of 4 months had elapsed since the last ablation procedure to assure that healing had occurred.
Immunohistochemistry
For the evaluation of Ki-67, COX-2, and p53 expression, a standard immunostaining assay involving biotin-avidin-linked peroxidase detection was used, as described previously.19 To ensure identical staining conditions for preablation and postablation biopsies, immunohistochemical staining for each individual biomarker was done together in single experimental run for all patients. Briefly, paraffin-embedded sections were deparaffinized, rehydrated, and placed in 3% hydrogen peroxide for 30 minutes to block endogenous peroxidase. Following antigen retrieval using a microwave protocol, the slides were incubated in 2% horse serum for 30 minutes and immunostained with monoclonal antibodies against p53 (1:50, DO-7, Dako Corp. Carpinteria, CA), COX-2 (1:500, C22420, Transduction Laboratories, Lexington, KY), or Ki-67 (1:50, MIB-1, Dako Corp.) for 60 minutes. After 3 rinses with PBS, the secondary antibody, biotinylated rabbit antimouse IgG antibody (1:400, E0413, Dako Corp.), was applied for 30 minutes. Slides were again rinsed with PBS and Vectastain ABC reagent (Elite PK-6100, Standard, Vector Laboratories, Burlingame, CA) was added according to the kit instructions. After 3 final PBS rinses, slides were immersed in 3,3′-diaminobenzidine, at a concentration of 0.25 mg/mL, activated with hydrogen peroxide for 5 minutes, rinsed, and lightly counterstained with hematoxylin. Isotype-matched immunocontrols were routinely included with each experiment, as described previously.21
Biomarker Expression Analysis
A simple grading system (+ to +++) routinely used in our laboratory was employed to grade the level of expression of individual markers. Positive staining of Ki-67 and COX-2 required staining of greater than 5% of the cells (focal staining = +; 5 to 50% cells stained = ++; >50% cells stained = +++). p53 expression was graded based on the intensity of staining in the nucleus of columnar cells (0 = no staining, + = mild staining, ++ = medium staining, and +++ = intense staining). Expression of individual biomarkers was evaluated prior to and after ablation in the columnar epithelium at the new squamocolumnar junctions. In addition, abnormal staining patterns, such as multilayer staining of squamous epithelium, were noted. Staining was evaluated independently by 2 experienced investigators. In case of disagreement, a third investigator reviewed the slides to arrive at a consensus.
Data Analysis
We evaluated staining pattern in multiple biopsies from each patient for each biomarker. The expression for each patient was measured in the nondysplastic columnar epithelium prior to ablation. Biomarker expression was also measured subsequent to ablation in columnar epithelium areas at the squamocolumnar junctions in the setting of incomplete ablation. For each biomarker, the percentage of patients with increased expression was calculated.
RESULTS
The patients’ characteristics, including the time after the last procedure and number of MPEC or APC sessions, are shown in Table 1. There were 18 males and 3 females, 32 to 84 years of age included in the study. The lengths of BE prior to ablation were 1 to 9 cm (median, 4.23 cm). All patients had nondysplastic IM in surveillance sessions prior to ablation as required by the clinical study. Complete ablation was defined as absence of any BE in any biopsy using a standard protocol of four-quadrant biopsies at every 2 cm and in a biopsy of any suspicious area (including any region that looked grossly like it may be BE). In contrast, incomplete ablation refers to the presence of BE, which may be residual or recurrent, in the largely squamous epithelium generated after ablation. Incomplete ablation may be evident grossly as reddish areas or only under the microscope.
TABLE 1. Patient Characteristics
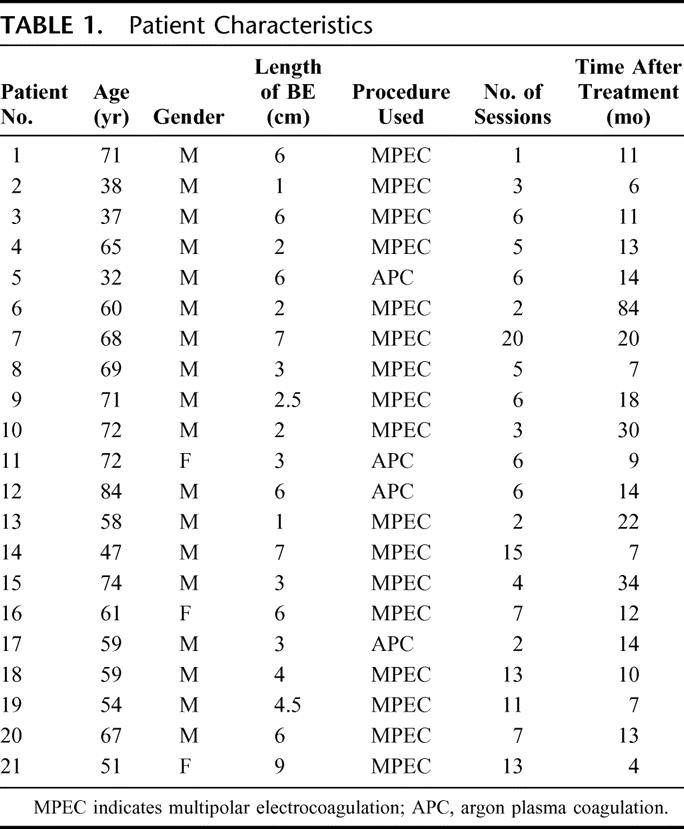
Ten of 22 patients (1–10) had no history of dysplasia ever being read in any surveillance biopsy. Eleven patients (11–21) were diagnosed once with low-grade dysplasia (LGD) in one previous session, with all others being ND in our thorough surveillance protocol. The majority of patients20 were evaluated at 6 to 84 months after treatment. Only one patient was evaluated at a shorter time period (4 months) after treatment.
As expected, the nondysplastic preablation columnar epithelium showed normal staining patterns of p53, COX-2, and Ki-67, ie, negative or mild staining for p53 and COX-2 together with a low proliferation rate in the majority of patients’ biopsies. The columnar epithelium showed positive cells for Ki-67 in the proliferative zone of BE crypts only; however, several fields were essentially negative with overall staining <5%.
In contrast, elevated staining for p53, COX-2, and Ki-67 was detected in columnar epithelium areas at the junctions between columnar and neo-squamous epithelium in the setting of incomplete ablation. The results of the study are summarized in Tables 2 and 3. Expression of Ki-67 was increased in 13 of 21 patients (62%) in columnar epithelium at the new junction. A markedly abnormal staining pattern with Ki-67 occurred postablation in the columnar glands with virtually every cell being positive in some glands (Fig. 1B). Additionally, intense multilayered staining of Ki-67 was observed in the adjacent squamous epithelium in all patients evaluated (Fig. 1B). Expression of COX-2 in the interstitium in the columnar epithelium at squamocolumnar junctions was increased in 8 of 21 patients (38%, Fig. 1D). No staining of COX-2 was detected in the squamous epithelium at the junctional area. Increased staining of p53 was observed in postablation junctions in the columnar cells in 8 of 21 cases (38%) (Fig. 1F). Importantly, in 9 patients, increased expression of at least 2 of the 3 biomarkers in the columnar portion of new squamocolumnar junctions was detected, and in 3 patients all 3 biomarkers were abnormally expressed. Biomarker abnormalities were noted in patients with no history of any prior dysplasia reading as well as those with an occasional LGD biopsy in the past. Clinically, of course, these patients are essentially equivalent.
TABLE 2. Changes in Staining Pattern After Ablation in Columnar Epithelium at Squamocolumnar Junctions Compared With Columnar Epithelium Prior Ablation
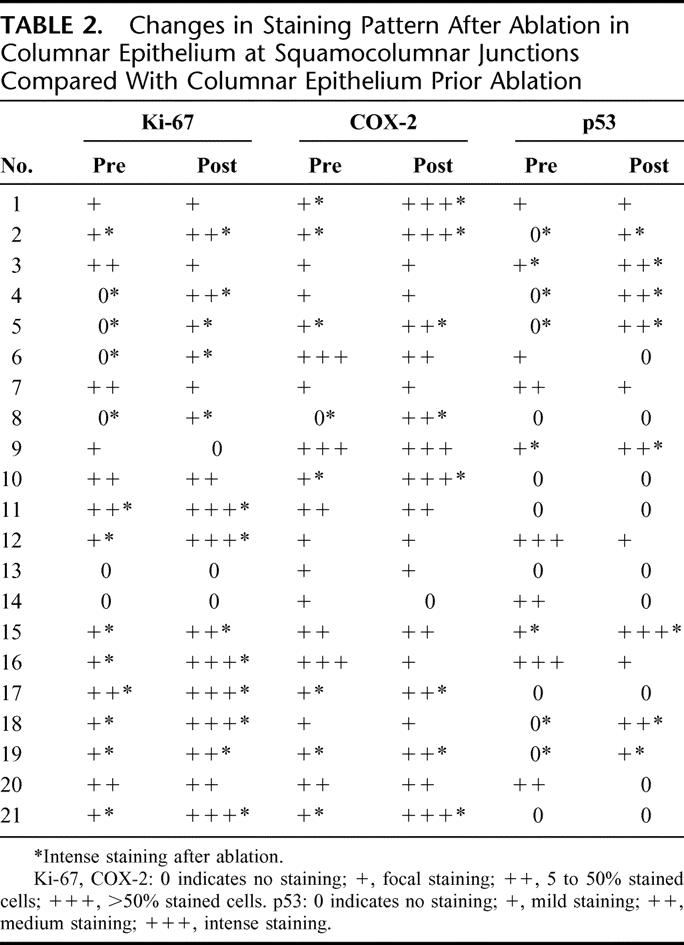
TABLE 3. Summary of Staining Pattern in Columnar Epithelium After Ablation at Squamocolumnar Junctions Compared With Columnar Epithelium Prior Ablation
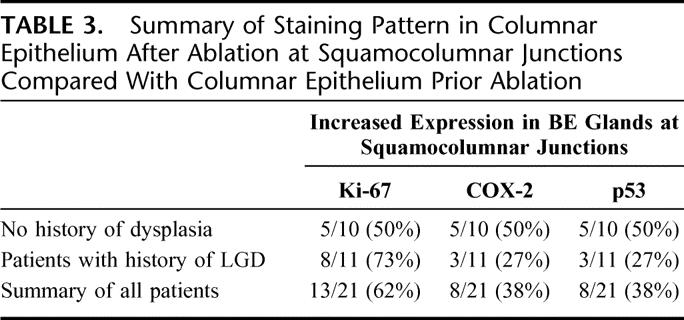
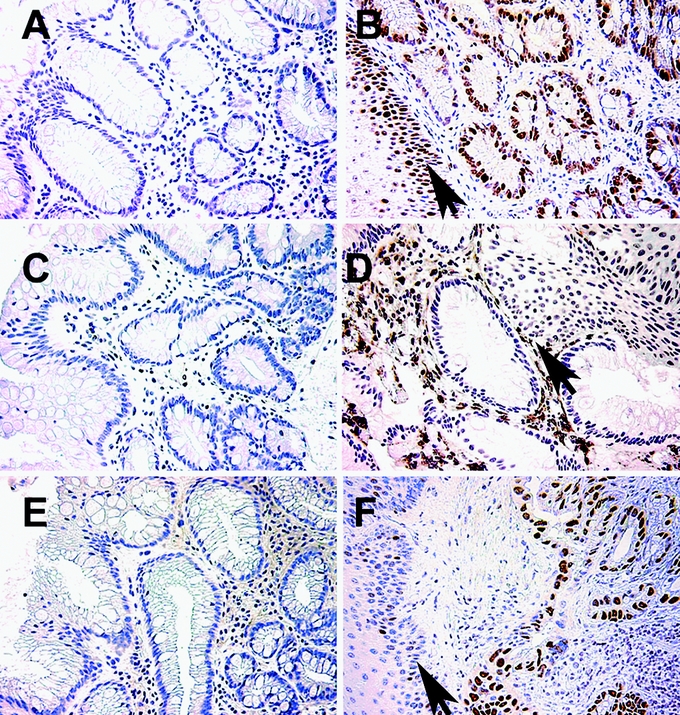
FIGURE 1. Expression patterns of Ki-67, COX-2, and p53 in BE patients prior and after partial ablation. Immunohistologic staining for Ki-67 (A, B), COX-2 (C, D), and p53 (E, F) of BE patient biopsies prior (A, C, E) and after MPEC ablation (B, D, F). Note the intense brown staining of tissues in the biopsies that were taken after ablation. Arrows indicate squamous epithelium.
DISCUSSION
BE is a well-known risk factor for the development of esophageal adenocarcinoma, which has become the predominant form of esophageal cancer in the United States and Western Europe.1,3,4 Recently, approaches have been developed to reverse Barrett’s IM to squamous epithelium.22 The methods incorporate reinjury along with acid suppression. Although increasingly used in practice, there is no evidence that these interventions actually reduce cancer risk in nondysplastic patients or those with LGD only. We have previously shown that complete ablation produces squamous epithelium that stains normally with a variety of biomarkers, but we had concerns regarding the partial ablation setting, based on our finding in so-called “squamous islands” often seen in patients being followed on PPI therapy.19 In this report, we describe the finding that incomplete ablation of BE by MPEC or APC in conjunction with PPI therapy results in abnormalities in the expression of biomarkers associated with cancer in the columnar epithelium at the new squamocolumnar junctions in a significant percentage of patients. Since partial ablation is very frequent, occurring in up to 30% to 50% of ablation therapy cases, these findings are important. This is especially true in the context of recent reports of cancers arising in these areas.10,14,15
Several patients who had undergone partial reversal showed increased expression of p53, COX-2, and/or the proliferation marker Ki-67 in biopsies taken after partial reversal compared with biopsies taken before ablation. The abnormalities were noted at the junctions of the new squamous epithelium and IM. These results raise concerns regarding the risk of neoplastic progression after incomplete ablation therapy.
p53 is a tumor suppressor gene, which activates the expression of genes that inhibit cell growth, repair DNA damage or induce apoptosis.23–25 Wild-type p53 is labile because of a high degradation rate, and is only expressed transiently. In contrast, mutated p53 has a longer half-life and can be detected by immunohistochemistry.23,24 p53 mutation and/or p53 protein accumulation are common in esophageal adenocarcinoma.26–28 In addition, it has been shown that there is a significant correlation between p53 expression, the degree of dysplasia in BE biopsies, and progression to adenocarcinoma.29–34 In the present study, we found increased p53 staining in the columnar glands at neo-squamocolumnar junctions in postablation samples in 8 of 21 cases. It is well recognized that positive p53 immunohistochemistry does not always correlate with the presence of p53 mutations.35,36 In this study, we did not perform p53 mutation analysis, and further studies are planned to assess whether the increased p53 staining is due to accumulation of mutated p53 or overexpression of wild-type p53.
Ki-67 antibody is used to detect proliferating cells in tissues since it binds a nuclear antigen expressed during the G1, S, G2, and M phase of the cell cycle but not in early G0.37,38 Increased cellular proliferation is thought to play an integral part in the progression of various tissues to cancer, including BE.37,39 Our findings indicate that, in partially reversed Barrett’s esophagus, the expression of Ki-67 is increased in the glandular tissue and squamous epithelium at the neo-squamous and IM interfaces. In contrast, the nondysplastic columnar epithelium before ablation showed a low proliferation rate in the majority of patients’ biopsies at the base of BE crypts, which corresponds to the naturally occurring proliferative zone. COX-2, a rate-limiting enzyme in prostaglandin synthesis, is an early-response gene that is induced rapidly following stimulation by growth factors or cytokines. Recent evidence has implicated COX-2 in colorectal, gastric, and esophageal carcinogenesis.40,41 COX-2 expression is increased in BE and correlates with the severity of dysplasia.41,42 Most of the staining is in the interstitial areas. Importantly, we found markedly increased COX-2 staining in the interstitial cells at the junctions of neo-squamous epithelium and IM in incompletely reversed BE patients. Since the abnormal staining patterns were noted in the patients’ biopsies at least 4 months postablation, these findings are not a reflection of wound healing. These data suggest that partially reversed BE is associated with increased proliferation, abnormal p53 expression, increased COX-2 expression, and possibly increased risk of neoplastic progression.
Krishnadath et al reported 3 BE cases with high-grade dysplasia and biomarker abnormalities at baseline; after incomplete ablation these abnormalities were found to persist in the residual IM.35 The crucial difference in our study, however, is the absence of abnormalities before ablation, as expected in nondysplastic BE, but increased expression of cancer-associated biomarkers after ablation. These changes were concentrated at the new squamocolumnar junctions. In other words, the patients in our study are essentially nondysplastic cases without abnormal biomarker patterns at baseline and at a very low cancer risk. After ablation therapy, areas of incomplete ablation are now associated with abnormal expression of biomarkers indicating instability, and possibly increase risk to develop esophageal adenocarcinoma.
We hypothesize that these findings are likely the result of a treatment strategy that results in unstable tissue types, especially at the new junctions. This could initiate a complex cascade of stress-inducible signaling molecules in an attempt to return the cells to their previous equilibrium. Further studies using other biomarkers, such as aneuploidy or p53 mutations, are clearly warranted to further characterize these junctions where cells with changing or mixed phenotypes are located.
The most direct approach to assess the effects of ablation on cancer risk would be a prospective, randomized, controlled trial comparing ablated cases with untreated controls. But this would be logistically and practically impossible in a group of patients with nondysplastic BE. Although the cancer incidence in BE patients is much greater than normal, cancer is still a relatively infrequent event with estimated rates of 0.5% per year. The number of cases, duration of follow-up, and the dropout of subjects either by choice or comorbidity are just a few of the factors that preclude such a trial. We have therefore used putative biomarkers to evaluate postablation epithelia to look for normal or abnormal expression. Complete ablation is defined as no visual BE and no residual presence of IM in a multiple biopsy protocol. However, it is recognized that, despite thoroughness, an area with IM may be missed. Similarly, incomplete ablation refers to the persistence of IM. It should be noted that, although this is often referred to as residual IM, it could very well represent recurrence of IM.
Although BE is associated with elevated risk for the development of esophageal adenocarcinoma, patients with nondysplastic BE are generally at very low risk to develop malignant disease. The data from this study suggest that partial reversal of BE lesions is associated with the creation of biomarker abnormalities in nondysplastic BE patients. Thus, such ablative procedures may not result in decreased risk for the development of esophageal cancer compared with nonablation in this group. Our findings raise serious concerns regarding ablation in nondysplastic BE patients, since there are no available means to determine, at the time of ablation, whether the procedure will be complete or incomplete. Thus, we suggest that ablation should be restricted to clinical trials except, perhaps, in patients with high-grade dysplasia, who are not candidates for esophageal resection. Patients with dysplastic disease who have undergone ablation should be regularly followed and carefully evaluated for BE recurrence after ablation. New techniques are being developed such as a balloon-based radiofrequency system to improve efficacy of ablation. Perhaps these new approaches will reduce the incidence of incomplete ablation.43
Therefore, at the very least, patients undergoing ablation need to be closely followed. Further studies are necessary to determine appropriate intervals and technique of follow-up after ablation. Since a prospective, randomized trial would be logistically impractical, we strongly advocate that a method for systemic follow-up of ablated patients be considered, eg, establishing a registry of patients who have undergone ablation.
Footnotes
Supported by NIH Grant Nos. CA72008 and CA23074, ADCRC Grant Nos. 10016 and 6002, NCI SPORE Grant No. 1 P50CA95060-01, and VA Merit Review Grant No. 0114.
Reprints: Harinder Garewal, MD, PhD, Tucson Veterans Affairs Medical Center, Section of Hematology/Oncology, Tucson, AZ 85723. E-mail: hgarewal@azcc.arizona.edu.
REFERENCES
- 1.Cameron AJ. Epidemiology of columnar-lined esophagus and adenocarcinoma. Gastroenterol Clin North Am. 1997;26:487–494. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett’s esophagus, and esophageal cancer: clinical applications. JAMA. 2002;287:1982–1986. [DOI] [PubMed] [Google Scholar]
- 3.Falk GW. Barrett’s's esophagus. Gastroenterology. 2002;122:1569–1591. [DOI] [PubMed] [Google Scholar]
- 4.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett’s's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–215. [PubMed] [Google Scholar]
- 5.Sarr MG, Hamilton SR, Marrone GC, et al. Barrett’s's esophagus: its prevalence and association with adenocarcinoma in patients with symptoms of gastroesophageal reflux. Am J Surg. 1985;149:187–193. [DOI] [PubMed] [Google Scholar]
- 6.Sampliner RE, Hixson LJ, Fennerty MB, et al. Regression of Barrett’s's esophagus by laser ablation in an antacid environment. Dig Dis Sci. 1993;38:365–368. [DOI] [PubMed] [Google Scholar]
- 7.Overholt BF. Acid suppression and reepithelialization after ablation of Barrett’s's esophagus. Dig Dis. 2000;18:232–239. [DOI] [PubMed] [Google Scholar]
- 8.Fennerty MB. Endoscopic ablation of Barrett’s's esophagus. Curr Gastroenterol Rep. 1999;1:210–213. [DOI] [PubMed] [Google Scholar]
- 9.Urosevic P, Kiroff GK. Ablation of Barrett’s's epithelium: the promise and the problems. Dis Esophagus. 2002;15:30–38. [DOI] [PubMed] [Google Scholar]
- 10.Walker SJ, Selvasekar CR, Birbeck N. Mucosal ablation in Barrett’s's esophagus. Dis Esophagus. 2002;15:22–29. [DOI] [PubMed] [Google Scholar]
- 11.Sampliner RE, Faigel D, Fennerty MB, et al. Effective and safe endoscopic reversal of nondysplastic Barrett’s's esophagus with thermal electrocoagulation combined with high-dose acid inhibition: a multicenter study. Gastrointest Endosc. 2001;53:554–558. [DOI] [PubMed] [Google Scholar]
- 12.Michopoulos S, Tsibouris P, Bouzakis H, et al. Complete regression of Barrett’s's esophagus with heat probe thermocoagulation: mid-term results. Gastrointest Endosc. 1999;50:165–172. [DOI] [PubMed] [Google Scholar]
- 13.Pacifico RJ, Wang KK. Role of mucosal ablative therapy in the treatment of the columnar-lined esophagus. Chest Surg Clin North Am. 2002;12:185–203. [DOI] [PubMed] [Google Scholar]
- 14.Bonavina L, Ceriani C, Carazzone A, et al. Endoscopic laser ablation of nondysplastic Barrett’s's epithelium: is it worthwhile? J Gastrointest Surg. 1999;3:194–199. [DOI] [PubMed] [Google Scholar]
- 15.Van Laethem JL, Cremer M, Peny MO, et al. Eradication of Barrett’s's mucosa with argon plasma coagulation and acid suppression: immediate and mid term results. Gut. 1998;43:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berenson MM, Johnson TD, Markowitz NR, et al. Restoration of squamous mucosa after ablation of Barrett’s's esophageal epithelium. Gastroenterology. 1993;104:1686–1691. [DOI] [PubMed] [Google Scholar]
- 17.Biddlestone LR, Barham CP, Wilkinson SP, et al. The histopathology of treated Barrett’s's esophagus: squamous reepithelialization after acid suppression and laser and photodynamic therapy. Am J Surg Pathol. 1998;22:239–245. [DOI] [PubMed] [Google Scholar]
- 18.Gross CP, Cruz-Correa M, Canto MI, et al. The adoption of ablation therapy for Barrett’s's esophagus: a cohort study of gastroenterologists. Am J Gastroenterol. 2002;97:279–286. [DOI] [PubMed] [Google Scholar]
- 19.Garewal H, Ramsey L, Sharma P, et al. Biomarker studies in reversed Barrett’s's esophagus. Am J Gastroenterol. 1999;94:2829–2833. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Weston AP, Cherian R, et al. Ablation of Barrett’s's esophagus using MPEC or APC: a randomized controlled trial. Am J Gastroenterol. 2002;97:533. [Google Scholar]
- 21.Bernstein C, Bernstein H, Garewal H, et al. A bile acid-induced apoptosis assay for colon cancer risk and associated quality control studies. Cancer Res. 1999;59:2353–2357. [PubMed] [Google Scholar]
- 22.DeMeester SR, DeMeester TR. The diagnosis and management of Barrett’s's esophagus. Adv Surg. 1999;33:29–68. [PubMed] [Google Scholar]
- 23.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. [DOI] [PubMed] [Google Scholar]
- 24.Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann NY Acad Sci 2000;910:121–137; discussion 137–129. [DOI] [PubMed]
- 25.Bernstein C, Bernstein H, Payne CM, et al. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002;511:145–178. [DOI] [PubMed] [Google Scholar]
- 26.Younes M, Lebovitz RM, Lechago LV, et al. p53 protein accumulation in Barrett’s's metaplasia, dysplasia, and carcinoma: a follow-up study. Gastroenterology. 1993;105:1637–1642. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald RC, Triadafilopoulos G. Recent developments in the molecular characterization of Barrett’s's esophagus. Dig Dis. 1998;16:63–80. [DOI] [PubMed] [Google Scholar]
- 28.Barrett’s MT, Sanchez CA, Prevo LJ, et al. Evolution of neoplastic cell lineages in Barrett’s oesophagus. Nat Genet. 1999;22:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnadath KK, Tilanus HW, van Blankenstein M, et al. Accumulation of p53 protein in normal, dysplastic, and neoplastic Barrett’s's oesophagus. J Pathol. 1995;175:175–180. [DOI] [PubMed] [Google Scholar]
- 30.Klump B, Hsieh CJ, Holzmann K, et al. Diagnostic significance of nuclear p53 expression in the surveillance of Barrett’s's esophagus: a longitudinal study. Z Gastroenterol. 1999;37:1005–1011. [PubMed] [Google Scholar]
- 31.Gimenez A, de Haro LM, Parrilla P, et al. Immunohistochemical detection of p53 protein could improve the management of some patients with Barrett’s esophagus and mild histologic alterations. Arch Pathol Lab Med. 1999;123:1260–1263. [DOI] [PubMed] [Google Scholar]
- 32.Gimenez A, Minguela A, Parrilla P, et al. Flow cytometric DNA analysis and p53 protein expression show a good correlation with histologic findings in patients with Barrett’s's esophagus. Cancer. 1998;83:641–651. [DOI] [PubMed] [Google Scholar]
- 33.Skacel M, Petras RE, Rybicki LA, et al. p53 expression in low grade dysplasia in Barrett’s's esophagus: correlation with interobserver agreement and disease progression. Am J Gastroenterol. 2002;97:2508–2513. [DOI] [PubMed] [Google Scholar]
- 34.Moskaluk CA, Heitmiller R, Zahurak M, et al. p53 and p21(WAF1/CIP1/SDI1) gene products in Barrett’s esophagus and adenocarcinoma of the esophagus and esophagogastric junction. Hum Pathol. 1996;27:1211–1220. [DOI] [PubMed] [Google Scholar]
- 35.Krishnadath KK, Wang KK, Taniguchi K, et al. Persistent genetic abnormalities in Barrett’s's esophagus after photodynamic therapy. Gastroenterology. 2000;119:624–630. [DOI] [PubMed] [Google Scholar]
- 36.Coggi G, Bosari S, Roncalli M, et al. p53 protein accumulation and p53 gene mutation in esophageal carcinoma: a molecular and immunohistochemical study with clinicopathologic correlations. Cancer. 1997;79:425–432. [DOI] [PubMed] [Google Scholar]
- 37.Polkowski W, van Lanschot JJ, Ten Kate FJ, et al. The value of p53 and Ki67 as markers for tumour progression in the Barrett’s's dysplasia-carcinoma sequence. Surg Oncol. 1995;4:163–171. [DOI] [PubMed] [Google Scholar]
- 38.Yu CC, Woods AL, Levison DA. The assessment of cellular proliferation by immunohistochemistry: a review of currently available methods and their applications. Histochem J. 1992;24:121–131. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto Y, Arai N, Mieno H, et al. Adenocarcinoma complicating Barrett’s's esophagus: an analysis of cell proliferation. J Gastroenterol. 2001;36:410–414. [DOI] [PubMed] [Google Scholar]
- 40.Kandil HM, Tanner G, Smalley W, et al. Cyclooxygenase-2 expression in Barrett’s's esophagus. Dig Dis Sci. 2001;46:785–789. [DOI] [PubMed] [Google Scholar]
- 41.Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s's metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2001;96:990–996. [DOI] [PubMed] [Google Scholar]
- 42.Wilson KT, Fu S, Ramanujam KS, et al. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]
- 43.Ganz RA, Utley DS, Stern RA, et al. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc. 2004;60:1002–1010. [DOI] [PubMed] [Google Scholar]


