Abstract
Objective:
To examine whether tumor-selective infiltration, activation, and cytotoxic activity of tumor infiltrating T lymphocytes (TIL) can be demonstrated in situ in colorectal cancer samples.
Summary Background Data:
Recent studies indicated a correlation between the presence of TIL and an improved prognosis in colorectal cancer. However, tumor-selective activation and cytotoxic activity of CD8+ TIL in situ in colorectal cancer patients have not yet been examined.
Methods:
Tumor samples from 49 patients and corresponding normal mucosa samples from 23 patients with colorectal cancer (UICC stages II–IV) were examined for TIL. Two-color fluorescence immunohistochemistry and multicolor flowcytometric (FACS) analysis were used for quantification of CD8+ T cells and measurement of their activation status (CD69-expression) and cytotoxic activity (CD107a-expression) in situ. Presence of tumor antigen-reactive T cells in tumor, blood, and bone marrow was evaluated by IFN-γ Elispot analysis.
Results:
While absolute numbers of CD8+ T cells were similar, CD4+ T helper cells were significantly increased in tumor tissue compared with normal mucosa. There was a significantly higher proportion of activated and cytotoxically active CD8+ TIL in colorectal cancer compared with normal mucosa. Increased activation, cytotoxic activity, and functional reactivity of TIL were correlated with the presence of functional tumor antigen-reactive T cells in the blood and bone marrow. The proportion of activated TIL decreased significantly with higher tumor stage.
Conclusions:
Tumor-selective activation and cytotoxic activity of CD8+ TIL and tumor-selective migration of CD4+ T helper cells were demonstrated in colorectal cancer for the first time. Our data support the immunogenicity of colorectal cancer and suggest clinical significance of tumor-specific immune responses.
This study examines the activation status of cytotoxic tumor infiltrating T lymphocytes (TIL) in colorectal cancer patients and demonstrates for the first time tumor-selective activation and cytotoxicity in situ. Correlation of the activation status of TIL with systemic tumor antigen-specific T-cell immunity and with tumor stage suggests a potential clinical significance.
Colorectal cancer belongs to the most common malignancies in the western world. The treatment of choice remains surgical resection, which provides cure in a considerable number of patients.1 Nevertheless, additional treatment options besides standard therapies (including surgery, radiotherapy, and chemotherapy) seem warranted to further improve survival of patients with colorectal cancer.2 New approaches include immunotherapeutic strategies as there is growing evidence in recent years supporting the existence of cancer immunosurveillance.3 However, immune suppression has also been demonstrated in cancer patients and in tumor-bearing animal models as well.3
The potential influence of immune-cell infiltrates in various neoplasms, including colorectal cancer, on the prognosis of patients is debated controversially.4 While the mere presence of tumor-specific CD8+ T cells (cytotoxic T cells) in the peripheral blood was not correlated with improved clinical outcome, several studies indicated a correlation between the numbers of tumor infiltrating CD8+ T lymphocytes (TILs) and an improved prognosis in colorectal cancer.5–8 A recently published study by Pagès et al confirmed that the presence of high levels of tumor infiltrating memory T cells within colorectal cancer tissue is associated with the absence of pathologic evidence of early metastatic invasion of the tumor and with prolonged survival.9 However, it is still unclear if an improved prognosis is directly related to CD8+ TIL-mediated tumor cell lysis in situ or rather an epiphenomenon of favorable pathomorphologic features of these tumors.
CD69 is a cell surface antigen that is expressed on T cells following activation.10 During the process of target cell killing, CD107a, a vesicle membrane protein, is transiently mobilized to the cell surface of effector CD8+ cells. A study by Rubio et al demonstrated that the identification of T cells with CD107a (CD107am) expression on their plasma membrane is a reliable test for T cells, which have exerted cytotoxic activity against tumor cells.11
The aim of this study was to examine tumor-selective infiltration, activation (CD69) and cytotoxic activity (CD107a) of CD8+ T cells in situ within colorectal cancer tissue of patients in different UICC stages.
MATERIALS AND METHODS
Patients
Tissue samples from 49 patients (33 male, 16 female; median age, 68 years) with colorectal cancer (UICC stages II–IV) undergoing curative or palliative resection of the primary tumor at the Department of Surgery at the University of Heidelberg were included in this study. Informed consent was obtained from all patients; the study protocol was approved by the ethics committee of the University of Heidelberg.
According to the UICC tumor classification, 20 patients were in stage II, 13 patients in stage III, and 16 patients in stage IV. In 23 patients, corresponding tissue samples from cancer and normal mucosa were obtained. In 11 patients, blood and bone marrow samples were additionally taken during the operation. Patients with other malignant diseases in their medical history and patients with neoadjuvant therapy were not included in this study. Diagnosis of colorectal adenocarcinoma was confirmed by postoperative histopathologic examination of the specimen. Tumor stage and grading were classified according to the 6th edition of the TNM Classification of the International Union Against Cancer.12
Tissue Samples
Tissue samples were taken from the operation specimen immediately after surgical removal. Tumor samples were biopsied from the exophytic component, avoiding grossly necrotic areas, and mucosal tissue was sampled at a distance of at least 10 cm proximal to the cancer (distal in tumors of the proximal cecum). All tissues for immunofluorescence analysis were immediately stored in cryotubes, shock frozen in liquid nitrogen, and stored at −80°C. Fresh tissue samples were transferred into phosphate-buffered saline (PBS) and used for FACS-analysis or IFN-γ Elispot-assays.
Immunofluorescence
A double-staining indirect immunofluorescence technique was used for analysis of T cell coreceptors (CD8, CD4) and T cell markers for early activation (CD69) and cytolytic activity (CD107a membrane expression; CD107am) of tumor infiltrating lymphocytes. Briefly, consecutive cryostat tissue sections (5 μm) were mounted on glass slides and fixed in cold acetone (−20°C) for 5 minutes. After blocking with normal chicken serum, staining with primary antibodies was performed. Sections were incubated with rabbit anti-human antibody CD8 (clone H-149; Santa Cruz Biotechnology) and one of the following mouse antihuman antibodies: CD4 (clone 18–46), CD69 (clone FN50), pancytokeratin (clone C11), (all Santa Cruz); and CD107a (clone H4A3; BD Pharmingen, Heidelberg, Germany). After washing steps with PBS, slides were incubated with the corresponding secondary fluorophore-labeled antibody: Alexa Fluor 488 chicken anti-mouse and Alexa Fluor 594 chicken anti-rabbit (Molecular Probes, Leiden, The Netherlands). Visualization of nuclei was performed with 4′,6-diamidin-2-phenyl-indol solution. Autofluorescence was blocked with NH4ACCuSO4 solution.
Microscopic analysis was done separately by 2 independent observers, who had no knowledge of the clinical outcome of the patients. The evaluation of stained sections and count of positive cells was done with a fluorescence microscope (Axioplan 2; Zeiss, Jena, Germany) and with imaging software (Axio Vision 3.0; Zeiss). The exact size of tissue areas on the slides were measured by a scanning program (Analysis; Zeiss).
Multicolor Flow Cytometry
For preparation of single cell suspensions, tumor and corresponding normal mucosa tissue samples were mechanically dissected and washed with PBS. The cell suspension was passed through a sterile 40-μm Nylon Filter (BD Falcon, Heidelberg, Germany) and a single cell suspension was obtained. Cells were pelleted and suspended in FACS buffer. After blocking with human IgG (Venimmun-N, Aventis, Germany), cells were stained with the following fluorophore-conjugated mouse monoclonal antibodies (mAbs) against human antigens: FITC-CD4 and PE-CD4 (clone RPA-T4), FITC-CD8 and PE-CD8 (clone HIT8a), PE-CD69 (clone FN50), and PE-CD107a, and FITC-CD107a (clone H4A3) from BD Pharmingen; and FITC-CD3 and PE-CD3 (clone SK7) from BD Biosciences, Heidelberg, Germany. Propidium iodide-negative (PI-negative) viable cells were gated and then analyzed by multicolor flow cytometry using a FACSCalibur with Cell-Quest software (BD Biosciences). FlowJo software (Tree Star, San Carlon, CA) was used to analyze 20,000 events. Data were expressed as dot plots or histograms.
Generation of Dendritic Cells and T Cells
EDTA-buffered peripheral blood and bone marrow samples were obtained during operation as previously described.13 Mononuclear cell populations (MNCs) were separated from peripheral blood and bone marrow samples over a Ficoll gradient (Amersham Pharmacia, Uppsala, Sweden). Dendritic cells (DCs) were generated from bone marrow precursors in the presence of 50 ng/mL GM-CSF (Behringwerke, Marburg, Germany) and 1000 U/mL IL-4 (PromoCell, Heidelberg, Germany) in X-Vivo 20 serum-free medium as previously described.14 DCs were enriched after 14 days by depletion of contaminating cells using magnetic beads labeled with mAbs against CD3, CD19, and CD56 (Dynal, Oslo, Norway). Peripheral blood monocyte-derived DCs were generated by isolation of the adherent cell fraction after 7 day culture using the same culture and cytokine medium as for BM. To obtain T lymphocytes, bone marrow and peripheral blood MNCs were incubated in RPMI-1640 medium (PAA Laboratories, Pasching, Austria) supplemented with 10% AB Serum, IL-4 60 U/mL (PromoCell) and IL-2 100 U/mL (Chiron, Ratingen, Germany) for 7 and 14 days, respectively. T lymphocytes were isolated after depletion of B cells, NK cells, and myeloid precursors with magnetic beads (Dynal Negative Isolation Kit) as recommended by manufacturer. In some patients, CD8+ tumor infiltrating lymphocytes (TILs) were isolated with MACS microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) after obtaining a single cell suspension from the tumor. Prior to use in functional assays, T cells were cultured for 24 hours in cytokine-free medium.
ELISPOT Assays
To determine systemic tumor-reactive T cell immunity, we isolated T cells from peripheral blood and bone marrow (as an organ particularly relevant for the induction and accumulation of tumor antigen-reactive memory T cells in various cancers15–17) and stimulated them with tumor antigen presenting DCs. As source of tumor antigen we used either autologous tumor cell lysates or defined synthetic polypeptides of the tumor associated tumor antigen mucin1 (MUC1), which is expressed by the majority of colorectal cancers.18 Briefly, DCs were pulsed for 20 hours with autologous tumor cell lysate (obtained after 5 freeze/thaw cycles15) or synthetic polypeptides (aa1-100ss and aa(137–157)5tr)17 derived from MUC1 at a concentration of 200 μg protein/mL/106 T cells. Antigen-pulsed DCs were coincubated for 40 hours with autologous T cells or TIL (DC:TC/TIL ratio = 1:5) in IP-Elispot plates (Millipore, Moselheim, France) coated with mAbs against IFN-γ (clone 1-D1K; Mabtech, Sweden) and analyzed for corresponding cytokine secretion according to the manufacturer's protocol. Spot-forming cells were quantified using KS ELISPOT reader (Zeiss) and CTL ImmunoSpot Analyzer and ImmunoSpot software (Cellular Technology, Cleveland, OH). Spots measured in the presence of DCs pulsed with autologous PBMC-lysate (as control for autologous tumor cell lysate) or human immunoglobulin (Endobulin,17 as control for synthetic MUC1-polypeptides) were considered as nonspecific background (negative control). Three wells per test group were quantified. Test samples were considered to contain tumor-reactive T cells when spot numbers in test triplicates significantly exceeded spot numbers in negative control triplicates (P < 0.05).
Statistics
Statistical computations were done using the software package JMP (JMP, Cary, NC) and SPSS (SPSS, Chicago, IL). IFN-γ Elispot-assays were evaluated using 2-sided Student t test. Continuous variables were compared using the Wilcoxon Test.
RESULTS
T-Cell Infiltration, Activation, and Functional Activity in Colorectal Carcinoma
TIL were examined in fresh tissue samples from 49 patients with colorectal cancer (UICC stages II–IV). In 23 patients, corresponding tissue samples from normal mucosa were additionally examined. Two color-fluorescence-immunohistochemistry and multicolor flowcytometry were used for quantification of CD8+ T cells, their expression of the early activation marker CD69 and of their exerted cytotoxic effector activity in situ by use of a new marker of cytotoxic activity, CD107a.
We detected a mean value of 2.1 ± 4.9 CD8+ tumor infiltrating T cells/mm2 and 2.6 ± 2.2 CD8+ mucosa infiltrating T cells/mm2 in tumor tissue and normal mucosa, respectively (P = not significant) (Fig. 1A). Immunohistochemistry revealed a strong and significant enrichment of CD4+ T helper cells in tumor specimens compared with corresponding normal mucosa resulting in decreased proportions of cytotoxic CD8+ T cells among total T cells in tumor tissue (median value, 3.3%; interquartile range (IQR), 1.4–12.8) compared with normal mucosa (median value, 18.1%; IQR, 12.3–41) (P = 0.0001) (Fig. 1B). The same results were obtained by flowcytometric analysis (P = 0.01, data not shown). Tumor infiltrating CD8+ T cells showed a significant higher proportion of early activated T cells (median value, 12.5%; IQR, 5–25) as demonstrated by their expression of CD69 (Fig. 2A) compared with their counterparts in normal mucosa (3.3%; IQR, 2.3–8.3) (P = 0.002).
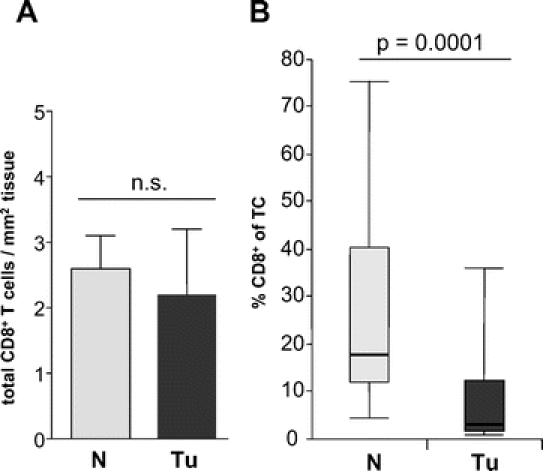
FIGURE 1. CD8+ T cell infiltration in 23 colorectal carcinomas (Tu) and 23 normal mucosa samples (N). A, Mean ± SEM CD8+ T cell counts per mm2 tumor tissue or normal mucosa. n.s., no significant difference between groups. B, Box and whisker plots of the percentage of CD8+ T cells among total (CD4+ and CD8+) T cells (TC) as determined by 2-color fluorescence immunohistology. Upper and lower endpoints of the whiskers represent the maximum and minimum percentages. The upper and lower edges of the boxes represent the 75th and 25th percentiles. The line inside the box represents the median.
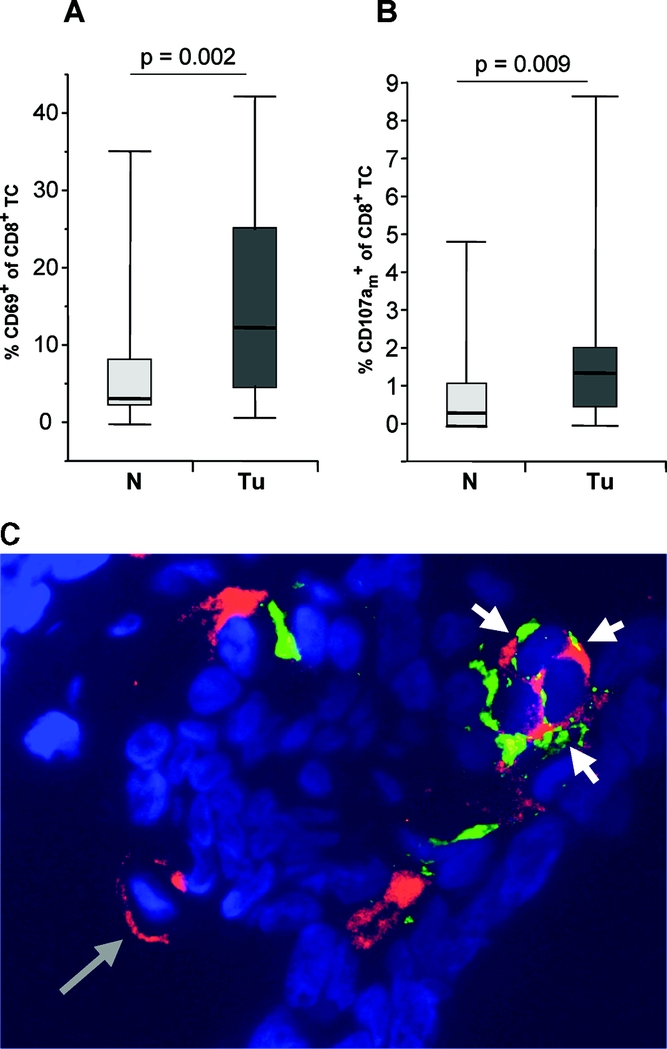
FIGURE 2. Selective activation and cytotoxic activity of tumor infiltrating CD8 T cells. Box and whisker plots of the percentage of the proportions of CD69+ (A) and CD107am+ (B) cells among CD8+ T cells analyzed in 23 colorectal carcinomas (Tu) and 23 normal mucosa samples (N) of corresponding patients using 2-color fluorescence immunohistology (A) or multicolor flow cytometry (B). Upper and lower end points of the whiskers represent the maximum and minimum percentages. The upper and lower edges of the boxes represent the 75th and 25th percentiles. The line inside the box represents the median. (a similar difference was detected by 2-color immunohistology of the same tissues; data not shown) C, One representative histologic tumor section stained for CD8 (red) and CD107a (green). Nuclei are counterstained in blue color. A single CD8+ T cell is depicted by a long gray arrow. Cytotoxically active CD8+ T cells with membranous expression of CD107am are depicted by short white arrows. Original magnification ×200.
We hypothesized that such selective activation of cytotoxic T cells in tumor tissue was due to specific recognition of antigens on tumor cells which might result in their increased cytotoxic activity in situ. To assess such functional activity, we evaluated the proportion of CD8+ T cells that carried the vesicle membrane protein CD107a on their outer cell membrane (CD107am+) as a reliable marker of recent degranulation (ie, cytotoxic activity).11 As shown in Figure 2B and C, we detected a low, but significantly higher proportion of CD107am+ CD8+ T cells in tumor tissue (median value, 1.38%; IQR, 0.5–2.0) compared with corresponding normal mucosa (median value, 0.3%; IQR, 0–1.1) of the same patients (P = 0.009).
Accumulation of Activated Cytotoxic CD8+ TILs in Patients With Systemic Tumor-Specific T-Cell Immunity
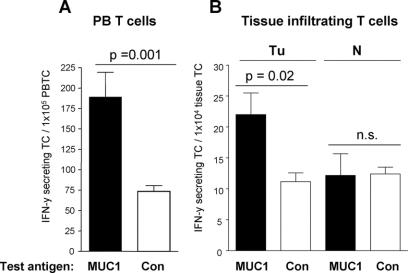
We speculated if the presence of tumor antigen reactive T cells in the systemic circulation or in lymphoid organs is related to the accumulation, activation and functional activity of tumor infiltrating CD8+ T cells in colorectal cancer tissue. Using IFN-γ Elispot analysis, we detected functional tumor antigen-reactive T cells in the blood and/or bone marrow of 5 of 11 tested patients (Fig. 3A). In 6 patients, we also purified TIL and tested them for reactivity against MUC1 by IFN-γ Elispot-analysis. Functional antigen-reactive TIL were found in 3 of 6 patients. As shown in Figure 3B, functional tumor specific T cells were selectively enriched in tumor tissue compared with normal mucosa.
FIGURE 3. MUC1-reactive T cells in the blood and tumor tissue of colorectal carcinoma patients; results of one representative patient. Numbers of T cells from peripheral blood (PBTC, A), tumor tissue (Tu, B), or corresponding normal mucosa (N, B) secreting IFN-γ in response to short-term stimulation with tumor associated test antigen MUC1 (black bars) as measured by Elispot assay. IFN-γ spots measured in the presence of irrelevant control antigen (Con, white bars) are considered as non specific background. P, significant difference between triplicate wells containing test antigen or control antigen. n.s., no significant difference between groups.
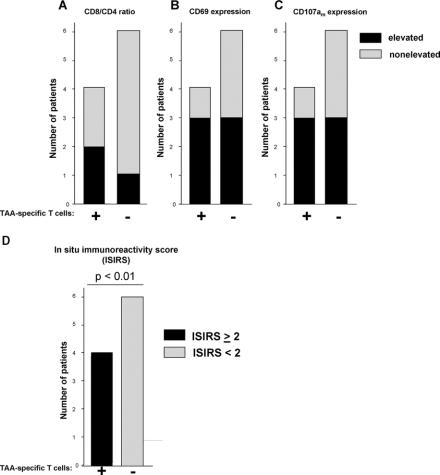
A comparison of the presence of systemic tumor reactive T cells with the immunohistologic evaluation of tumor infiltrating CD8+ T cells revealed increased CD8/CD4 ratios and increased proportions of activated (CD69+) and functionally active (CD107am+) CD8+ T cells in those patients who contained tumor antigen-reactive T cells in their blood or bone marrow (Fig. 4A–C). As surrogate parameter of in situ CD8+ T cell immune reactivity, we established a score (in situ immunoreactivity score; ISIRS) resulting from the addition of values being elevated (1) or not elevated (0) above the mean of the total patient population. The score included CD8/CD4 ratio of TIL, proportion of CD69+ among CD8+ TIL and proportion of CD107am+ among CD8+ TIL. Interestingly, all patients who contained systemic tumor antigen-reactive T cells showed an increase of at least 2 of these 3 parameters of CD8+ T cell infiltration (ISIRS of ≥2) while all Elispot-negative patients had ISIRS scores below 2 (Fig. 4D).
FIGURE 4. Enhanced reactivity of CD8+ tumor infiltrating lymphocytes (TIL) in patients with systemic tumor antigen specific immunity. Ten patients were evaluated for presence of tumor antigen-reactive (TAA) T cells in their bone marrow or peripheral blood and stratified into 2 groups of patients containing (+, n = 4) or lacking (−, n = 6) TAA-specific T cells. For each group, the number of patients with elevated numbers of CD8+ TIL compared with the mean values in the total population is shown in black with regard to the proportions of CD8+ among total TIL (A), CD69+ among CD8+ (B), and CD107am+ among CD8+ (C). The number of patients with nonelevated proportions are shown in gray. D, All patients were assessed according to a combined score for in situ immunoreactivity (ISIRS) of TIL. The score results from the addition of values ascribed to the presence (1) or lack (0) of parameters elevated above the mean of the total patient population. Three parameters were assessed, including CD8/CD4 ratio, proportion of CD69+ among CD8+ and proportion of CD107am+ among CD8+. All patients with systemic tumor-specific immunity presented with ISIRS ≥2 (black), while all patients lacking tumor-specific immunity presented with ISIRS <2 (gray). P, significant difference between patients containing or lacking tumor reactive T cells in PB or BM.
Correlation of CD8 T-Cell Activation In Situ With Disease Stage
We correlated activation parameters of CD8+ TIL with the disease stage of all 49 colorectal cancer patients to further clarify their clinical relevance. The proportion of activated CD69+among CD8+ TIL decreased significantly with higher tumor stage: 25.5% (IQR,13.8–32) in stage II, 17% (IQR, 3.5–29) in stage III, and 9.5% (IQR, 4.3–19.5) in stage IV (Fig. 5). Absolute CD8+ TIL numbers and their proportion of cytolytically active T cells were not correlated with tumor stage (CD107am+/CD8+ ratio, stage II: 25%, IQR, 21–40; stage III: 34%, IQR, 21–51; stage IV: 21%, IQR, 17–49; P = 0.5, data not shown).
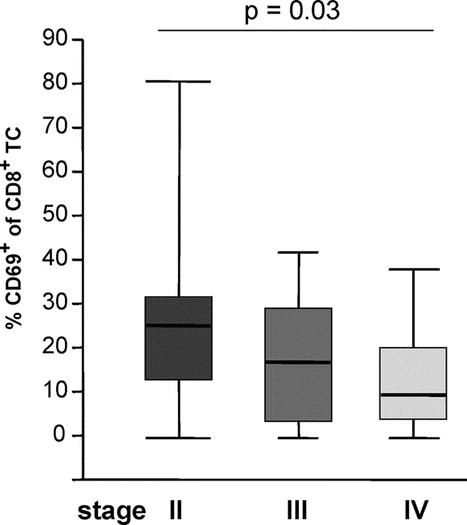
FIGURE 5. Decreased activation status of CD8 TIL in advanced tumor stages. Box and whisker plots of the percentage of CD69+ cells among CD8+ T cells analyzed in 49 colorectal carcinomas according to tumor stages II, III, and IV using 2-color fluorescence immunohistology. Upper and lower endpoints of the whiskers represent the maximum and minimum percentages. The upper and lower edges of the boxes represent the 75th and 25th percentiles. The line inside the box represents the median.
DISCUSSION
This study for the first time shows tumor-selective activation and cytotoxic activity in situ of tumor infiltrating CD8+ T cells in colorectal cancer. Furthermore, the data demonstrate tumor tissue-selective infiltration of CD4+ T helper cells in colorectal cancer. This enhancement of CD4+ T cells might be partly explained by the fact that CD4+ T-cell help is required during the primary antigen-specific response to imprint CD8+ T cells with the ability to develop into long-living functional memory cells.19 Such requirement of direct T-cell help at the site of CD8 effector function has previously been demonstrated in a mouse lymphoma model and a recent study confirmed the prognostic value of tumor infiltrating CD4+ T cells in head and neck cancer.20,21 However, further studies are needed to better characterize the function and phenotype of tumor infiltrating CD4+ T cells in colorectal cancer.
Only few studies have examined the activation status of TIL in colorectal cancer patients, but these studies failed to demonstrate tumor-specific activation of cytotoxic CD8+ TIL.8,22,23 Since we compared cancer and normal mucosa samples from the same patient, we were able to demonstrate significantly enhanced activity (CD69+) and cytotoxicity (CD107am+) of CD8+ T cells in tumor tissue indicating a tumor-selective immunologic phenomenon. On the other hand, cytotoxic T cells from normal mucosa also expressed activation markers in our study and, therefore, the tumor specificity of TIL may be questioned. We assume that the activated CD8+ T cells in normal mucosa represent memory T cells with various antigen specificity homing to the gut mucosa.
Recent data have expanded the concept that inflammation is a critical component of tumor progression.24 Additionally, many cancers arise from sites of infection, chronic irritation, and inflammation. Therefore, it is still unclear if the presence of TIL may represent the result of an inflammatory response that facilitates either tumor progression or a protective host response against cancer cells. We prefer the latter hypothesis as our study demonstrates a significant correlation between early tumor stages and enhanced activation of TIL. There might be a protective local immune response in colorectal cancer (especially in earlier tumor stages), preventing further tumor growth and spread. However, this hypothesis has to be substantiated by further analysis of activation status of TIL in patients with early tumor stages as in our analysis data from patients with UICC stage I were not included. Alternatively, host immune response against cancer cells may decrease with increasing tumor growth. Pagès et al could demonstrate that T cell responses within colorectal cancer are significantly associated with the absence of early metastatic invasion (eg, vascular emboli, lymphatic invasion, and perineural invasion) in the tumor and result in a significantly prolonged survival of the patients.9 However, a clear correlation between activation, functional capacity of TIL in situ, and improved survival cannot be drawn from the study. Additionally, previous studies showed a significant correlation between the number of tumor infiltrating CD8+ T cells and prognosis suggesting a clinical significance of the local host antitumor response in colorectal cancer.6
Several studies correlated the presence of cytotoxic TIL in colorectal cancer with microsatellite instability status of tumors as microsatellite instable tumors may be more immunogenic than microsatellite stable tumors.8,25–27 However, the question remains whether the improved prognosis in colorectal cancer is directly associated with specific activity of TIL against tumor cells in situ. Our results suggest the ability of colorectal cancer cells to recruit specific tumor antigen-reactive T cells from the circulation and to activate them. This conclusion differs from other published data.28 We detected TILs responding to MUC1, a characteristic colorectal cancer antigen in ELISPOT assays. Interestingly, such TILs were detected in patients with preexisting systemic anti tumor immunity. Spontaneous systemic anti-tumor immunity in blood and bone marrow has already been demonstrated for several malignancies, eg, pancreatic and breast cancer.15,17 Nagorsen et al found a natural T-cell response against specific tumor-associated antigens in part of colorectal cancer patients.29 This is in accordance with our results (5 of 11 responders in blood and/or bone marrow). However, there are also patients who did not display systemic antitumor T-cell immunity. This observation may be explained by immune escape or immune deviation mechanisms or by regulatory T cells suppressing host anti-tumor T-cell response.30
CONCLUSION
This study, for the first time, shows a functional reactivity of tumor-infiltrating T cells against tumor antigens in colorectal cancer patients. It was also, for the first time, demonstrated that there is tumor-specific activation and cytotoxic activity of CD8+ TIL and tumor-selective migration of CD4+ T helper cells in colorectal cancer. Since activated CD8+ TILs are detected at a significantly higher proportion in early tumor stages, our data support the immunogenicity of early stage colorectal cancer. One potential drawback of recent immunotherapeutic trials may be the fact that only patients with advanced tumor stages were included. Therefore, further studies regarding clinical significance of adjuvant immunotherapy in colorectal cancer should be performed, even in patients with earlier tumor stages to adequately investigate the potential implication of adjuvant immunotherapeutic strategies.
Discussions
Dr. Kaspar Z'graggen: Ladies and gentleman, I would like to thank the organization for giving me the opportunity to comment. I have seen the manuscript previously, and I do not think that I would have been able to make sensible comments without it. It is very complex technology, and you need a powerful collaboration with an immunology laboratory to do this kind of study. Dr. Weitz and his group have undertaken a beautiful study.
Now, tumor immunology has been a field for basic scientists over at least the last 15 years, and it was, until recently, considered not to be very important. However, it's now time to seriously consider these papers because immunotherapy is implemented rapidly into clinical practice and will also define what we do as surgeons in oncology over the next few years.
Immunotherapy and chemotherapy have become standard treatments over the last few years, and the next wave of tumor immunology findings will lead us to more specific therapies. Of course, colorectal cancer is one of the most prevalent cancers and one of the important fields of development.
I do have 3 questions which are relatively short.
Firstly, the absolute T-cell number increases within normal mucosa. The number of CD8 cells is stable compared with normal mucosa. Do you have any data on other cell populations and on their significance?
Secondly, does cytotoxic cell function relate to other clinically significant tissue factors? You have demonstrated a correlation with stage, but is there a correlation with important factors such as tumor grading, lymph vessel invasion, and vascular invasion? Does a correlation exist between these factors, which we currently work with and tumor immunology in the local tumor?
The third question regards systemic immunity. You have investigated systemic immunity by analysis of blood and bone marrow, which is a relatively new concept. There is good evidence that blood and bone marrow are important players in tumor response. How do you explain that, in some patients, systemic immunity cannot be demonstrated? In particular, since you use either specific antigens or tumor lysate to pulse the dendritic cells?
Dr. Juergen Weitz: Thank you, Dr. Z'graggen, for these excellent questions. I will answer your second question first because the answer is the shortest.
Correlation of the cytotoxic T-cell function to other factors besides the TNM stage: We do not have this data yet, but you are right: this is important, especially after the paper in the New England Journal of Medicine last year showing a correlation of CD8 T memory cells to these factors. We did not do this analysis so I cannot comment on this; however, this is an important point that we will have to address in the future.
Now, to your first question regarding other cells besides the CD8 fraction of TILs. Most of the TILs are CD4 cells. The question is: what do those CD4 cells, which are T helper cells do in the tumor tissue? As you know, the CD helper cells can be divided into 3 fractions, and I think the numbers 1 and 3 are important here. The number 1 helper cells are important for the cytotoxic CD8 cells to actually kill the tumor cells so, if these cells are in the tumor, this would be good because they would help. Actually, the CD8 cells exert the cytotoxic function. Unfortunately, there is one study showing that patients who have high CD4 numbers have a worse prognosis so this may not be the full story. So what does it mean? I talked about the 3 different fractions of T helper cells. The T helper cells 3 are actually regulatory T cells, so they are characterized by being CD4, CD25, FoxP 3 positive. So these are regulatory T cells and they are actually immunosuppressant. The functional and quantitative balance between both cell types may determine the efficiency of antitumor responses in situ. I personally think the tumor actually works against the immune system to somehow suppress the answer. This is a hypothesis that needs to be addressed in further experiments. So that's my answer to your first question.
Now, the last question regarding the systemic immunity in these patients. That's a very difficult question to answer because, until now, we always thought that the battle between the immune system and the tumor actually takes place in the tumor. As you might be aware, the group of Philip Beckhove, who is an author of this paper, has worked extensively on breast cancer, and they could show that this is not the case. In fact, the bone marrow is one site where the memory T cells are primed. This might also be the case for colorectal cancer. This is speculation that might or might not be true. Certainly, reasons that determine the induction or absence of antitumor responses have not been characterized enough. It will be a highly interesting and important challenge to learn to understand this complex topic in the future.
Dr. Gerald O'Sullivan: First of all, thank you for bringing this to the Association. I enjoyed your paper very much. I think this area is important and I am very reluctant to criticize a study that has, as coauthors, such a galaxy of the superstars of the tumor immune fraternity.
I think you confirm an observation that has been made in animal studies, that you get progression of the primary tumor yet there is coexistence of a concomitant immunity which controls the progression of micrometastases to metastases. This has already been shown in a number of studies in colorectal cancer, eg, the Jass immune infiltrate is of prognostic significance and our own work that showed disappearance of micrometastases in the immune favorable group and a paper more recently in the New England Journal of Medicine, which also demonstrated that the presence of CD8 memory T cells are of prognostic significance.
You have partly answered the question that I was going to put to you, which was: why did you not look within the CD4 population for the Fox P3 gene, the CD25 positive cells (T Reg Cells)? In animal studies, it has been clearly shown that tumors, as they grow, particularly as the bulk of the tumor increases, seem to accumulate selectively T reg cells, and this explains the disparity between the presence of an active systemic immune response and the failure of that response locally which allows the tumors to progress.
The last question I wanted to ask is: can you speculate why, in some people, we do not see an immunologic reaction to the tumor, as you have shown, and is there anything that we could do to create one because, clearly, it would be of prognostic importance? Thank you very much.
Dr. Juergen Weitz: Thank you for your important questions.
Coming to your first question, I have to repeat what I have just said. We are just beginning to understand the role of regulatory T cells in the tumor and I do not think this is a static system; it's a constant battle. You know the term “immuno-editing,” so the immune system and the tumor are constantly fighting and, therefore, yes, in higher tumor stage, probably regulatory T cells accumulate, but I have no data here to prove this hypothesis. I can only say that we are now able to find these cells in the tumor and that we are in the process of examining this important topic further.
To your second question: why do we not have systemic immunity in all patients? Again, this was partly answered before. We do not really understand this. Clearly, this is important because we have to understand how the tumor fights the immune system. Once we understand this, then we may be able to influence this in a therapeutic way. We have to do more experiments and then we'll find out, hopefully.
Footnotes
Reprints: Jürgen Weitz, MD, PhD, Section of Surgical Oncology, Department of Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany. E-mail: Juergen.Weitz@med.uni-heidelberg.de.
REFERENCES
- 1.Weitz J, Koch M, Debus J, et al. Seminar: Colorectal Cancer. Lancet. 2005;365:153–165. [DOI] [PubMed] [Google Scholar]
- 2.Mocellin S, Mandruzzato S, Bronte V, et al. Vaccines for solid tumours. Lancet Oncol. 2004;5:681–689. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. [DOI] [PubMed] [Google Scholar]
- 4.Parmiani G. Tumor-infiltrating T cells: friend or foe of neoplastic cells? N Engl J Med. 2005;353:2640–2641. [DOI] [PubMed] [Google Scholar]
- 5.Chiba T, Ohtani H, Mizoi T, et al. Intraepithelial CD8+ T-cell count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;61:3491–3494. [PubMed] [Google Scholar]
- 7.Menon AG, Janssen-van Rhijn CM, Morreau H, et al. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Laboratory Investigation. 2004;84:493–501. [DOI] [PubMed] [Google Scholar]
- 8.Phillips SM, Banerjea A, Feakins R, et al. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469–475. [DOI] [PubMed] [Google Scholar]
- 9.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994;12:456–465. [DOI] [PubMed] [Google Scholar]
- 11.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T-cells. Nat Med. 2003;9:1377–1382. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Wittekind C. UICC: TNM Classification of Malignant Tumors, 6th ed. London: Wiley, 2002. [Google Scholar]
- 13.Solomayer EF, Feuerer M, Bai L, et al. Influence of adjuvant hormone therapy and chemotherapy on the immune system analysed in the bone marrow of patients with breast cancer. Clin Cancer Res. 2003;9:174–180. [PubMed] [Google Scholar]
- 14.Bai L, Beckhove P, Feuerer M, et al. Cognate interactions between memory T cells and tumor antigen-presenting dendritic cells from bone marrow of breast cancer patients: bidirectional cell stimulation, survival and antitumor activity in vivo. Int J Cancer. 2003;103:73–83. [DOI] [PubMed] [Google Scholar]
- 15.Feuerer M, Beckhove P, Bai L, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–458. [DOI] [PubMed] [Google Scholar]
- 16.Beckhove P, Feuerer M, Dolenc M, et al. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz-Winnenthal FHVC, Zgraggen K, Galindo L, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–10087. [DOI] [PubMed] [Google Scholar]
- 18.Ajioka Y, Allison LJ, Jass JR. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J Clin Pathol. 1996;49:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. [DOI] [PubMed] [Google Scholar]
- 20.Gao F, Khammanivong V, Liu WJ, et al. Antigen-specific CD4+ T-cell help is required to activate memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 2002;62:6438–6441. [PubMed] [Google Scholar]
- 21.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. [DOI] [PubMed] [Google Scholar]
- 22.Ostenstad B, Lea T, Schlichting E, et al. Human colorectal tumour infiltrating lymphocytes express activation markers and the CD45RO molecule, showing a primed population of lymphocytes in the tumour area. Gut. 1994;35:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulder WC, Bloemena E, Stukart J, et al. T cell receptor and granzyme B expression in mononuclear cell infiltrates in normal colon mucosa and colon carcinoma. Gut. 1997;40:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidoboni M, Gafa R, Viel A, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prall F, Dührkop T, Weirich V, et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–816. [DOI] [PubMed] [Google Scholar]
- 28.Diederichsen ACP, Hjelmborg JvB, Christensen PB, et al. Prognostic value of the CD4+/CD8* ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immun. 2003;52:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagorsen D, Keilholz U, Rivoltini L, et al. Natural T cell response against MHC class I epitopes of epithelial cell adhesion molecule, her-2/neu, and carcinoembryonic antigen in patients with colorectal cancer. Cancer Res. 2000;60:4850–4854. [PubMed] [Google Scholar]
- 30.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. [DOI] [PubMed] [Google Scholar]