Abstract
Objective:
To compare the efficacy and safety of early, nasogastric enteral nutrition (EN) with total parenteral nutrition (TPN) in patients with predicted severe acute pancreatitis (SAP).
Summary Background Data:
In SAP, the magnitude of the inflammatory response as well as increased intestinal permeability correlates with outcome. Enteral feeding has been suggested superior to parenteral feeding due to a proposed beneficial effect on the gut barrier.
Methods:
Fifty patients who met the inclusion criteria were randomized to TPN or EN groups. The nutritional regimen was started within 24 hours from admission and EN was provided through a nasogastric tube. The observation period was 10 days. Intestinal permeability was measured by excretion of polyethylene glycol (PEG) and concentrations of antiendotoxin core antibodies (Endocab). Interleukins (IL)-6 IL-8, and C-reactive protein (CRP) were used as markers of the systemic inflammatory response. Morbidity and feasibility of the nutritional route were evaluated by the frequency of complications, gastrointestinal symptoms, and abdominal pain.
Results:
PEG, Endocab, CRP, IL-6, APACHE II score, severity according to the Atlanta classification (22 patients), and gastrointestinal symptoms or abdominal pain did not significantly differ between the groups. The incidence of hyperglycemia was significantly higher in TPN patients (21 of 26 vs. 7 of 23; P < 0.001). Total complications (25 vs. 52; P = 0.04) and pulmonary complications (10 vs. 21; P = 0.04) were significantly more frequent in EN patients, although complications were diagnosed dominantly within the first 3 days.
Conclusion:
In predicted SAP, nasogastric early EN was feasible and resulted in better control of blood glucose levels, although the overall early complication rate was higher in the EN group. No beneficial effects on intestinal permeability or the inflammatory response were seen by EN treatment.
Early, nasogastric enteral nutrition in patients with predicted severe acute pancreatitis was feasible and resulted in better blood-glucose control as compared with isocaloric total parenteral nutrition. No benefits on intestinal permeability or the acute inflammatory response were seen by enteral nutrition.
The mortality rate in patients with severe acute pancreatitis (SAP) is reported in the range of 9% to 27%.1,2 Mortality has 2 peaks, ie, “early” during the first week, when the systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) develop and “late” after 1 to 3 weeks, when mortality often is caused by MODS together with infections and sepsis.3,4 The production of cytokines, such as interleukins (IL)-6 and IL-8, increase early on during the course of SAP and play a dominant role in the development of SIRS.5 The magnitude of the inflammatory response correlates with the development of MODS and death.6
The second peak in mortality usually involves MODS together with infections, which are frequently caused by gram-negative bacteria.7 The predominance of gram-negative bacteria found in pancreatic infections supports the theory on the gut fuelling the disease process.8 Gut barrier injury results in potential translocation of endotoxin and bacteria through the epithelial layer to the lamina propria, mesenteric lymph nodes, and the systemic circulation and thereby cause sepsis and infections also at distant sites. Bacterial translocation has been demonstrated in experimental acute pancreatitis, but is still not proven in humans. Indirectly, there is evidence on translocation by the findings of bacteria of enteric origin in patients with infected necrotic pancreatic tissue.9 Possibilities to measure translocation are not directly available, and this has resulted in the frequent use of intestinal permeability as a mode of evaluating gut barrier function. Intestinal permeability may play an important role in the pathophysiology of SAP and clinical prospective studies have shown that increased gut permeability correlates with increased levels of endotoxin and also the grade of severity of pancreatitis.10,11
Therapies that aim to preserve and restore intestinal barrier function and thereby improve outcome have included enteral nutrition (EN). In experimental studies, enteral feeding preserves the gastrointestinal mucosa and microbial ecology, reduces bacterial translocation, and maintains immunocompetence of the host.12 Comparisons of enteral versus parenteral nutrition in patients with SAP have pointed at a reduction of infectious complications, length of hospital stay, and costs.13–17
In SAP, enteral feeding is usually delivered via the nasojejunal route, which is more inconvenient as compared with a nasogastric position of the tube. The insertion of jejunal tubes involves radiographic screening and endoscopic placement, which delays the start of EN; moreover, proximal dislocation of the tube is frequent.18 The rationale for using the jejunal route or alternatively fasting the patients is that nutrients passing the duodenum induce a cholecystokinin release that stimulates pancreatic enzyme secretion and, therefore, is thought to cause exacerbation of the pancreatitis and potential tissue injury.19 However, the relevance of the concept of “put the pancreas at rest” is not truly proven in clinical studies. The exocrine pancreatic secretion is suppressed during the course of experimental acute pancreatitis;20 and in a recent clinical randomized study of 49 patients with SAP, nasogastric feeding was not found to exacerbate the pancreatitis process.21
Proper timing is probably crucial for achieving success with therapeutic interventions, including modulation of inflammatory mediator production and release. In SAP, plasma concentrations of IL-6 peaks about 36 hours after onset of pain and organ dysfunction develop most commonly on the second or third day.22 Potentially, a therapeutic window exists up to about 48 and 72 hours from pain onset, ie, in the time phase usually required for the development of remote organ dysfunction.23
The present study aimed to evaluate the efficacy and safety of early, nasogastric, enteral nutrition as compared with total parenteral nutrition in patients with predicted SAP.
METHODS
Protocol
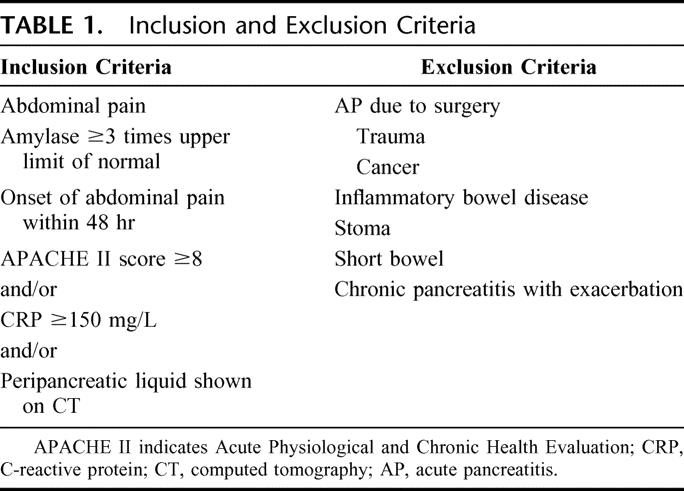
This prospective randomized study was conducted between June 2002 and December 2004. Adults (≥18 years of age) admitted to Lund University Hospital with the clinical diagnosis of acute pancreatitis were considered for inclusion. Inclusion and exclusion criteria are summarized in Table 1. 24–26 Fifty patients were recruited: 26 in the TPN (total parental nutrition) group and 24 in the EN group. One patient from each group was considered as protocol violators (not fulfilling set criteria for study nutrition) due to surgery performed after study inclusion on day 2 in 1 case and a dislocated tube that the patient did not accepted to be replaced in the other. Written informed consent was obtained from all participating patients. The local ethic committee of the University of Lund approved the study protocol.
TABLE 1. Inclusion and Exclusion Criteria
Patients were assigned to receive either TPN or EN and the nutritional support to start within 24 hours from admission. The nutritional regimen per protocol aimed to be isocaloric between groups with the energy target of 25 cal/kg per day based on admission weight. In both groups, standard formulas without specific immunomodulating nutrients were used. TPN (Kabiven PI, Fresenius-Kabi, Uppsala, Sweden) was infused via a peripheral or central venous catheter. EN (Fresubin original, Fresenius-Kabi) was administered via a nasogastric tube (Flocare, Nutricia Healthcare SA, Châtel-St. Denis, Switzerland). The initial rate of EN was 25 mL/hr and gradually increased daily up to 100 mL/hr if tolerated and needed. The aim was to reach full nutrition within 72 hours. If a patient was unable to tolerate the prescribed rate of enteral feeding, the rate was reduced by 50% and gradually increased again when tolerated. To maintain isocaloric groups, the TPN group did not receive Kabiven on day 1 since the amounts of delivered in EN patients initially were small. Fluids, such as crystalloids or colloids, were added in both groups to fulfill the individual's needs of fluid and energy (in case of reduced rate). Oral feeding was reintroduced when amylase and CRP levels had decreased and abdominal pain had resolved. Regular hospital diet was introduced gradually, in general initially starting with liquid and then solid food. Patients were monitored daily for nutritional supply, gastrointestinal symptoms (nausea, vomiting, and diarrhea) and pain by visual analog scale (VAS) performed at rest. Patients were treated according to clinical routine including pain control, symptomatic and organ supportive treatment and, when indicated, restrictive indications for surgery. Broad-spectrum antibiotic therapy was used according to current recommendations.27 The observation period was 10 days and follow-up was conducted after 3 months.
The primary endpoint was intestinal permeability measured by excretion of polyethylene glycol (PEG) in urine. Concentrations of antiendotoxin core antibodies (Endocab) for immunglobulin M (IgM) were also used as an indirect marker for intestinal permeability. IL-6, IL-8, and C-reactive protein (CRP) were used as markers of the systemic inflammatory response. Morbidity and feasibility of the nutritional route were evaluated by the frequency of complications, hyperglycemia, gastrointestinal symptoms, and abdominal pain. Power calculations were based on published data10,28 and a sample size calculation showed that 42 patients would be required to demonstrate a difference of 10% between groups in PEG excretion at the 5% level of significance with a power of 80%.
Data are presented as median and interquartile range. Comparisons between groups were performed using the χ2 tests for binary data or Fisher exact test for small samples. Continuous variables were compared with the Mann-Whitney U test. P values of less than 0.05 were considered significant. Statistical analyses were performed with SPSS version 12.0.2. (SPSS, Inc., Chicago, IL). Patients had to receive the study diet for at least 48 hours to be counted in the calculations of outcome data and the 2 groups were compared on an intention-to-treat basis.
Assignment
The patients were randomly divided into 2 groups and allocation concealment was by the use of sealed, numbered envelopes. The assignment was balanced with the use of blocks of four.
Blinding Procedures
It was not possible to blind the present study because of the nature of the treatment arms. Physicians and nurses from the staff collected patient data and fulfilled the study documentation in an attempt to minimize observer bias. A data analyst from the Competence Centre for Clinical Research at the Lund University Hospital performed the statistical analyses on the primary and secondary endpoints.
Analysis
Intestinal permeability was assessed noninvasive by measuring urinary excretion of an orally administered marker. The substance PEG is nontoxic, not normally absorbed, not naturally present in urine, nondegradable by bacteria, and permeates the epithelial layer paracellularly.29 The patients were given 40 g of PEG (Macrogolum 3000, M=3000 Da, Apoteksbolaget, Stockholm, Sweden) on day 1, 3, and 7. PEG was dissolved in 150 mL of water and administered either orally or through a nasogastric tube. Urine was collected over 24 hours, the volume was measured, and the sample was stored in −20°C and subsequently PEG was quantified using liquid chromatography and mass spectrometry as detector (Hewlett Packard 1100 series LC system, Esquire-LC trap mass spectrometer, Bruker Daltonics Inc., Billerica, MA).30 Urinary PEG excretion was expressed as the percentage of the administered dose. Samples from 10 healthy volunteers were used as controls.
Blood samples were collected after the inclusion in the study (baseline) and after 12 hours, 1, 3, 5, and 7 days. The samples were centrifuged at 2200g (3200 rpm; rotor diameter, 19.1 cm) at 10 minutes, plasma collected and stored at −70°C for subsequent analysis. Determination of EndoCab for immunglobulin M (IgM) levels by enzyme linked immunosorbent assay (ELISA) was used as an indirect measure of lipopolysaccharide (LPS) exposure. An increase in systemic LPS levels results in decreased levels of unbound antibodies.31 Values were expressed as median units/mL (MU/mL) (HyCult Biotechnology, Uden, Netherlands). Samples from 10 healthy volunteers were used as controls. Levels of IL-6 and IL-8 were measured by ELISA (Quantikine, R&D Systems Europe, Abingdon, UK).
Clinical Data
Clinical data that were collected included age, gender, etiology, time from onset of pain to baseline, weight at admission, APACHE II on day 1 and 3, total parental nutrition, enteral nutrition, fluid administration, energy delivery, route of nutrition, hyperglycemia (defined as blood glucose ≥10 mmol/L), insulin treatment, gastrointestinal symptoms (nausea, vomiting, diarrhea), abdominal pain, days until intake of oral food, pain recurrence after refeeding, antibiotic prophylaxis, surgery, complications, mortality, length of hospital stay, days at the intensive care unit, and compliance to protocol.
RESULTS
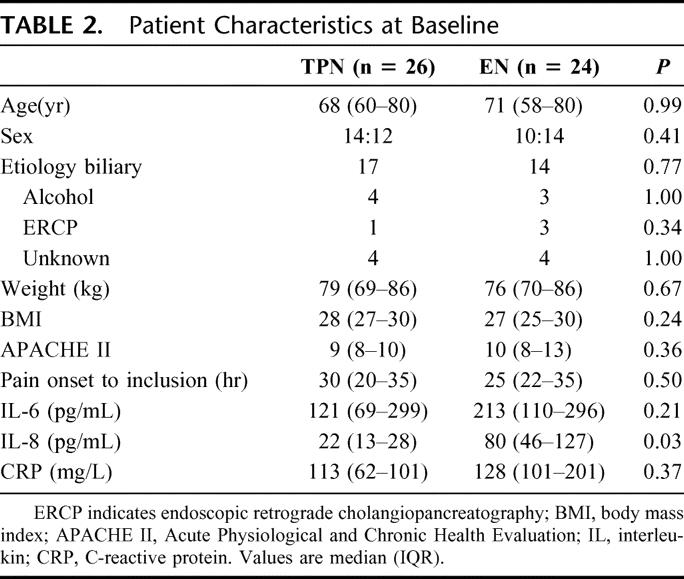
At inclusion, the groups were comparable with respect to clinical characteristics such as age, sex, etiology, weight, BMI, APACHE II, and time from onset of pain to baseline (Table 2). According to the Atlanta classification system,32 22 (46%) patients were defined as severe and 26 (54%) as mild of the finally evaluated 48 patients. In the TPN group, 8 of 25 (32%) patients were defined as severe, and in the EN group, 14 of 23 (61%) patients were severe (P = 0.08).
TABLE 2. Patient Characteristics at Baseline
Intestinal Permeability
PEG excretion was assessed in 40 of the 48 (83%) patients. Missing samples were equally distributed between the groups. The median PEG excretion in the TPN group was 1.2% (0.3–2.3) as compared with 1.6% (0.7–3.2) in the EN group; P > 0.30 at baseline, 0.6% (0.4–1.0) in the TPN group versus 2.0% (1.1–3.9) in the EN group; P = 0.003 on day 3 and 1.1% (1.0–1.9) in the TPN group versus 2.0% (1.0–3.8) in the EN group; P > 0.30 on day 7. No significant differences were found in Endocab IgM levels at any time point between the TPN and EN group. For all patients EndoCab concentrations decreased between day 2 and 5.
Systemic Inflammatory Response
The concentrations of IL-6, IL-8, and CRP at baseline are shown in Table 2. No significant differences were found in IL-6 or CRP levels between the treatment groups. The baseline concentrations of IL-8 were significantly higher in the EN group as compared with the TPN group (22.3 [13.3–27.8] vs. 79.8 [46.3–127.3] pg/mL; P = 0.03). For all patients, the IL-6 peak early, maybe even before admission and CRP peaked as expected, later with maximal concentrations on day 3. At every time point, when comparing mild and severe pancreatitis patients, both IL-6 and CRP median concentrations were significantly higher in severe disease, eg, on the day for their peak values were 100 (55–210) versus 275 (158–315) pg/mL; P = 0.001 for IL-6 at baseline and 143 (79–199) versus 278 (230–332) mg/L; P < 0.001 for CRP on day 3.
Nutritional Outcome
The nutrition per protocol was initiated in median 17 (range, 10–24) hours after admission in the TPN group and 19 (range, 14–24) hours in the EN group. The energy delivery per protocol was 1300 (1230–1530) calories/day in the TPN group versus 1250 (1100–1530) calories/day in the EN group (P > 0.30). The nutritional goal of 25 kcal/kg per day was achieved in 66% (based on median weight for each group) in both groups. Intake of liquid or solid food without TPN/EN supplement was achieved in median on day 6 (range, 5–9) in both groups. By the time when oral food was reintroduced, 13 of 25 (52%) patients in the TPN group and 12 of 22 (55%) patients in the EN group still had limited abdominal pain, but no patient interrupted their oral feeding because of pain relapse.
Route
The enteral nutrition was delivered through a clinifeeding tube in 18 of 24 (75%) patients, while 6 (25%) patients received their enteral feeding in an already placed nasogastric tube. TPN was administered via the peripheral route in all patients except for 2 patients who received a central venous catheter. Five of 24 (21%) patients in the EN group received central venous catheters for the administration of fluids and drugs.
Feasibility
There were no complications associated with insertion of the nasogastric tubes. In no patient, EN had to be withdrawn. In 3 of 23 (13%) patients, the feeding had to be interrupted for a maximum of 12 hours due to gastric retention. No patients demonstrated any signs of aspiration. The number of gastrointestinal symptoms was 23 in the TPN group and 17 in the EN group and did not statistically differ between the groups (P > 0.30). Abdominal pain, evaluated by VAS, was in median 6 (4–8) in the TPN group and 7 (6–8) in the EN group on day one. No significant differences were shown on any day when comparing TPN and EN patients.
Clinical Outcome
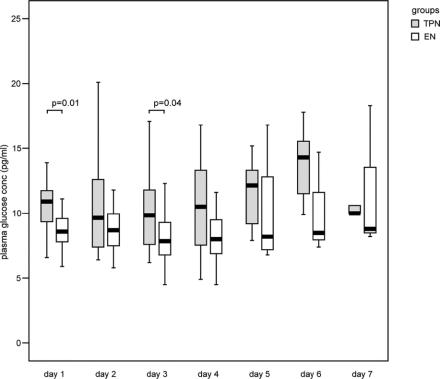
The length of hospital stay was in median 7 days (range, 6–14 days) in the TPN group and 9 days (range, 7–14 days) in the EN group (P = 0.19). A total of 6 of 50 (12%) patients were admitted to the intensive care unit (2 patients in the TPN group and 4 patients in the EN group), 5 due to organ failure and 1 patient due to severe pain. No significant difference was seen between the groups in the frequency of antibiotic prophylaxis (17 of 25 vs. 18 of 23; P > 0.30). One patient in each group underwent surgery during hospital stay; cholecystectomy (on day 2) and necrosectomy (after 10 weeks), respectively, was performed. The incidence of hyperglycemia at any time point during nutritional support and during the first 7 days was significantly higher in TPN patients (21 of 26 vs. 7 of 23; P < 0.001). The concentrations of plasma glucose are shown in Figure 1. One patient in the EN group had diabetes mellitus prior to admission and was therefore excluded in the calculations of hyperglycemia. No significant differences were shown between the groups concerning the number of patients treated with insulin (9 patients in the TPN group vs. 3 patients in the EN group; P = 0.10). Insulin was administered at a blood glucose level of in median 16 mmol/L (14–19 mmol/L) in both groups.
FIGURE 1. Plasma concentrations of glucose during nutritional support were significantly increased on day 1 and 3 in patients in the TPN group compared with those in the EN group. Boxes represent medians and interquartile ranges. Statistical significance at P < 0.05.
Complications
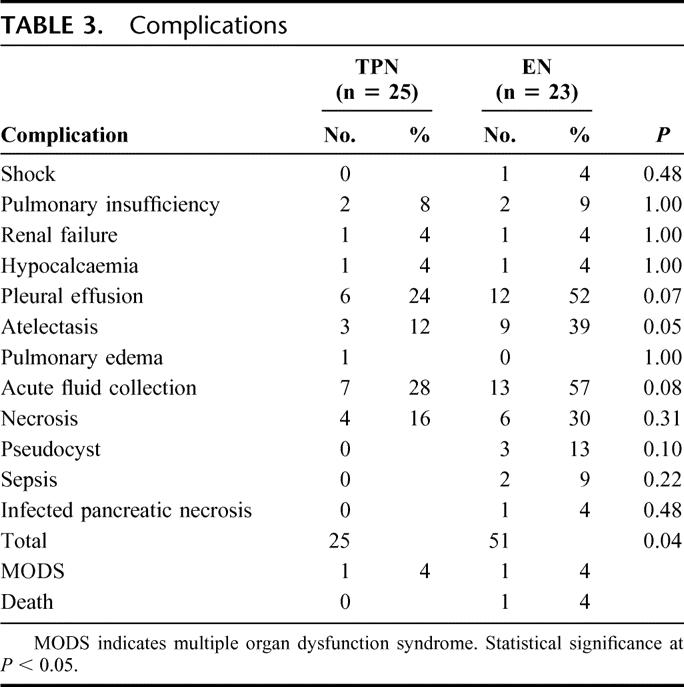
Twenty-six patients developed complications: 10 of 26 (40%) in the TPN group and 16 of 23 (70%) in the EN group (P = 0.05). Pulmonary complications and the total number of complications were significantly more frequent in EN patients (Table 3). Three septic complications were found in the EN group and none in the TPN group (P = 0.10). In both groups, most of the complications were diagnosed early, ie, within the first 3 days. Thus, in the TPN group 18 of 25 (72%; P = 0.01) and in the EN group 41 of 51 (80%; P < 0.001) of the total complications were early. Late complications did not differ between groups, being 7 of 25 (28%) in the TPN group and 10 of 51 (20%) in the EN group (P > 0.30 in both groups). Multiple organ failure, defined as 2 or more failing organ systems,32 was found in 2 (4%) patients, 1 in each group. One death in the EN group occurred on day 3 in a 91-year-old woman, caused by circulatory failure. The overall mortality rate was thus 2% (1 of 48).
TABLE 3. Complications
Follow-up
By the time of follow-up after 3 months, 23 of 25 (92%) patients in the TPN group and 18 of 22 (82%) in the EN group (P > 0.30) had no symptoms left related to their SAP. Symptoms in the 6 patients with some complaints were pain, fever, or pathologic liver function tests. Three of these patients had underlying pseudocysts, all in the EN group.
DISCUSSION
Knowledge from clinical studies on the efficacy of EN on intestinal gut barrier function in SAP is limited. In the present study, it does not seem that early EN without supplements renders any benefits on gut barrier function, as evaluated by urinary excretion of orally administered PEG and systemic levels of EndoCab, in patients with SAP. On day 3, the intestinal permeability (measured by PEG) was increased in the group that received EN. The permeability parameters in our study do not fully support the otherwise frequently suggested benefits provided by EN on the gut, including restoration of permeability changes. Instead, the present findings support the results presented by Powell et al, demonstrating that intestinal permeability was not favored by EN and permeability instead significantly increased by day 4 after initiated EN.33 EN per se may increase the demands on mucosal blood supply and this might contribute to the leakage over the endothelial barrier and interstitial edema formation, thereby facilitating gut barrier permeability. Powell et al administered a minimal dose of nutrition and it may not have been sufficient to influence on the gut mucosal barrier. In the present study, the amounts of EN administered were 66% of the estimated energy target, which is in the upper range of what has been achieved in other studies comparing EN with TPN.13–17 There are other factors than administered volumes that could influence on the efficacy of enteral feeding on gut barrier function; such as time of insertion, composition, and duration of feeding but so far, no precise clinical recommendations exist. The present study evaluated the effects of a standard composition inserted early by the nasogastric route; thus, the formula did not contain fibers and glutamine, substances suggested to be beneficial for the epithelial cells and the structure of the mucosa.34
Previous studies have reported that gut permeability increases mainly early in the course of acute pancreatitis.10,11 In the present study, the hypothesis that early intervention by EN would influence on early gut permeability was tested. However, no beneficial effects were found. The concentrations of EndoCab were lower than normal between day 2 and 5 in both groups. It may be that the consumption of antibodies increased, ie, gut permeability of endotoxin was increased and higher concentrations of endotoxin reached into the systemic circulation. Experimentally, gut permeability increased by fasting as compared with EN, although without increasing bacterial translocation.35 In humans, pathways for endotoxins and bacteria through the intestinal barrier are not fully understood.
In a trial by Windsor et al, it was suggested that acute inflammatory markers were modulated by EN in acute pancreatitis.14 In previous studies on EN in SAP, the time interval prior to initiation of nutritional support has been poorly defined, usually varying from 48 to 72 hours from admission and furthermore, the time of pain onset has not been stated.13–17 In the present study, the curves for IL-6 and CRP for all patients peaked in accordance with what has been reported in the literature and the time for the insertion of nutrition (in median 17 hours in the TPN and 19 hours in the EN group after admission) was within the suggested potential therapeutic window for modulating the peaks of IL-6 and CRP. However, no significant differences were seen between the treatment groups. This absence of an influence on the inflammatory response (studied up to 7 days), despite early inserted EN may, eg, be due to that potential modulation of gut-associated immune-competent cells is not enough to influence on the systemic inflammatory response. Furthermore, specific, known immunomodulating supplements (eg, glutamine, arginine, and omega-3 fish oils) to EN may be required. These aspects have to be investigated in future studies.
In the present study, the nasogastric route was feasible in the aspect of frequency of gastrointestinal complications and abdominal pain. A larger number of overall complications were shown in the EN group than in the TPN group. In both groups, most of the complications were diagnosed during the first 3 days and most frequent were pleural effusions, atelectasis, and peripancreatic fluid collections. It is unlikely that the route of nutrition could have an impact on the development of these early complications. In the study by Eatock et al, the nasogastric route for administration of enteral feeding in SAP was suggested to be safe, although the number of local or systemic complications were not reported.21 No other infectious complications were found except for 2 cases of sepsis and one infected pancreatic necrosis in the EN group. Side effects of central venous catheters, such as line infections, are reported also in SAP.16 Some previous studies comparing EN with TPN in SAP have reported a reduction in infectious complications in the EN group, although the numbers of patients with central venous lines within the groups were not reported.13,15 In the present study, only a total of 7 patients had central venous catheters, since TPN mostly was delivered through a peripheral catheter and this might have influenced on the rate of infectious complications.
Hyperglycemia is common in SAP, and in the present study the incidence of hyperglycemia was significantly lower in the EN group. A recent trial in critical illness, practicing strict glucose control with glucose levels maintained below 6 mmol/L, has pointed at an improved outcome with decreased morbidity and mortality.36 In the present study, the median blood glucose levels were 16 mmol/L when insulin therapy was initiated and not all patients with hyperglycemia received insulin. The effects of normoglycemia in SAP have not yet been studied, but potentially this concept might further improve outcome also in patients with SAP.
The varying definitions of SAP, as well as the fact that no reliable, simple method of severity prediction at admission exists, complicates study design and makes comparisons between studies difficult.37 In the present study, only 22 of 50 patients were finally severe as classified by the Atlanta classification system, which indicates that the used inclusion criteria overestimated severity. In the study performed by Ammori et al comparing intestinal permeability between mild and severe acute pancreatitis,10 the subgroup of patients who developed MODS had a significantly higher excretion of PEG as compared with patients with severe disease who developed single-organ failure or local pancreatic complications. The lower number of deaths and incidence of MODS in the present study might be a reason for the absence of significance in intestinal permeability when comparing the mild and severe pancreatitis groups.
CONCLUSION
In predicted SAP, nasogastric early EN was feasible and resulted in better control of blood glucose levels, although the early complication rate was higher in the EN group. Intestinal permeability was overall not influenced by EN, and PEG-measured permeability actually increased on day 3 in the EN group. Furthermore, no effects on the inflammatory response were seen by the EN treatment. In current literature and guidelines, enteral feeding is recommended as the preferred route in SAP, although mechanisms and details in the management, such as initiation time, route, composition, and volumes of the nutrition, are not fully addressed.38,39 In the present study, early insertion of EN does not seem to be crucial, at least not when considering our results. If so, this would allow sufficient time for the clinician to define true severity of the disease (within 2–3 days) prior to defining demands and route of administration of nutritional support. EN has its role in the management of patients with SAP and may very well be provided nasogastrically, thereby facilitating the handling of the patients. Factors that need further clarification are, however, potential benefits of various supplements to EN and whether late or prolonged EN might contribute to an improved outcome. It may very well be that future EN management could be “tailored” as comes to specific composition of the nutritional formula to patients identified as being at high risk for complications.
ACKNOWLEDGMENTS
The authors thank Axel Mie, Department of Clinical Chemistry, University of Lund, Sweden, for technical expertise and assistance.
Discussions
Dr. Helmut Friess: I would like to thank the Society for giving me the opportunity of discussing this interesting paper. First of all, I would like to thank Roland Andersson for this interesting and excellent presentation on the important topic of nasogastric feeding in patients with predicted severe acute pancreatitis. The authors have to be congratulated on this well-designed randomized study. To improve our clinical work, we have to rely on studies like this, which, even nowadays, are carried out too infrequently. The authors have compared nasogastric enteral feeding with total parenteral nutrition in 50 patients with predicted severe acute pancreatitis. The overall complication rate was higher in the enteral nutrition group, and enteral nutrition resulted in a better control of blood glucose levels. Roland, I would like to ask you some questions.
First, I would like you to elaborate more on the power calculation of your study. What was the rational for using one human and one animal study as the basis for this power calculation? In addition, at which time point did you expect the 10% difference in your primary endpoint? I would also like your comments on the outcome of the patients. You included patients with predicted severe acute pancreatitis as stated in your inclusion criteria. It is surprising that, in this group of patients, the median hospital stay was 7 to 9 days and that only 12% of the patients were admitted to an intensive care unit. One could argue that most of the patients, although predicted to have severe acute pancreatitis, had no severe acute pancreatitis but rather a mild form of the disease.
Previous studies comparing enteral versus parenteral nutrition in patients with severe acute pancreatitis have pointed at a reduction of infectious complications. How do you explain that you could not observe a similar effect in your study?
In your study, the incidence of hypoglycemia was significantly lower in the enteral nutrition group. How relevant do you judge this effect, especially with respect to a recently published study of Van den Berghe on intensive insulin therapy in critically ill patients published in the New England Journal of Medicine?
And the last question. The increase in complications in the enteral group was largely attributable to a higher incidence of pulmonary complications. Do you have any explanation for this effect? Could you also comment on the risk of aspiration, especially in the patients treated with enteral nutrition? Thank you.
Dr. Roland Andersson: Thank you, Helmut. A lot of clever comments, as always. Especially asking a surgeon about statistics is not always that comfortable so we went to a statistician! There are limited background data as to how to estimate outcome and, the assumption we made, was made on day 5. If you look in the literature, this is as good as you get, and the number we calculated was 42 to reach any statistical significance.
You may consider the study underpowered, which you may very well do. Concerning the actual true severe pancreatitis patients that came out when reviewing the final analysis, there are no deterrents whatsoever to any benefits. If you don't get any trends in this number of patients, I think that this is fair enough.
Concerning predicted severity, yes, I honestly showed that less than half came out as true severe if you grade the patient afterward according to the Atlanta classification. The reason for having these types of inclusion criteria for severe disease was that they were collected from the literature to get what we thought would be clinically relevantly severe patients. We obtained a mix of moderate to severe patients in this way. We are aware of that. Still, this is a single-center study, but it would be a considerable challenge to fulfill it, and I think this series is probably more severe than the previous one concerning the grading.
And then, the comment on the short stay in severe patients. Well, I agree that true severe disease was only found in less than half the patients. The hospital stay is short in Sweden in general. Fast track surgery is applicable also on patients with acute illness: we try to do so. There are a limited number of beds; we have to have a high turnover and we are not paid per day. To address the waiting list and all that, and to be efficient, we have to look at logistics.
Infections and complications in general were few, and we have seen that before. There are a number of studies from our institution on the overall outcome and infectious complications are limited. There are many aspects to that—I think what I said in the beginning—the early aggressive fluid resuscitation is one of the major things decreasing both morbidity and mortality in these patients. We still use frequent, prophylactic antibiotics. We're going to compare early enteral nutrition together with probiotics in the future against antibiotics, and we will see if it's as efficient.
Hypoglycemia—yes, it is important, as shown by Grete Van de Berge in 2001 in critical illness and reproduced by several others. Evidently, also of some relevance after major abdominal surgery to keep blood glucose level at below 6, we had intensive insulin treatment, and I think that's relevant. I think that patients with acute pancreatitis are nonspecific as when you talk about patients with critical illness. They need similar types of interventions, and it's a question of getting large enough materials. I think this has some relevance; and if a simple thing like enteral nutrition can contribute to that, that's fine.
Your last question, as I noted, was the number of pulmonary complications, and they were all noted within 3 days after admission. My personal belief is that I don't think that the nutrition had any impact on the pulmonary complications. I think that's merely attributed because most of these had atelectasis or pleural effusion. Probably that is attributed to the fact that they tended to be more severe than the parenteral group. We were afraid of aspiration pneumonia as a potential complication, but we had no pneumonias in this series. These patients were quite intensively checked concerning gastric retention to avoid that type of complication.
Dr. Peter Neuhaus: Yes, I enjoyed the presentation. Obviously, these patients were not as severely ill as we surgeons expected. In Berlin, we had good cooperation with your former colleague, Stig Bengmark, also using early enteral nutrition with probiotics for postoperative patients and also for pancreatitis. Now, this is focused mainly on the prevention of infectious complications, which were rare in your study. You probably undertook a CT scan on all your patients. When you saw fluid collections, did you perform a needle aspiration? I wanted to ask whether you saw bacterial infections of these collections because that is one way to distinguish between severe conditions and sterile collections, which do not need any further treatment.
Dr. Roland Andersson: We have been treating patients in a conservative way during the years when central Europe had a very aggressive attitude toward the management of severe cases. We have not used FNA to see if there were any positive cultures or not. It is clear that infected pancreatic necrosis worsens the prognosis or increases the severity enormously, but we never used FNA. So, these assessments were based on clinical grounds. There was only one necrosectomy undertaken in this group during a follow-up of 3 months. In our 1 patient, I undertook necrosectomy 4 months after her acute attack and, actually, I was misled by the radiologist in that particular case because they said there was only a fluid collection and that there were no infectious signs. When I went in there, I removed maybe 80% of her pancreas but it was sterile. She was in the enteral group.
Probiotics, yes, they are addressed in Attila Olah's studies in a setting where they have a more aggressive surgical attitude. I think it has its role together with probiotics. Probiotics and symbiotics together with enteral nutrition have to challenge the antibiotics because antibiotics are not without problems in this setting.
Dr. Peter Friend: A very brief question. I wanted to pick up on this issue of hypoglycemia. Hypoglycemia is an almost inevitable association with parenteral nutrition, and it is manageable always by an insulin infusion. I wouldn't regard it, therefore, as a complication but rather an inevitable component of that particular arm of treatment and I'm not sure, therefore, whether it should be used as an outcome parameter.
Dr. Roland Andersson: We observed a difference already by day 1 when they did not get complete parenteral nutrition but only some. Day 1 was focused on fluid resuscitation and not nutrition, but blood glucose was affected already on day 1 when we had reached only two thirds of what we actually had estimated. The goal was to reach 25 calories per kilo and we reached two thirds of that. Similarly, in both groups, patients received about 1300 kilocalories each and, as these patients were not that critically ill, I think they were capable of taking that up peripherally also. As for our data, I mean, there's place for improvement because blood glucose levels were high so we are addressing that now to improve even further, yes.
Mr. Chris Russell: Thank you very much. I enjoyed hearing the results of this study but have that feeling of a slightly unsatisfactory outcome because we don't really know where to take these observations next. As you have shown that there is no difference, do you use this negative result to say that enteral nutrition now has no advantage, or are you going on to do further studies on this subject? In other words, what is your policy of treatment now?
Dr. Roland Andersson: If you're a believer in enteral nutrition, as we were and we are, I would still not consider this as a negative result. I would consider this as being a quite realistic result. Being realistic, I mean if you think that enteral nutrition, because these were plain formulas, no supplements, no glutamine or fibers, no immune modulators whatsoever, will effect a fulminant systemic inflammatory response, this may not be possible to completely achieve. So we are going on with this, and it has its benefits. It is feasible, it is less costly as a nutritional formula, and it probably gives better glucose control, which is a good thing and there is a place for improvement. So, the next step will be the addition of probiotics and the various supplements to tailor this type of treatment more. Also remember it's not an either/or situation. We increase gradually the enteral nutrition during the first 3 days. We start with 25 mL per hour on day 1, to reach a full regimen within 3 days. Meanwhile, we can supplement parenterally, so it is both.
Footnotes
Supported by the Swedish Nutrition Foundation, Swedish Research Council (Grant No 11236), Foundation for Gut and Intestinal Research, and Fresenius-Kabi AB.
Reprints: Roland Andersson, MD, PhD, Department of Surgery, Clinical Sciences Lund, Lund University Hospital, S-221 85 Lund, Sweden. E-mail: roland.andersson@med.lu.se.
REFERENCES
- 1.Gloor B, Muller CA, Worni M, et al. Late mortality in patients with severe acute pancreatitis. Br J Surg. 2001;88:975–979. [DOI] [PubMed] [Google Scholar]
- 2.Appelros S, Lindgren S, Borgstrom A. Short and long term outcome of severe acute pancreatitis. Eur J Surg. 2001;167:281–286. [DOI] [PubMed] [Google Scholar]
- 3.Buter A, Imrie CW, Carter CR, et al. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298–302. [DOI] [PubMed] [Google Scholar]
- 4.Blum T, Maisonneuve P, Lowenfels AB, et al. Fatal outcome in acute pancreatitis: its occurrence and early prediction. Pancreatology. 2001;1:237–241. [DOI] [PubMed] [Google Scholar]
- 5.Norman JG, Fink GW, Denham W, et al. Tissue-specific cytokine production during experimental acute pancreatitis: a probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783–1788. [DOI] [PubMed] [Google Scholar]
- 6.McKay CJ, Gallagher G, Brooks B, et al. Increased monocyte cytokine production in association with systemic complications in acute pancreatitis. Br J Surg. 1996;83:919–923. [DOI] [PubMed] [Google Scholar]
- 7.Hartwig W, Werner J, Uhl W, et al. Management of infection in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:423–428. [DOI] [PubMed] [Google Scholar]
- 8.Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract: the ‘undrained abscess’ of multiple organ failure. Ann Surg. 1993;218:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beger HG, Bittner R, Block S, et al. Bacterial contamination of pancreatic necrosis: a prospective clinical study. Gastroenterology. 1986;91:433–438. [DOI] [PubMed] [Google Scholar]
- 10.Ammori BJ, Leeder PC, King RF, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252–262. [DOI] [PubMed] [Google Scholar]
- 11.Juvonen PO, Alhava EM, Takala JA. Gut permeability in patients with acute pancreatitis. Scand J Gastroenterol. 2000;35:1314–1318. [DOI] [PubMed] [Google Scholar]
- 12.Flint R, Winsor J. The role of the intestine in the pathophysiology and management of severe acute pancreatitis. Hepatobiliary. 2003;5:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalfarentzos F, Kehagias J, Mead N, et al. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84:1665–1669. [PubMed] [Google Scholar]
- 14.Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olah A, Belagyi T, Issekutz A, et al. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–1107. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Assi S, Craig K, O'Keefe SJ. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: results of a randomized comparative study. Am J Gastroenterol. 2002;97:2255–2262. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Patel K, Calder PC, et al. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II > or = 6). Pancreatology. 2003;3:406–413. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood JK, Lovelace HY, McClave SA. Enteral nutrition in acute pancreatitis: a survey of practices in Canadian intensive care units. Nutr Clin Pract. 2004;19:31–36. [DOI] [PubMed] [Google Scholar]
- 19.Cassim MM, Allardyce DB. Pancreatic secretion in response to jejunal feeding of elemental diet. Ann Surg. 1974;180:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niederau C, Niederau M, Luthen R, et al. Pancreatic exocrine secretion in acute experimental pancreatitis. Gastroenterology. 1990;99:1120–1127. [DOI] [PubMed] [Google Scholar]
- 21.Eatock FC, Chong P, Menezes N, et al. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am J Gastroenterol. 2005;100:432–439. [DOI] [PubMed] [Google Scholar]
- 22.McKay CJ, Imrie CW. The continuing challenge of early mortality in acute pancreatitis. Br J Surg. 2004;91:1243–1244. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343–351. [DOI] [PubMed] [Google Scholar]
- 24.Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201–205. [DOI] [PubMed] [Google Scholar]
- 25.Wilson C, Heads A, Shenkin A, et al. C-reactive protein, antiproteases and complement factors as objective markers of severity in acute pancreatitis. Br J Surg. 1989;76:177–181. [DOI] [PubMed] [Google Scholar]
- 26.Robert JH, Frossard JL, Mermillod B, et al. Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers. World J Surg. 2002;26:612–619. [DOI] [PubMed] [Google Scholar]
- 27.Golub R, Siddiqi F, Pohl D. Role of antibiotics in acute pancreatitis: a meta-analysis. J Gastrointest Surg. 1998;2:496–503. [DOI] [PubMed] [Google Scholar]
- 28.Ryan CM, Schmidt J, Lewandrowski K, et al. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology. 1993;104:890–895. [DOI] [PubMed] [Google Scholar]
- 29.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. [DOI] [PubMed] [Google Scholar]
- 30.Palmgren JJ, Toropainen E, Auriola S, et al. Liquid chromatographic-electrospray ionization mass spectrometric analysis of neutral and charged polyethylene glycols. J Chromatogr A. 2002;976:165–170. [DOI] [PubMed] [Google Scholar]
- 31.Barclay GR. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263–272. [PubMed] [Google Scholar]
- 32.Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. [DOI] [PubMed] [Google Scholar]
- 33.Powell J, Murchison T, Fearon KC, et al. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. Br J Surg. 2000;87:1375–1381. [DOI] [PubMed] [Google Scholar]
- 34.Buchman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453–460. [DOI] [PubMed] [Google Scholar]
- 35.Kansagra K, Stoll B, Rognerud C, et al. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1162–G1170. [DOI] [PubMed] [Google Scholar]
- 36.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 37.Sandberg AA, Borgstrom A. Early prediction of severity in acute pancreatitis: is this possible? JOP. 2002;3:116–125. [PubMed] [Google Scholar]
- 38.Gurusamy KS, Farouk M, Tweedie JH. UK guidelines for the management of acute pancreatitis. Gut 2005;54(suppl 3):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier R, Ockenga J, Pertkiewicz M, et al. ESPEN guidelines on enteral nutrition: pancreas. Clin Nutr. 2006;2:275–284. [DOI] [PubMed] [Google Scholar]