Abstract
Objective:
To evaluate whether ischemic preconditioning (IP) with continuous clamping or intermittent clamping (IC) of the portal triad confers better protection during liver surgery.
Summary Background Data:
IP and IC are distinct protective approaches against ischemic injury. Since both strategies proved to be superior in randomized controlled trials (RCTs) to continuous inflow occlusion alone, we designed a RCT to compare IP and IC in patients undergoing major liver resection.
Methods:
Noncirrhotic patients undergoing major liver resection were randomized to receive IP with inflow occlusion (n = 36) or IC (n = 37). Primary endpoints were postoperative liver injury and intraoperative blood loss. Postoperative liver injury was assessed by peak values of AST (alanine aminotransferase) and ALT (aspartate aminotransferase), as well as the area under the curve (AUC) of the postoperative transaminase course. Secondary endpoints included resection time, the need of blood transfusion, ICU, and hospital stay as well as postoperative complications and mortality.
Results:
Both groups were comparable regarding demographics, ASA score, type of hepatectomy, duration of inflow occlusion (range, 30–75 minutes), and resection surface. The transection-related blood loss was 146 versus 250 mL (P = 0.008), and when standardized to the resection surface 1.2 versus 1.8 mL/cm2 (P = 0.01) for IP and IC, respectively. Although peak AST, AUCAST, and AUCALT were lower for IC, the differences did not reach statistical significance. Overall (42% vs. 38%) and major (33 vs. 27%) postoperative complications as well as median ICU (1 vs. 1 day) and hospital stay (10 vs. 11 days) were similar between both groups.
Conclusions:
Both IP and IC appear to be equally effective in protecting against postoperative liver injury in noncirrhotic patients undergoing major liver resection. However, IP is associated with lower blood loss and shorter transection time. Therefore, both strategies can be recommended for noncirrhotic patients undergoing liver resection.
Ischemic preconditioning and intermittent clamping have been shown to confer protection against ischemic liver injury, both in experimental models and in randomized controlled trials. Therefore, we conducted a randomized controlled trial in 73 unselected patients undergoing major liver resection and found similar degrees of postoperative liver injury for both strategies, but significantly less blood loss and transfusion amount as well as a shorter transection time in favor of ischemic preconditioning.
Liver resection has been increasingly performed over the last 2 decades worldwide due to improved postoperative outcomes and evidence that this approach offers the only curative option in many patients.1–3 Despite the success of liver surgery, excessive blood loss and the need for blood transfusion remain the most significant risk factors for poor outcome.2–4 Furthermore, the use of blood transfusion is associated with poorer long-term survival.4–6
Inflow occlusion by clamping of the portal triad (Pringle maneuver) has been used since the early 20th century to minimize blood loss during transection of the liver parenchyma,7–9 and became very popular during the 1980s.10 This strategy is particularly effective in preventing blood loss when associated with low central venous pressure (CVP).11,12 For example, we recently showed in a randomized controlled trial (RCT) that liver transection under inflow occlusion with the clamp crushing technique is associated with lower blood loss and reduced requirement for perioperative transfusions, than resection performed with more sophisticated transection devices claimed to enable safe surgery without the need for inflow occlusion.9 Currently, many surgeons worldwide use routine or selective inflow occlusion in patients undergoing major liver resection. A recent Japanese survey revealed that only the minority (7%) of surgeons never use inflow occlusion, whereas 25% apply a Pringle maneuver on a routine basis even in cirrhotic patients.13
However, the Pringle maneuver induces ischemic injury in the remnant liver, which is directly related to the duration of inflow occlusion and associated with increased morbidity and mortality.14 Ischemic preconditioning (IP) and intermittent clamping (IC) of the portal triad are the only clinically established protective strategies against liver injury due to prolonged ischemia.15 The protective effects of both strategies have been demonstrated in comparison with continuous inflow occlusion in several experimental studies16–21 and at the highest level of evidence in randomized clinical trials.22–24 On the other hand, a direct comparison between IP and IC in patients undergoing liver resection is currently not available. In a mouse model of warm ischemia, we found that both IC and IP were highly and equally protective against ischemic injury up to 75 minutes of hepatic ischemia with improved animal survival.18 However, IC proved superior for ischemic insults exceeding 75 minutes of duration.
Therefore, in view of the established benefits of IC and IP in patients undergoing liver resection and the lack of clinical comparison between both approaches, we designed a RCT comparing both strategies in noncirrhotic patients undergoing major liver resection. Endpoints included the degree of reperfusion injury, intraoperative blood loss, transection time, and the incidence and severity of postoperative complications.
MATERIALS AND METHODS
Experimental Design
Between January 2005 and November 2005, 73 consecutive patients undergoing major liver resection were randomized to receive IP with continuous clamping (Pringle maneuver) or IC (Fig. 1). Eligibility criteria included only major hepatectomies defined as the removal of more than 2 segments. Living donors for liver transplantation, patients with combined local ablation such as radiofrequency or cyroablation or the need for total vascular hepatic exclusion, and those with major concomitant extrahepatic procedure (eg, colectomy) were not included in the study. Data regarding primary and secondary endpoints were prospectively collected.
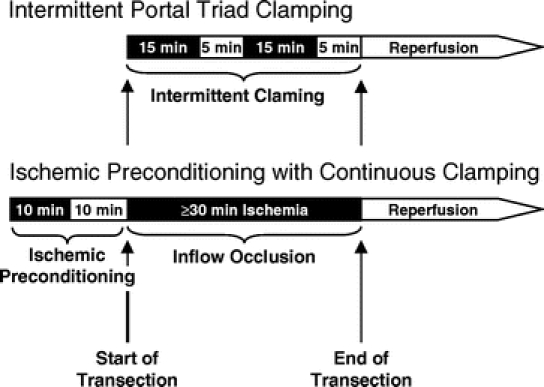
FIGURE 1. Treatment protocol of the intermittent portal triad clamping and ischemic preconditioning with continuous clamping. Inflow occlusion and reperfusion are symbolized by black and white boxes, respectively.
Surgical Procedure
The study was approved by the ethic committee of the University Hospital Zurich. Informed consent was obtained from each patient prior to surgery. Each patient was randomized during surgery in the operating room using sealed envelopes (40 for each strategy). Anatomic hemi-hepatectomy was performed in a standardized manner with a selective devascularization of the resected specimen, as previously described.23 Resections were performed with the requirement of a low CVP anesthesia (0–5 mm Hg) and the supervision of same hepatobiliary surgeon (P.-A.C.). Each patient underwent an intraoperative ultrasound to define the tumor localization in relation to the major vascular and biliary structures. Liver transection was performed by parenchyma crushing using a small Kelly clamp (3-mm-diameter tip) as recently described.9 Small vessels (<2 mm) were coagulated with the irrigated bipolar forceps set at 120 W, while all other structures including major intrahepatic bile ducts were ligated or clipped. A stapler device was only used for the transection of the hepatic veins. According to randomization, transection was performed either under continuous (IP group) or intermittent (IC group) portal triad inflow occlusion. The time of continuous inflow occlusion (IP group) and intermittent clamping (IC group) was adapted to the need, but had to exceed 30 minutes, as this is the minimal ischemic period with detectable postoperative liver injury.8 Inflow occlusion was achieved by the tourniquet technique around the portal triad with a 4-mm mersilene tape. Separate clamping of aberrant left hepatic arteries was carefully performed when present. Protective strategies were applied as follow.
IP With Continuous Clamping
IP was performed before starting the liver transection by a 10-minute inflow occlusion followed by a 10-minute reperfusion period. This 20-minute period was not included in the transection (resection) time because this time was always simultaneously used for other procedures such as intraoperative ultrasound, dissection, or mobilization and transection was always started after IP. Afterward, continuous inflow occlusion was subsequently initiated at the beginning of liver transection and was maintained until the transection was completed (Fig. 1). If continuous occlusion exceeded 75 minutes, it was discontinued for 10 minutes, as our previous animal study showed poorer results with IP for prolonged duration of ischemia.18
IC
Intermittent portal triad clamping was performed by cycles of a 15-minute inflow occlusion followed by 5-minute reperfusion. The length of the transection determined the number of repeated cycles (Fig. 1). In contrast to IP, resection and ischemia time were different for IC.
Prophylactic postoperative drainage was only used when a bilioenteric anastomosis was performed.
Outcome Measures
Each patient was followed for 3 months. The primary endpoints were postoperative hepatocyte injury and blood loss during parenchyma transection. The degree of postoperative hepatic injury was assessed by daily measurements of postoperative AST, ALT, bilirubin, and prothrombin time. To characterize the transaminase-based postoperative hepatic injury, peak values as well as the area under the curve (AUC) of the postoperative course of AST and ALT were determined for the first 5 postoperative days. The integration of the AUC was performed using the GraphPad PRISM software (GraphPad Software, Inc., San Diego, CA). The reference area, which is defined by the normal cutoff values of AST (50 U/L) and ALT (52 U/L), was subtracted from the total integrated AUC.
Transection time was defined as the duration between the beginning and the end of parenchyma transection. Another time was recorded until complete satisfaction with the hemostasis. Blood loss was carefully monitored until the beginning of the parenchyma transection, during transection, and after hepatectomy. The amount of blood loss was estimated taking into account the suction volume after subtraction of rinse fluids and weighing the swabs that were used during transection (assuming that 1 mL of blood = 1 g). The cut surface was put on a sponge and the area of liver transection surface was drawn on a sheet of paper. The paper area was cut and weight to calculate the surface area according to the paper density (80 mg/m2). The data for blood loss were standardized to the transection area expressed as mL/cm2. The speed of transection was calculated as transection area divided by transection time (cm2/min).
Secondary endpoints included the transection time, the degree of liver damage by serial postoperative bilirubin and prothrombin levels, the need of blood transfusion, and morbidity and mortality according to a new classification of complications by severity.25 The indications for red blood cell (RBC) transfusion were a massive hemorrhage (>1500 mL) during surgery or a hemoglobin level <7 g/dL within 48 hours following surgery. An abdominal US was performed at postoperative day 5 and a CT scan at 3 months to assess fluid collections.
Histologic Evaluation
Nontumorous liver tissue was evaluated by a single pathologist (W.J.), who was blinded for the postoperative outcome of the patients. The presence of steatosis was evaluated using hematoxylin and eosin-stained sections. The percentage of hepatocytes with fat droplets was determined and the degree of steatosis was graded as 0 (no steatosis or <10%), 1 (steatosis, 10%–29%), and 2 (≥30%).
Statistical Analysis
The analysis was performed in an “intention-to-treat” manner. Summary statistics are expressed as mean ± SEM or median (range), as appropriate. The sample size calculation was based on the primary endpoints postoperative AST and ALT and blood loss. According to previous published data9 and previous experience with both protective strategies,8,23 the sample size calculation was performed with the expectation of a 30% difference in postoperative peak serum AST/ALT levels or blood loss during parenchyma transection in at least one group with a level of statistical significance of 0.05 and a power of 0.80, using a posthoc 2-sample t test with a Bonferroni correction. These calculations indicated to include 35 patients in each group. For qualitative variables, comparisons between groups were performed by the χ2 test followed by the posthoc 2 × 2 Fisher exact test, when needed. All the calculations were performed with the SPSS 12.0 statistical package (SPSS, Inc., Chicago, IL).
RESULTS
Was the Randomization Process Adequate and Were the 2 Groups Comparable?
Eighty-two consecutive patients were considered for this study. One patient refused to sign the consent form. Another 8 patients did not reach the randomization due to the intraoperative decision of a nonresectional approach or the combination with other large abdominal procedures such as an additional Whipple procedure. Of the 73 randomized patients, 38 were men and 35 were women with a mean age of 57.1 ± 1.6 years.
Among the demographic parameters, the mean age (56.5 vs. 58.5 years) and BMI (24.6 vs. 25.2 kg/m2) were comparable between the IP and IC groups. However, a trend toward a difference in gender distribution was observed between the groups, which may occur in 6% of nonstratified randomizations. The preoperative risk profile and preoperative laboratory tests including liver function tests were similar in both groups. Prior to liver resection, chemotherapy was applied in 24 (33%) patients with 12 patients in each group (Table 1).
TABLE 1. Patient Characteristics
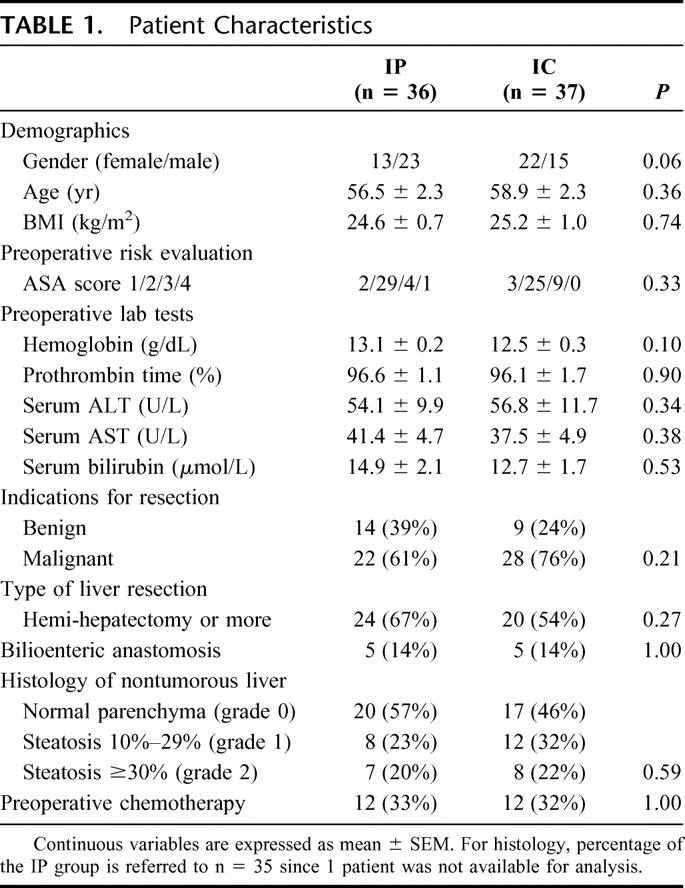
Twenty-six patients were operated for liver metastases from a colorectal carcinoma, 8 for cholangiocarcinoma, 5 for gallbladder carcinoma, 4 for hepatocellular carcinoma, and 7 for various kinds of metastatic diseases. In 23 patients, liver resection was performed for benign diseases, such as liver adenoma, focal nodular hyperplasia, echinococcosis, or hemangioma. The malignant and benign diagnoses were distributed homogeneously between the groups.
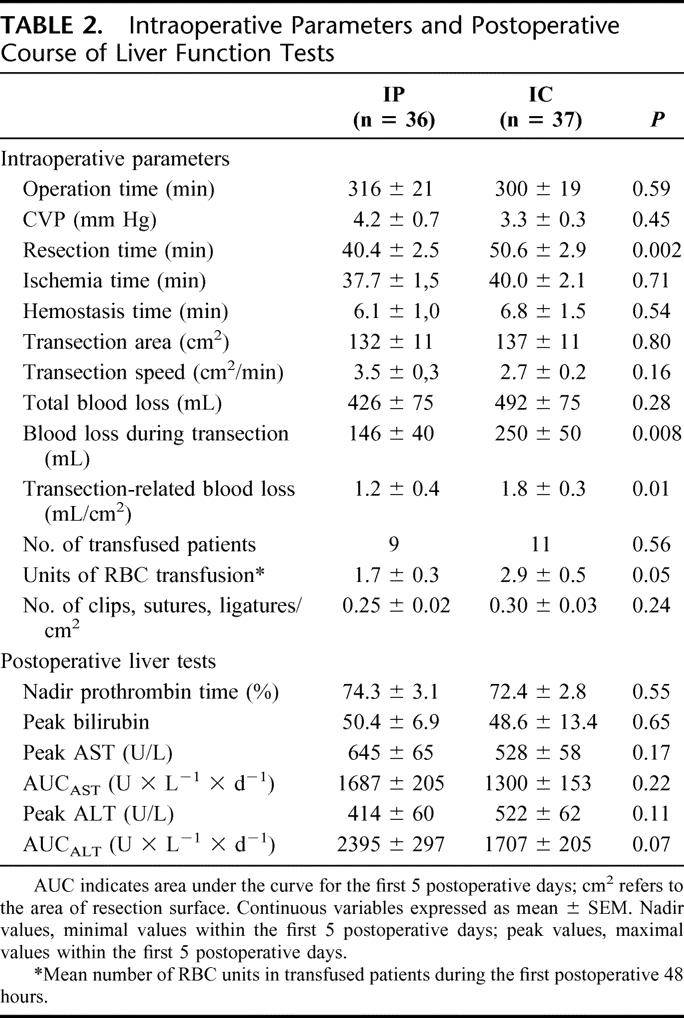
Forty-four patients (60%) underwent a hemi-hepatectomy or more, of whom 22 had an anatomic right (SV–VIII) hemi-hepatectomy, 8 had a left (SII–IV) hemi-hepatectomy, 8 had an extended right hemi-hepatectomy (SIV–VIII), and 6 had an extended left liver resection (SI–IV). Five patients of each group had an additional bilioenteric anastomosis. The type of hepatectomy, need of a bilioenteric anastomosis, and transection surface area (132 vs. 137 cm2) were comparable between the IP and IC groups (Tables 1, 2).
TABLE 2. Intraoperative Parameters and Postoperative Course of Liver Function Tests
Were Transection Time, Blood Loss, and Transfusions Influenced by IP and IC?
Liver transection was performed under low CVP (≤5 mm Hg) in the majority of patients (n = 65, 89%). In 8 patients (11%), CVP was higher than 5 mm Hg during transection mainly because of poor tolerance due to cardiac comorbidity. The overall mean transection time was 45.7 ± 2.0 minutes (median, 41 minutes; range, 30–106 minutes). The mean transection speed was 3.1 ± 0.2 cm2/min. IP was associated with a significantly shorter transection time than IC (40.4 vs. 50.6 minutes, P = 0.002), while the ischemia time was comparable in both groups. Although not statistical significant, a tendency for a faster transection speed was observed in the IP group (3.5 vs. 2.7 cm2/min., P = 0.16) (Table 2).
The overall mean blood loss was 460 ± 53 mL (median, 300 mL; range, 10–2000 mL). The total blood loss was 426 versus 492 mL (P = 0.28) and the blood loss during transection 146 versus 250 mL (P = 0.008) for IP and IC, respectively. When standardized to transection surface area, the mean blood loss during transection was also significantly lower in the IP group than in the IC group (1.2 vs. 1.8 mL/cm2, P = 0.01). In 12 of 37 (32%) patients with IC, parenchymal transection was interrupted due to significant bleeding during the 5-minute reperfusion cycles, while this did not occur in the IP group (P < 0.001).
Clips, sutures, or ligatures were used during parenchyma transection as additional methods to avoid hemorrhage and bile leaks. As shown in Table 2, the cumulative number of clips, sutures, and ligatures used per area of liver resection surface was not significantly different between the 2 groups (P = 0.24). The hemostasis time from the end of transection until completion of hemostasis was not different between both groups. Additional fibrin-based hemostasis methods such as fibrin glue (Tissucol) and fibrinogen-coated collagen patches (TachoSil) were only used in 2 patients. Twenty patients (27%) received RBC transfusions within the first 48 hours following surgery; 9 patients in the IP and 11 patients in the IC group. Transfused patients in the IC group received in mean one RBC unit more than transfused patients in the IP (2.9 vs. 1.7 units, P = 0.05).
How Effective Were Both Protective Strategies Against Postoperative Liver Injury?
The data of the postoperative liver injury are summarized in Table 2 and Figure 2. Although the mean transection time in the IP group was approximately 10 minutes shorter than in the IC group, the mean cumulative ischemia time of the 15-minute clamping cycles in the IC group was comparable with the mean ischemia time of continuous clamping in the IP group (40.0 vs. 37.7 minutes, P = 0.71). The peak of the transaminases occurred between 6 hours after surgery and the third postoperative day. In most patients, AST and ALT levels returned to normal values within 7 days. IC resulted in lower peak AST values than IP (528 vs. 645 U/L), whereas the opposite constellation was observed for peak ALT (522 vs. 414 U/L). However, these differences were not statistically significant. When the AUC was compared, IC resulted in lower mean AUCAST and AUCALT values compared with IP; however, again, both differences did not reach statistical significance (Table 2). When postoperative liver function was assessed by serum bilirubin and prothrombin time, no differences were found in mean peak values of both parameters between IP and IC.
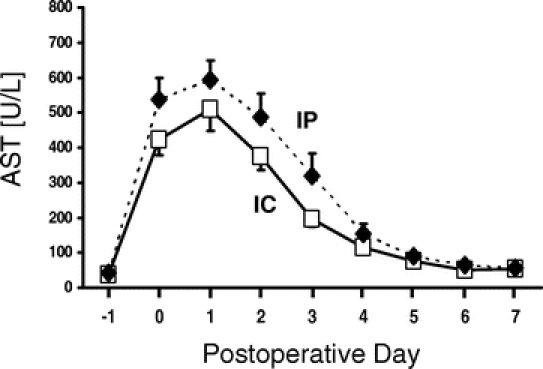
FIGURE 2. Postoperative liver injury assessed by serial measurements of serum AST for IP and IC clamping group. Day −1 and day 0 refer to the preoperative day and the postoperative period of the operative day, respectively. Data are mean ± SEM.
How Did Liver Steatosis and Age Influence the Liver Injury in Both Groups?
Histologic analysis was available on 72 of 73 resection specimens. Thirty-five patients (49%) had steatosis (≥10% of hepatocytes containing fat droplets), while steatosis was absent in the remaining 38 patients (51%). The distribution of patients with steatosis in the IP and IC group was comparable (43 vs. 54%, respectively). Also, the distribution of patients with grade 1 (11 vs. 17%) and grade 2 (10 vs. 11%) steatosis was similar in both groups reflecting comparable populations in IP and IC groups (Table 1).
For the entire study population, there was no correlation of postoperative liver injury and degree of steatosis. When steatosis was related to the degree of postoperative liver injury, steatotic patients (grades 1 and 2) in the IP group had a higher median AUCAST and AUCALT than patients without steatosis (grade 0). However, the observed differences were not statistically significant possibly due to the small sample size of the subgroups (Fig. 3). For IC, the median AUCAST was comparable in the nonsteatotic and steatotic subgroups (grades 1 and 2). A similar result was observed for AUCALT. These results suggest that nonsteatotic and steatotic livers are equally protected by IC (Fig. 3).
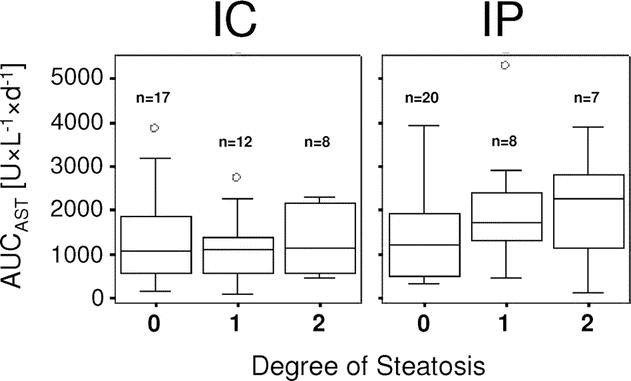
FIGURE 3. AUCAST for the first 5 postoperative days in relation to the degree of steatosis for IC and IP group. Steatosis was classified as grade 0 (<10%), grade 1 (10%–29%), and grade 2 (≥30%). AUCAST is presented as box plot with median, 25%, and 75% quartiles, and range of values.
One unexpected finding of our previous RCT was that IP efficiently protected younger patients (<60 years), while the protection was lost in older patients.23 Therefore, we reanalyzed our population by a stepwise age elimination of patients at 5-year intervals starting at 70 years. By this incremental analysis, we observed in the IP group a positive strong age-adjusted correlation with the mean AUCAST (R2 = 0.84). The increasing AUCAST with age implies that the protective effect of IP becomes weaker with increasing age in a population older than 60 years (Fig. 4). In contrast, the mean AUCAST remained unchanged in the different age populations of the IC group. When patients until 70 years were included, the mean AUCAST was lower for IC than for IP (1292 ± 171 vs. 1732 ± 242 U × L−1 × d−1; P = 0.22).
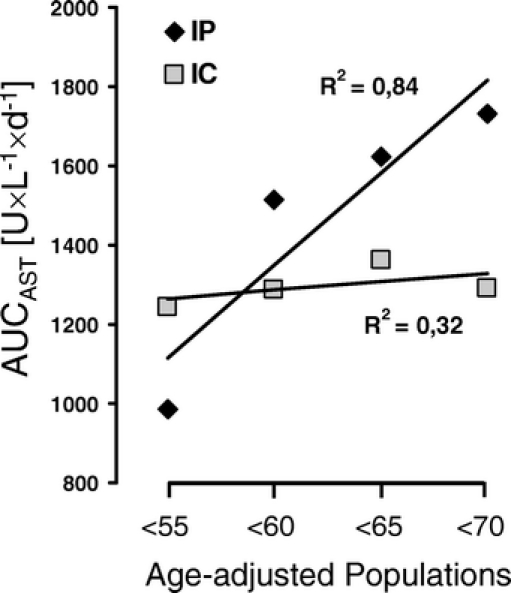
FIGURE 4. Age-adjusted correlation of AUCAST for the IP (solid rhomboid) and IC (open squares) group. Patients were backwards stepwise eliminated at 5-year intervals starting at the age of 70 years. The mean AUCAST were plotted against the different age groups <55, <60, <65, and <70 years. The degree of correlation is expressed by the correlation coefficient R2.
How Did Both Protective Strategies Impact on Postoperative Complications, ICU and Hospital Stays?
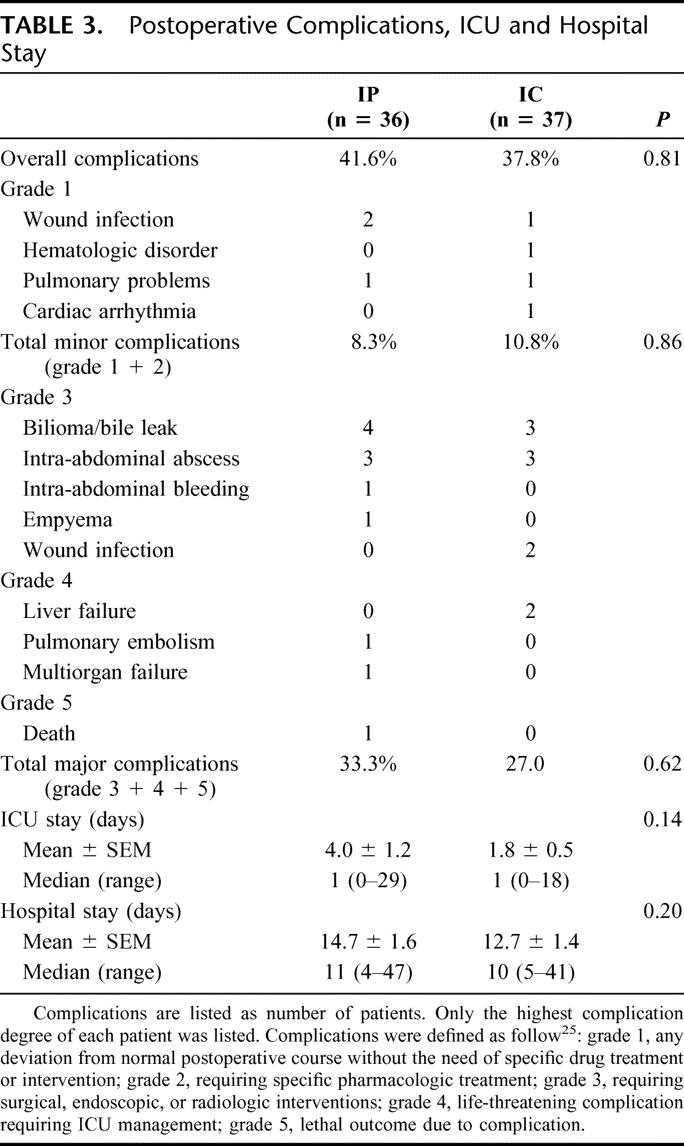
One patient who underwent an extended hemihepatectomy for a cholangiocarcinoma died at the 15th postoperative day. Death occurred due to intestinal ischemia followed by sepsis with multiorgan failure (mortality rate, 1.6%). Complications were ranked according to our recently reported classification of surgical complications.25 The overall complication rate of the entire study population was 40%. Thirteen patients (18%) developed postoperatively intra-abdominal symptomatic collections, including bilioma (n = 7) requiring percutaneous drainage or operative drainage in 4 patients (complication grade 3). Bilioma or bile leaks occurred in 4 patients in the IP group and in 3 patients in the IC group. There was no significant difference between IP and IC regarding overall (42% vs. 38%; P = 0.81), minor (8% vs. 11%, P = 0.86), and major (grade 3 or greater) complications (33% vs. 27%; P = 0.62) (Table 3). Also, the overall median ICU (1 vs. 1 day) and hospital stays (10 vs. 11 days) were not significantly different between both groups.
TABLE 3. Postoperative Complications, ICU and Hospital Stay
DISCUSSION
This is the first randomized controlled trial comparing IP with continuous inflow occlusion and IC of the portal triad in patients undergoing major liver resection. We found that IP was associated with a lower blood loss during parenchymal transection and significantly shorter transection time than IC. The degree of postoperative liver injury was comparable between the 2 groups but appeared higher in patients older than 65 in the IP group. Furthermore, neither overall nor major complications were significantly different between both protective strategies.
Because of the nature of a RCT, the findings of the study provide the highest level of evidence. The study protocol was designed based on our large experimental18,20,21,26–28 and clinical8,23 experience with both protective strategies with careful attention to minimize potential bias. Patients with cirrhosis were excluded from the trial to secure homogeneity between both groups since there might be evidence that IP and IC have different effects in this high-risk population. We included only major resection (>2 segments) to minimize the risk of type II errors. The involved anesthetists are well experienced to conduct this type of surgery under a maintained CVP below 5 mm Hg in most cases. Each procedure was performed by the same surgical team with a standardized transection technique. The surgical team was only informed intraoperatively about randomization after the decision was made to proceed with liver resection. Finally, the descriptive statistical analysis of both groups revealed a high degree of accordance regarding age, BMI, preoperative risk evaluation (American Society of Anesthesiologists, ASA), laboratory tests, and chemotherapy, as well as type of hepatectomy, need of a bilioenteric anastomosis, and transection surface area.
On the other hand, the study design of the RCT is inherently associated with a few limitations. There might be a concern that the study was performed without a control group of patients undergoing liver resection under continuous inflow occlusion only. We did not include such a group since the protective effects of both IP and IC versus continuous inflow occlusion have been previously established at the highest level of evidence.22–24 We focused our trial on which protective strategy is best, and felt that the addition of a control group is no longer ethically justifiable. Another limitation is that the sample size calculation was based only on 2 variables, namely, postoperative liver injury and blood loss. Thus, the sample size might not be sufficient to detect differences in other clinical outcome variables such as mortality, complications, ICU, and hospital stay. Finally, the study was not designed to look at long-term survival.
The first clinical attempt to minimize ischemic injury during liver resection was performed by interrupting long ischemic intervals with multiple short periods of reperfusion by Makuuchi et al in the 1980s.29 Belghiti et al showed in a RCT that IC of the portal triad by cycles of short ischemia (15 minutes) and reperfusion (5 minutes) provides a significant degree of protection during hepatic resection compared with patients with continuous clamping of the portal triad.22 As a drawback, the study disclosed a higher intraoperative blood loss for IC. The protective phenomenon of IP was initially discovered in the myocardium in the 1980s.30 Subsequently, the protection by IP against ischemic injury has been shown in many other organs including the liver.26 The protective effect of IP involves many different mechanisms, including inhibition of apoptosis.15,18,23 A recent cDNA microarray study in humans demonstrated that IP triggers the overexpression of IL-1Ra, iNOS, and Bcl-2, which counteracts the ischemia-induced pro-inflammatory and pro-apoptotic activation.31 We recently showed that IP significantly reduced the postoperative liver injury with preservation of cellular ATP content in patients undergoing major liver resection.23 Because of the absence of a direct comparison between IP and IC, we compared both strategies in a murine model of ischemic liver injury.18 Both IC and IP proved to be highly protective against ischemic injury in mice; however, prolonged ischemia of 120 minutes was better tolerated through IC. Based on this observation and the available RCTs on IC22 and IP,23 we initiated the current trial to identify the best protective strategy for major liver resection.
The central finding of this study is that there was no major difference in postoperative liver injury between IP and IC. The degree of the ischemic injury was not only based on peak or individual transaminase values, but also on the AUC for the first 5 postoperative days. The AUC has the advantage that it takes into account the slope of the postoperative transaminase drop. Although we found a slightly better postoperative profile regarding peak and AUC transaminase values for the IC group, this difference was not statistically significant and appears irrelevant in the context of equal postoperative liver function, assessed by peak bilirubin and nadir prothrombin time in both groups (Table 2). Thus, these data confirm our previous experimental findings that both strategies provide a similar protection for ischemia times not exceeding 75 minutes.18
There is growing evidence that liver steatosis is associated with reduced outcome after hepatic resection.1,32,33 One major finding of both RCTs comparing IP23 or IC22 versus continuous clamping was the highly protective effect of both strategies in steatotic livers. In our study, the protection conferred by IC was fully preserved in steatotic livers, even if high-grade steatosis (≥30%) was present (Fig. 3), while this protective effect was weaker in the IP group, as reflected by higher mean AUC of AST and ALT. These data may imply that IC should be preferred in patients with severe steatosis and expected prolonged ischemia time.
An unanticipated finding in our previous RCT was that IP conferred a high degree of protection in patients <60 years, while this effect was lost in older patients, particularly in those >70 years of age.23 Therefore, we performed an incremental age-adjusted analysis, where patients were stepwise eliminated at 5-year intervals starting at an age of 70 years. Thereby, we demonstrated that patients older than 65 years are less protected by IP while the protective effect of IC was maintained also in the older population (Fig. 4). This observation confirmed our previous results23 and implies that IC might be the preferred strategy in the older population (eg, >65 years of age) undergoing liver resection.
Another important finding of this study was the significantly lower blood loss during transection in the IP group. Even when transection-related blood loss was standardized to the transection surface area, IP was still associated with a significantly lower blood loss. Transfused patients in the IP group received significantly less blood units than those in the IC group. Similar findings were also observed in the RCT by Belghiti et al who reported greater blood loss during transection for IC than for the continuous clamping group.22 Intraoperative bleeding is not only associated with poor early2–4 and late4–6 outcome but impairs the optimal visibility of the transection plane and may make transection imprecise. Because of serious bleeding during the repeated reperfusion intervals, we had to interrupt the parenchymal transection to prevent blood loss in one third of the patients with IC while a “bloodless” transection was possible in the other two thirds of patients. This resulted in a significantly longer transection time and a slower transection speed. Although the transfusion amount was in favor of the IP group, the total blood loss was similar between the groups. The explanation of this finding is that the mean total blood loss was calculated for the entire study population, whereas the mean number of RBC units refers only to transfused patients (Table 2). Therefore, the total and transection-related blood loss is not directly reflected in the transfusion amount.
Postoperative mortality and morbidity were evaluated according to a standardized classification system of complications by severity.25 The mortality of the entire population was 1.6%, and is in the lower range of published reports.2,3 This favorable result was achieved in a nonselected population, including extensive liver resections (eg, cholangiocarcinoma) and many patients with underlying steatosis. Complications were documented in 40% of patients with an incidence of severe complications (grades 3–5) of 30%. The rate of overall and major complications was not significantly different between IP and IC. Symptomatic collections requiring an intervention occurred in 18% of our patients, in which biliomas or bile leaks were detected in 9.6%. Bile leaks remain still a challenging complication and are reported to occur in 5% to 14% of patients who undergo hepatectomy.34–36 The median ICU and hospital stay were similar in the IP and IC groups (Table 3), with figures comparable to those reported in other large series.2,3
The impact of IP and IC on long-term outcome including tumor recurrence was not evaluated in our study. There is clinical evidence that blood loss and transfusions promote tumor recurrence after hepatic resection.6,37 This observation would favor the strategy IP with continuous clamping. A recent experimental study found accelerated tumor growth following vascular clamping.38 Although IC showed an anti-growing tumor effect in this model, blood loss and transfusions in the clinical situation may abrogate the suggested experimental effect of IC in humans.
CONCLUSION
This RCT demonstrates that IC and IP are equally effective to minimize postoperative liver injury in noncirrhotic patients undergoing major liver surgery. Findings favoring IP are a significantly lower blood loss, lower transfusion amount, and shorter transection time. The comparison also suggests that IC may be superior in patients with severe steatosis and older than 65 years. Thus, we would conclude that both strategies can be used based on personal preference, but IP may be preferable in younger patients (eg, <65 years of age) with an expected inflow occlusion less than 75 minutes and in absence of severe steatosis. IC may be selected in older patients or in those with severe steatosis.
Discussions
Dr. Daniel Jaeck: Congratulations, Dr. Petrowsky, for this nice presentation and thank you also for sending me the manuscript of your paper. I think this is another nice prospective study in your field of expertise, and this study brings some answers to questions that liver surgeons continue to debate. We have already, and you mentioned them, at least 2 prospective randomized trials on hepatic pedicle clamping. One from Belghiti's group (Ann Surg. 1999;229:369–375) and you just reminded us of this study, which clearly showed that there is better hepatic tolerance to intermittent over continuous clamping, especially in patients with abnormal liver parenchyma. The second, from your group (Ann Surg. 2003;232:156–162) comparing continuous clamping with or without ischemic preconditioning, which you also recorded. So, logically, the study that we expected was a comparison between 2 groups: with and without ischemic preconditioning in the intermittent setting. The difficulty I feel, when you compare intermittent with ischemic, is in fact that you compare intermittent with continuous clamping. You add ischemic preconditioning in your continuous clamping, but you don't know exactly what is a part of the continuous clamping and what is a part of the ischemic preconditioning. So, in my opinion, the better study would have been to compare the intermittent setting with and without ischemic preconditioning. This is my first point.
Secondly, you mention that there was more bleeding with intermittent clamping, and this has been emphasized in Belghiti's study. The reason is that there is bleeding during the declamping period, and in the paper of Belghiti, they said that the best way to avoid this bleeding would be to secure ligature of vessels on both sides including the specimen. This will take time and prolong the ischemia time. So, in fact, during the intermittent clamping, how did you try to reduce the bleeding of the cut edge of the liver?
Thirdly, in both groups, you showed that, in fact, ischemic preconditioning had no protective effect in patients older than 65 and with severe steatosis. So my question concerns steatosis: do you perform systematic biopsy before deciding which procedure you will use?
And finally, in your opinion, what is the real impact of your comparative study at a time when bloodless transection techniques are available without clamping, for instance, using Tissuelink dissector or other devices which reduce the interest of the clamping.
Dr. Henrik Petrowsky: Professor Jaeck, thank you very much for your comments and questions. Your first question addressed the design of the study. Our goal was to compare, as you mentioned, 2 established protective protocols, namely, intermittent clamping versus ischemic preconditioning with continuous clamping. We did not attempt designing a new protocol, and we also omitted a control group with continuous clamping alone in view of previous randomized trials showing significant protection from both ischemic preconditioning and intermittent clamping, when compared with continuous clamping alone. Your second question is how we can reduce bleeding during intermittent clamping? This study was a follow-up of our trial presented last year at the ESA meeting, where we showed that the crushing clamping technique under inflow occlusion is superior to other transection strategies using CUSA, hydrojet, or TissueLink, which are all marketed for use without the need for inflow occlusion. Thus, logically we chose the crushing technique and ligated all structures belonging to the remnant liver; only larger vessels of more than 3 mm were ligated on both sides. It might indeed be useful, as you suggest, to meticulously ligate all small vessels on both sides, but this would obviously extend the duration of resection. Your third question is related to the use of biopsy to identify steatosis. At this point, we do not perform liver biopsy for this purpose. I would like to emphasize that this study was not powered for steatosis; therefore, one should remain cautious in drawing a conclusion. Ischemic preconditioning was also protective in patients with steatosis; the data only suggest that intermittent clamping might be superior. Finally, you challenged the need of inflow occlusion in the presence of available “bloodless” transection devices. Here again, I would like to refer to our last trial, in which we found that the clamp crushing technique is superior to other “bloodless” devices in terms of duration of transection, blood loss, and costs. Of note, in this study, about one third of patients treated with either of the so-called “bloodless” devices required a Pringle maneuver due to excessive intraoperative bleeding. Therefore, we think that there is still an important role for inflow occlusion in performing major liver resection.
Dr. Peter Neuhaus: I have 2 questions. First, do you use any additional drugs for ischemia protection, because we have employed steroids routinely for many years at the beginning of the operation? We have shown experimentally that the advantage of preconditioning is abolished when you use steroids. The same can be done with aprotinin, but that's more expensive.
The other question is regarding living donor liver transplantation. We, you, and others divide the liver without clamping and with a blood loss of 300 to 500 mL at the most during this dissection of the right lobe. In Berlin, we do 90% of liver resections without ischemia. Would you agree now, after the study is finished, that most liver resections can be done without ischemia?
Dr. Henrik Petrowsky: Professor Neuhaus, many thanks for your questions. First, we do not use steroid or other drugs in our liver resection, and thus I cannot comment on their impact on ischemic preconditioning. Your second point is similar to the last remark from Professor Jaeck about the need for inflow occlusion in liver surgery. Similarly to your experience, we perform also right living donor hepatectomies without inflow occlusion, and none of these patients has required blood transfusion until today. However, our experience is different for major hepatectomies performed mostly for large tumors. We do think that the use of inflow occlusion is beneficial in many cases of difficult liver resections. Although a number of surgeons challenge the need for inflow occlusion, the situation in the “real world” seems different. I would like to quote a recently published survey from Japan, where HPB surgeons were asked whether they use inflow occlusion. Most answered that they use inflow occlusion routinely or selectively, while only 7% of Japanese surgeons answered that they would never use it.
Dr. Peter Neuhaus: Can I just comment that the Japanese surgeons have mainly cirrhotic patients? I was mainly referring to the normal liver with metastasis and other pathologies.
Dr. Pierre Clavien: Professor Borel-Rinkes is conducting such a survey in Europe, and I would like to ask him whether he could share some of the results.
Dr. Inne Borel-Rinkes: Just a very short comment. We are currently performing a survey among the members of the European HPB Society, and I have just talked with Dr. Clavien also to include as many people as possible, including ESA members, who are experienced liver surgeons. It's still very preliminary, but, from the results, it is surprising that many more surgeons use some form of clamping also in noncirrhotic cases, more than we would have thought at the beginning of the questionnaire. So I would encourage everybody to complete the 5-minute questionnaire. It would be interesting to be able to get these results from a Western European prospective.
Dr. Olivier Farges: My question is, along the same lines, that no clamping is an alternative to clamping. You have mainly looked at serum transaminase levels in your study, which are supposed to reflect what happens in the liver. How confident are you, however that, with transaminase levels, you actually measure the impact of clamping? As a matter of fact, if you try to correlate duration of clamping, which is possible because now many patients are not clamped, with the increase in transaminases levels, there tends to be no correlation at all. My second point is that you have not considered what happens before the liver as a result of clamping, in other words, intestinal ischemia. Have you been able to monitor the suffering of the intestine, for example, by looking at carbon dioxide expiration or acidosis? This might not have had a measurable clinical impact in your study because you have used classic clinical endpoints and because the number of patients is small but it could result, for example, in abdominal pain or speed of return of bowel movements.
Dr. Henrik Petrowsky: I did not show these data in my presentation, but we also failed to show a strong correlation between ischemia time and postoperative AST levels. There are many factors in the clinical setting that may influence transaminase levels, such as the extent of liver resection, the presence of steatosis, or the use of preoperative chemotherapy. However, transaminase levels remain a well-accepted marker of severe liver injury, and here we did not only assess peak AST levels, but also the area under the curve of the postoperative AST course. Additionally, we looked at many other markers, including prothrombin time, bilirubin levels, and clinical features such as encephalopathy. We did not look at injury to the intestine, and would agree that this is a valuable endpoint to investigate in the future.
Dr. James Garden: You stated right at the beginning of your presentation that one of the most important aspects of minimizing blood loss is controlling the central venous pressure. In demonstrating a difference between your 2 study groups, I just wondered if you could make a comment on whether or not the anesthetists were able to maintain that CVP below 5 mm Hg or whether the 2 techniques that you employed posed difficulties for them at the top end of the table.
Dr. Henrik Petrowsky: Thank you for your questions, Professor Garden. I did not show the data on CVP, but this information is available in the paper. We measured CVP in each patient during surgery; and in most cases, it could be kept below 5. There was no significant difference in CVP between both groups.
Dr. Pierre Clavien: I would like to conclude with 2 points. First, a number of discussants questioned the need for inflow occlusion. Most surgeons, worldwide, still use inflow occlusion for difficult cases of liver resection to prevent blood loss; therefore, efforts to minimize ischemic injury are still warranted. As there is currently sufficient evidence that both intermittent clamping and ischemic preconditioning strategies are protective, the results of this study might be of value for those who choose to perform liver resections under inflow occlusion and low CVP. The second point is that such a study is a good example of a successful translational research. These strategies have been well investigated in various rodent models with, additionally, the availability of more convincing endpoints such as animal survival. Basically, most of the results gathered in experimental studies were also observed in the human situation and have enabled to design better trials. This point is made to highlight the need to support basic research in surgery.
Footnotes
Dr. Petrowsky and Dr. Selzner are the recipients of the Novartis fellowship in HPB surgery and liver transplantation at the Swiss HPB Center.
Reprints: Pierre-Alain Clavien, MD, PhD, Department of Visceral and Transplantation Surgery, Zurich University Hospital, Rämistrasse 100, CH-8091 Zürich, Switzerland. E-mail: clavien@chir.unizh.ch.
REFERENCES
- 1.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406–407. [DOI] [PMC free article] [PubMed]
- 3.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708; discussion 708–710. [DOI] [PMC free article] [PubMed]
- 4.Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869; discussion 869–870. [DOI] [PMC free article] [PubMed]
- 5.Tsao JI, Loftus JP, Nagorney DM, et al. Trends in morbidity and mortality of hepatic resection for malignancy: a matched comparative analysis. Ann Surg. 1994;220:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 7.Pringle J. Note on the arrest of hepatic hemorrhage due to trauma. Ann Surg. 1908;48:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavien PA, Yadav S, Sindram D, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesurtel M, Selzner M, Petrowsky H, et al. How should transection of the liver be performed? A prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814–822, discussion 822–823. [DOI] [PMC free article] [PubMed]
- 10.Huguet C, Nordlinger B, Bloch P, et al. Tolerance of the human liver to prolonged normothermic ischemia: a biological study of 20 patients submitted to extensive hepatectomy. Arch Surg. 1978;113:1448–1451. [DOI] [PubMed] [Google Scholar]
- 11.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya S, Jackson DJ, Beard CI, et al. Central venous pressure and its effects on blood loss during liver resection. Br J Surg. 1999;86:282–283. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima Y, Shimamura T, Kamiyama T, et al. Control of intraoperative bleeding during liver resection: analysis of a questionnaire sent to 231 Japanese hospitals. Surg Today. 2002;32:48–52. [DOI] [PubMed] [Google Scholar]
- 14.Wei AC, Tung-Ping Poon R, Fan ST, et al. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. [DOI] [PubMed] [Google Scholar]
- 15.Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. [DOI] [PubMed] [Google Scholar]
- 16.Isozaki H, Adam R, Gigou M, et al. Experimental study of the protective effect of intermittent hepatic pedicle clamping in the rat. Br J Surg. 1992;79:310–313. [DOI] [PubMed] [Google Scholar]
- 17.Hewitt G, Halliday I, McCaigue M, et al. Mortality, endotoxaemia and cytokine expression after intermittent and continuous hepatic ischaemia. Br J Surg. 1995;82:1424–1426. [DOI] [PubMed] [Google Scholar]
- 18.Rudiger HA, Kang KJ, Sindram D, et al. Comparison of ischemic preconditioning and intermittent and continuous inflow occlusion in the murine liver. Ann Surg. 2002;235:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang KJ, Jang JH, Lim TJ, et al. Optimal cycle of intermittent portal triad clamping during liver resection in the murine liver. Liver Transpl. 2004;10:794–801. [DOI] [PubMed] [Google Scholar]
- 20.Peralta C, Bartrons R, Riera L, et al. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol. 2000;279:G163–G171. [DOI] [PubMed] [Google Scholar]
- 21.Selzner N, Selzner M, Jochum W, et al. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39:55–61. [DOI] [PubMed] [Google Scholar]
- 22.Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clavien PA, Selzner M, Rudiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850; discussion 851–852. [DOI] [PMC free article] [PubMed]
- 24.Li SQ, Liang LJ, Huang JF, et al. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol. 2004;10:2580–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peralta C, Closa D, Hotter G, et al. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Commun. 1996;229:264–270. [DOI] [PubMed] [Google Scholar]
- 27.Yadav SS, Sindram D, Perry DK, et al. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223–1231. [DOI] [PubMed] [Google Scholar]
- 28.Rudiger HA, Graf R, Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J Hepatol. 2003;39:972–977. [DOI] [PubMed] [Google Scholar]
- 29.Makuuchi M, Mori T, Gunven P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987;164:155–158. [PubMed] [Google Scholar]
- 30.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 31.Barrier A, Olaya N, Chiappini F, et al. Ischemic preconditioning modulates the expression of several genes, leading to the overproduction of IL-1Ra, iNOS, and Bcl-2 in a human model of liver ischemia-reperfusion. FASEB J. 2005;19:1617–1626. [DOI] [PubMed] [Google Scholar]
- 32.Behrns KE, Tsiotos GG, DeSouza NF, et al. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. [DOI] [PubMed] [Google Scholar]
- 33.Kooby DA, Fong Y, Suridwinata A, et al. J Gastrointest Surg. 2003;7:1034–1044. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama H, Masuda H, Shibata M, et al. Incidence of bile leakage after three types of hepatic parenchymal transection. Hepatogastroenterology. 2003;50:1517–1520. [PubMed] [Google Scholar]
- 35.Nagano Y, Togo S, Tanaka K, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003;27:695–698. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka S, Hirohashi K, Tanaka H, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484–489. [DOI] [PubMed] [Google Scholar]
- 37.Asahara T, Katayama K, Itamoto T, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–680. [DOI] [PubMed] [Google Scholar]
- 38.van der Bilt JD, Kranenburg O, Nijkamp MW, et al. Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology. 2005;42:165–175. [DOI] [PubMed] [Google Scholar]


