Abstract
Objective:
To evaluate the outcome of reoperations in patients with duodenopancreatic neuroendocrine tumors (PETs) in a tertiary referral center.
Summary Background Data:
The management of reoperations in PETs is still controversial.
Methods:
A total of 125 patients with PETs that underwent surgery between 1987 and 2004 at our institution were retrospectively evaluated. The diagnosis of PETs was based on clinical symptoms, biochemical tests, and histopathology. Patients with at least one reoperation were analyzed regarding clinical characteristics, pathology, operations, and long-term follow-up.
Results:
A total of 33 patients with a median age of 42 years were identified for this study: 13 patients had gastrinomas, 12 patients had nonfunctional islet cell tumors, 6 patients had insulinomas, and 2 patients had vipomas; 24 patients had sporadic NETs, 9 patients had a MEN-1-syndrome; 27 patients had histologically verified malignant tumors; 33 initial operations and 50 reoperations were performed. The initial procedures comprised 27 resections of the primary tumor and 6 explorative laparotomies; 28 of all reoperations were resections of distant metastases, including 15 liver resections; 19 resections of the pancreas or duodenum were performed during reoperations. The overall morbidity and mortality was 45% and 4.8%, respectively. After a median follow-up of 124 months (range, 16–384 months), 27 of 33 patients are still alive, 12 without evidence of disease. All 6 patients with benign tumors are still alive. The 5-, 10-, and actuarial 25-year survival rate for patients with malignant tumors were 81%, 72%, and 36%, respectively. The survival rate was significantly related to the patients age at time of initial operation and better in patients younger than 50 years compared with patients older than 50 years (P = 0.0007), and the presence or development of metastases (none or lymph node metastases versus distant metastases: P = 0.01).
Conclusion:
We show that an aggressive surgical approach leads to long-term survival in patients with malignant PETs. Although long-term cure can only be achieved in a proportion of patients with malignant PETs, significant long-term palliation can be achieved.
An aggressive surgical approach for pancreaticoduodenal endocrine tumors (PETs) leads to long-term survival in patients with malignant PETs. Although long-term cure is rare, significant long-term palliation can be achieved.
Pancreaticoduodenal endocrine tumors (PETs) represent an important subset of pancreatic neoplasms. They account for 2% to 4% of clinically detected pancreatic tumors, can consist of single or multiple benign or malignant neoplasms,1 and are associated in 10% to 20% with multiple endocrine neoplasia type 1 (MEN-1). PETs present as either functional tumors, causing specific hormonal syndromes, such as Zollinger-Ellison syndrome (ZES) or organic hyperinsulinism, or as nonfunctional PETs with symptoms similar to patients with pancreatic adenocarcinoma.2
The natural history of PETs is highly variable. Small, benign neoplasms such as 90% of all sporadic insulinomas are readily curable by surgical resection. Most other functional and all nonfunctional PETs have a much less favorable prognosis. Approximately 50% to 80% of these neoplasms recur or metastasize, and up to one third of patients already have metastases at initial presentation.3 Historic controls with untreated liver metastases have a 5-year survival of only 20% to 30%.4 Also large retrospective series showed that liver metastases are the worst prognostic factors.5,6 Chemotherapy and radiation therapy have not demonstrated significant antitumoral effects.7 Even chemoembolization has not been shown to improve survival.
Reoperations for PETs may be indicated not only for synchronous or metachronous lymph node or liver metastases, but also for persistence after a failed primary operation, recurrence, or newly developing tumors. Despite accumulating data that have suggested that resection of PETs and their metastases in selected patients can prolong survival,8 there are very little data available regarding the outcome of reoperations in this setting. Therefore, we analyzed our strategy of an aggressive surgical approach in a tertiary referral center.
MATERIALS AND METHODS
A total of 125 patients that underwent surgery for PETs and/or metastases of PETs between 1987 and December 2004 at the Department of Surgery of the Philipps-University Marburg were retrospectively evaluated. The clinical records of all patients with at least one reoperation during this time range, either after primary operation at other hospitals or at our institution, were analyzed with special regard to patient demographics, clinical characteristics, pathologic findings, operative procedures, complications, and long-term follow-up.
The diagnosis of ZES was established by clinical symptoms, an elevated fasting serum gastrin level (>125 pg/mL), a positive secretin stimulation test defined as an increase of serum gastrin concentration to >200 pg/mL together with low pH in the stomach, and a positive immunohistochemistry for gastrin in the tumor cells. The diagnosis of insulinoma required a symptomatic hypoglycemia (<40 mg/dL) with concomitant endogenous hyperinsulinism (>20 μU/mL) during a supervised fasting test and a positive immunohistochemistry for insulin in the tumor cells. The diagnosis of vipoma was confirmed by watery diarrhea (>6 L/day) and a fasting vasoactive intestinal polypeptide serum concentration >130 pg/mL. Lesions were considered as nonfunctioning PETs if there were no clinical symptoms of hormonal excess present and plasma hormone levels except those of pancreatic polypeptide were within normal limits. Malignancy was determined on the basis of strict criteria of infiltrating growth, lymph node, or distant metastases. All tumors beside small insulinomas (<2 cm) without any signs of malignancy were treated by pancreatic resection with regional lymph node dissection. In all types of PETs, synchronous or metachronous liver metastases were resected simultaneously at our institution if 90% or more of metastatic tissue in the liver could be resected.
Patients who fulfilled the criteria of ZES, insulinoma, or vipoma underwent laparotomy, and patients with nonfunctional PETs were scheduled for exploration if the tumor size was >1 cm in diameter. In all patients, diffuse liver metastases were excluded by preoperative imaging. Preoperative imaging was comprised of abdominal ultrasonography (US), magnetic resonance imaging (MRI), computed tomography (CT), somatostatin-receptor-scintigraphy (SRS), endoscopic ultrasonography (EUS), and in earlier years, selective angiography (SA), selective portal venous sampling (PVS), and selective hepatic venous sampling after arterial stimulation (modified Imamura procedure).
Persistence or recurrence of the different types of PET was defined by the same criteria as for primary tumors. Patients with MEN-1 were followed annually by biochemical testing, abdominal CT, EUS, and SRS. In patients with sporadic PETs, at least an abdominal ultrasonography, an abdominal CT, and an SRS were performed. Biochemical testing was performed only in patients with primary functional tumors. Cure of ZES was defined as a normal fasting gastrin concentration (<125 pg/mL) and a negative secretin stimulation test postoperatively and at follow-up investigations. Insulinoma was considered to be cured when fasting serum glucose levels were >40 mg/dL and concomitant insulin levels were <20 μU/mL. Nonfunctional PETs were considered to be cured if there was no evidence of tumor upon imaging studies. Absence of metastases was also confirmed by imaging studies, primarily by SRS and CT.
Indications for abdominal reoperations were a failed primary operation without identifying a primary tumor, newly developed PETs, or local and distant metastases. Reoperations that were performed only because of complications were counted separately. The specific reoperation(s) performed was dependent on pattern of disease recurrence, as identified by imaging studies. Reoperative cases involved enucleation of the tumor(s) in the pancreatic head or neck, distal pancreatic resection, duodenotomy with tumor excision, pylorus-preserving pancreaticoduodenectomy, or resection of metastases alone.
Descriptive and explorative statistics were performed by using mean, median, standard deviation (SD), range, and frequencies. Survival curves were computed from the time of surgical exploration to either death or most recent contact by using the Kaplan-Meier method. Censored patients are patients who are alive at the end of the observation time. Log-rank test was applied to identify significant differences. Analyzing proportions χ2 test was used. P values <0.05 were considered as statistically significant. Data were analyzed using SPSS software (version 11; SPSS, Inc., Chicago, IL).
RESULTS
Clinical Characteristics and Pathology
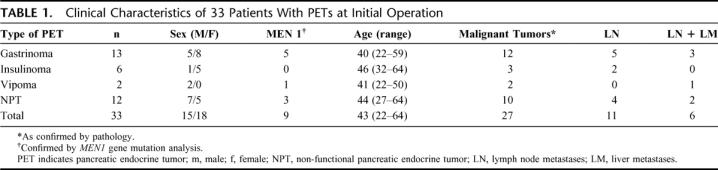
The clinical characteristics for the identified patients are given in Table 1. The patients with sporadic disease were older than patients with MEN-1 syndrome (45 vs. 36 years, P = <0.05); 27 (82%) patients had histologically verified malignant tumors as characterized by the presence of lymph node and/or distant metastases; 17 patients had local and/or distant metastases at initial operation, whereas 10 patients were classified as malignant because of angioinvasion or infiltrating growth. Six (18%) patients had no sign of malignancy at the termination of this study (Table 1). Three of these 6 patients had benign insulinomas, which were not identified at initial operation. The other 3 patients were MEN-1 patients. In the 24 patients with sporadic tumors, the median largest tumor size in all patients was 39 mm (range, 3–250 mm). Medium tumor size did not differ between patients with sporadic tumors (38 mm; range, 6–100 mm) and MEN-1-patients (mean, 39 mm; range, 3–250 mm). The tumors were distributed through the whole pancreas, with the majority resected from the pancreatic head. At initial operation, 11 of 33 (33%) patients had lymph node metastases only, 10 of them with sporadic tumors and 1 MEN-1 patient. Six (18%) patients presented with distant metastases, 1 of them a MEN-1 patients with a single lung metastasis. The median tumor size of patients who did not have distant metastases during the study period was 15 mm (range, 3–40 mm) versus 65 mm (range, 20–250 mm) in patients with distant metastases either at initial operation or during the study (P = <0.05). In the course of the study, distant metastases occurred in 17 (52%) patients, 10 (30%) patients had only lymph node metastases and 6 (18%) patients remained free of any metastases.
TABLE 1. Clinical Characteristics of 33 Patients With PETs at Initial Operation
Operative Procedures: Initial Operations
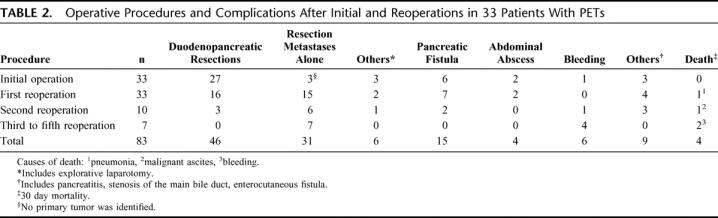
Of the 33 initial operations, 18 (55%) were performed at our institution and 15 (45%) at other hospitals; 7 of 9 MEN-1 patients were initially operated in our institution, whereas 13 of 24 initial procedures in sporadic patients wereperformed at other hospitals. The initial procedures comprised 27 resections of the pancreas or the duodenum. Among the patients with sporadic tumors, 11 distal pancreatic resections, 4 pancreaticoduodenectomies, and 3 tumor enucleations (Table 2) were performed.
TABLE 2. Operative Procedures and Complications After Initial and Reoperations in 33 Patients With PETs
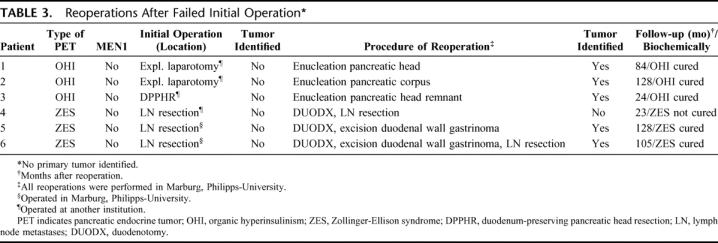
In 6 patients with sporadic tumors (3 with an insulinoma, and 3 with ZES), no primary tumor was identified at initial operation. This happened in 2 of 19 (11%) patients initially operated in our institution and in 4 of 15 (27%) patients initially operated at other hospitals. However, in all 3 ZES patients without identified primary tumor, lymph node metastases were resected (Table 3).
TABLE 3. Reoperations After Failed Initial Operation
MEN-1 ZES patients underwent duodenotomy with excision of duodenal gastrinomas as initial procedure alone, duodenotomy together with enucleation of pancreatic tumors, and/or distal pancreatic resection. The patient with the large malignant vipoma had a splenopancreatectomy with left colectomy as initial procedure. All MEN-1 patients with nonfunctioning PETs were treated with distal pancreatic resections and, if necessary, enucleation of tumors of the pancreatic head.
Operative Procedures: Reoperations
Overall, 50 reoperations were performed: 13 in patients with MEN-1 and 37 in patients with sporadic tumors. Twenty-three patients underwent only one reoperation, whereas 5 patients underwent two, 4 patients underwent three, and one patient underwent five reoperations, respectively. Forty-four (88%) of the reoperations were performed at our institution, including the final reoperation of all patients.
A total of 28 (56%) of all reoperations were resections of distant metastases, including 11 liver segmentectomies, 4 hemihepatectomies, 3 adrenalectomies, and 1 nephrectomy. Twenty-four of these resections were performed in patients with sporadic tumors, whereas only 4 reoperations for distant metastases were performed in MEN-1 patients, 3 of them in the patient with the large vipoma. Nineteen (38%) resections of the pancreas or duodenum were performed during reoperations (Table 2); the most common were tumor enucleation (n = 4), duodenotomies (n = 4), distal pancreatic resection (n = 3), and pylorus-preserving pancreaticoduodenectomies (n = 3). Eight of these operations were performed in MEN-1 patients. The 3 (6%) remaining operations were resections of local lymph node metastases, one of them in a MEN-1 patient, in whom a NPT was not enucleated because of the closeness to main pancreatic duct.
In all 3 patients with sporadic insulinoma, where the primary operation had failed, the tumor was detected by intraoperative ultrasound (IOUS) and/or palpation at first reoperation. Also, in 2 sporadic ZES patients with failed primary operation, a duodenal gastrinoma was excised after duodenotomy (Table 3). However, in 1 patient with ZES, no tumor was detected despite duodenotomy and separate palpation of the anterior and posterior duodenal wall, as well as meticulous palpation of the pancreas and IOUS.
Complications
The overall morbidity was 45%. The morbidity increased from 36% after the initial operation to 57% after third to fifth reoperation, which was statistically not significant (P = >0.05). The most frequent complication was transient pancreatic fistula, which occurred after 15 of 46 (32%) resections of the pancreas or duodenum (Table 2). All fistulas could be managed without reoperation. Fistula rate did not differ between patients with sporadic tumors and MEN-1 patients. After 83 operations, 3 patients (3.6%) had to undergo reoperation in the early postoperative period because of abdominal abscess, enterocutaneous fistula, and ischemic colitis. Altogether, after a total of 83 operative procedures, 4 patients died during the perioperative period, resulting in an overall mortality of 4.8%. All 4 patients had a sporadic tumor. The causes of death were respiratory failure, severe pneumonia, bleeding (n = 2), and decompensated ascites, respectively. The mortality increased from 3% after the first reoperation to 29% after third reoperation. Because of the low number of patients undergoing 3 or more reoperations, this was statistically not significant (P = >0.05).
Follow-up and Survival
After a median follow up of 124 (range, 16–384) months, 27 of 33 (82%) patients are still alive. Twelve of 33 (36%) patients are free of disease, 15 (45%) are alive with their disease, and 6 (18%) patients died. From the 27 patients with malignant disease, 9 (33%) patients were free of detectable disease, 4 patients after removal of lymph node metastases, 4 after removal of liver metastases, and 1 MEN-1 patient after surgical removal of a lung metastasis. Twelve (45%) patients were alive with disease and 6 (22%) were deceased.
Overall, after a median follow-up of 142 months (range, 36–384 months), 5 of 13 (38%) patients operated for ZES are biochemically cured, 3 of them with a MEN-1 syndrome. Five patients (38%) are alive with their disease, and 3 (24%) patients died due to liver metastases. Despite a negative secretin test, all 5 ZES-MEN-1 patients developed new nonfunctioning PETs (<1 cm) in the pancreatic remnant.
After a median follow-up of 145 months (range, 37–339 months), all 3 patients with benign insulinomas and 1 patient with a malignant insulinoma have been rendered euglycemic and asymptomatic. The other 2 patients with malignant insulinoma died. Only 1 of 12 (8%) patients with NPT died. Four patients with sporadic NPTs and 1 MEN-1 patient (42%) are free of disease and 6 (50%) live with their disease after a median follow-up of 142 months (range, 16–236 months). The MEN-1 patient with the malignant vipoma developed liver metastases.
The 5-, 10-, and actuarial 25-year survival rate was 84%, 76%, and 38%, respectively, for all patients (Fig. 1), and 81%, 72%, and 36%, respectively, for patients with malignant tumors. Besides the 4 patients who died during the perioperative period, 2 more patients were deceased due to diffuse liver metastases. All patients with benign tumors and all MEN-1 patients are still alive and the median survival was not reached in this group. The median actuarial survival for patients with malignant disease was 337 months.
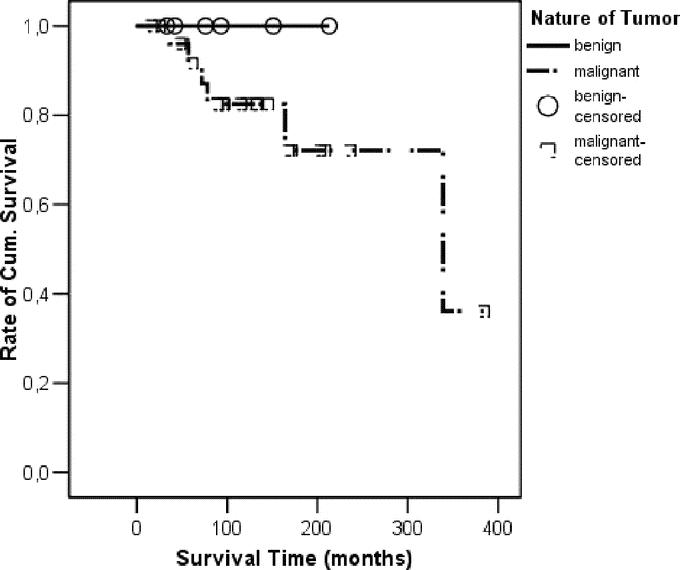
FIGURE 1. Kaplan-Meier survival curve related to the nature of the tumor. Whereas all patients with benign tumors are still alive, the patients with malignant lesions had a 5-, 10-, and actuarial 25-year survival rate of 81%, 72%, and 36%, respectively.
The survival rate was significantly related to the patients age at time of initial operation and was better in patients younger than 50 years compared with patients older than 50 years (P = 0.0007) (Fig. 2). We then compared patients who either had distant metastases at initial operation or developed distant metastases after it with patients who remained free of any metastases or had lymph node metastases at initial operation or developed only lymph node metastases (Fig. 3). We found a significant better survival in the latter group (P = 0.01).
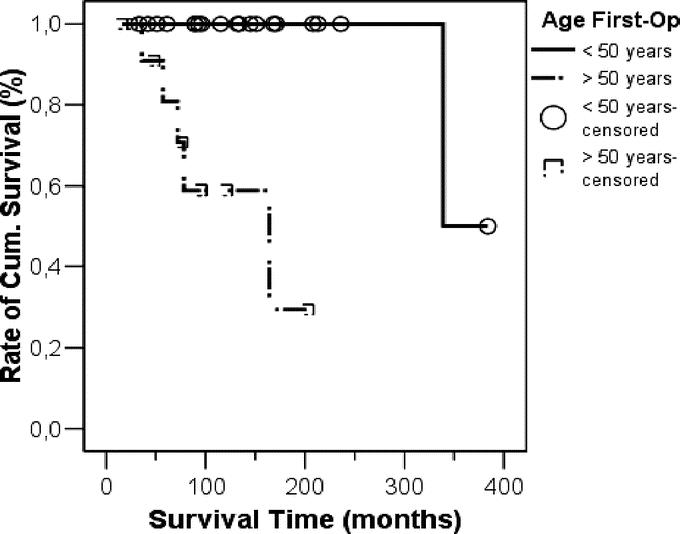
FIGURE 2. Kaplan-Meier survival curve comparing the patients’ age at time of initial operation. Patients younger than 50 years had a much better survival compared with patients older than 50 years (P = 0.0007).
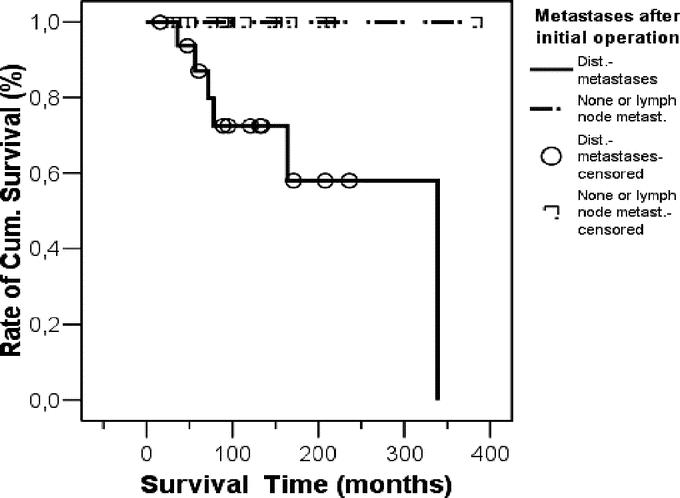
FIGURE 3. Kaplan-Meier survival curve comparing patients who either had distant metastases at initial operation or developed distant metastases with patients who remained free of any metastases or had lymph node metastases at initial operation or developed only lymph node metastases. Survival was significant better in the latter group (P = 0.01).
There was a trend toward greater survival related to the presence of metastases at time of initial operation (none or lymph node metastases vs. distant metastases: P = 0.13), genetic background of tumor (MEN-1 vs. sporadic: P = 0.16, Fig. 4), number of reoperations (1 vs. >1: P = 0.13), indication of reoperation (failed primary vs. recurrence/metastases: P = 0.12), and chemotherapy (P = 0.05). The survival rate was statistically not affected by gender (P = 0.84), type of tumor (functioning vs. nonfunctioning: P = 0.51) or dignity (benign vs. malignant: P = 0.35), the latter because of the small sample size of patients with benign tumors.
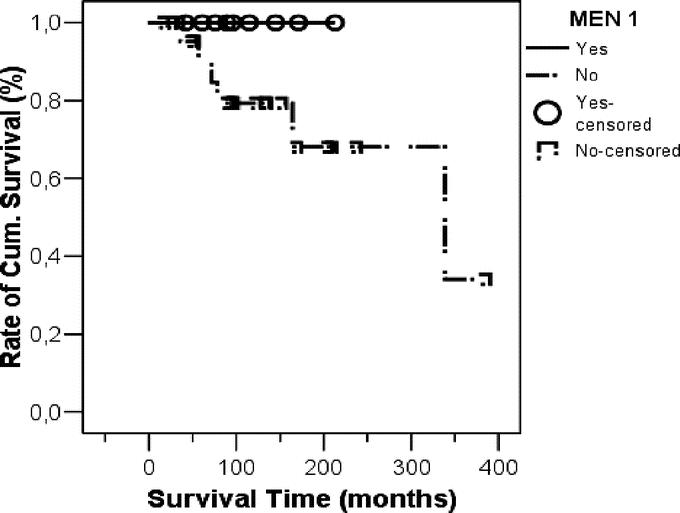
FIGURE 4. Kaplan-Meier survival curve comparing patients with different genetic background of tumor (MEN-1 vs. sporadic). Although no MEN-1 patient died, due to the low number of this group, the survival was statistically not significantly better than in patients with sporadic disease (P = 0.16).
DISCUSSION
The only potentially curative option for patients with PETs is complete surgical resection, as it was shown for insulinomas,9,10 gastrinomas,11,12 and nonfunctioning PETs.13,14 The rationale for surgical resection is clear. If the condition is untreated, the natural history of malignant PETs is one of slow but gradual progression, with the development of liver metastases and death of the patients.15,16 In a retrospective study from the National Institutes of Health, Fraker et al17 showed that 23% of patients with gastrinoma who did not have resection of their primary tumor developed liver metastases, whereas only 3% of patients who underwent operative resection of their gastrinoma developed liver metastases. Furthermore, elimination of a sometimes spectacular hormonal syndrome is of major interest. Therefore, aggressive resection of the primary tumor in patients with PETs is highly recommended by different groups.7,18–21 In this study, the initial operation not only included resection of the primary pancreaticoduodenal tumor but concomitant resections of synchronous hepatic metastases, as done by others.8 In patients with locally advanced primary tumors without distant metastases, a multivisceral resection should be considered.7
The indications for reexploration, either for recurrence or metastases, are largely undefined and controversial, even if there is more and more evidence that resection of metastases improves survival.4,8,22–24 In contrast to the very little data regarding reoperation for recurrent sporadic PETs,25,26 an increasing number of studies on surgical treatment of neuroendocrine liver metastases have been published.4,22,27–31 Although none of these studies was a randomized clinical trial, and most of them mixed patients with PETs and patients with midgut carcinoid tumors, important conclusions can be drawn. In these studies, a total of 118 patients with hepatic metastases from PETs were treated, mostly by surgical resection. There was an average operative mortality of 3% and a 5-year survival rate of 64%.4,22,27–31 In a recent study by Touzios et al,4 the median and 5-year survival were only 20 months and 25% for patients with their liver metastases treated in a nonaggressive way, versus >96 months and 72% for a group of patients who underwent hepatic resection and/or radiofrequency ablation of their liver metastases. In the present study 27 patients with metastases from PETs were treated surgically and a 5-, 10-, and actuarial 25-year survival rate of 81%, 72%, and 36%, respectively, was achieved. These are very encouraging numbers, compared with historic controls, where patients with metastatic PETs remained untreated and had a 5-year survival rate of only 30% to 40%.32,33 Remarkable are 2 facts. First, at the termination of the study, one third of the patients (n = 9) with malignant disease were free of disease: 4 patients after developing lymph node metastases and 5 patients even after developing metastases to the liver or lung. Second, the presence of lymph node metastases had no influence on survival (Fig. 3). Indeed, no patient with only lymph node metastases died during this study. This is concordant with a study by Yu et al, who showed, for patients with ZES, that only the presence of liver metastases is associated with a worse prognosis.6 In this study, patients with distant metastases had significantly larger primary tumors than patients without distant metastases (65 vs. 15 mm). Therefore, an early and aggressive operative strategy may prevent the development of distant metastases and thereby prevent worsening of the patient's prognosis. We also found that the survival rate was influenced by patient's age. Patients younger than 50 years at the time of initial operation had a significantly better survival than patients older than 50 years (Fig. 2). Although the group of the younger patients included all MEN-1 patients, this should encourage treating young patients with PETs aggressively, even if recurrent tumors and/or metastases occur. We found no difference in survival rate comparing gender (P = 0.84), although some groups reported an aggressive form of gastrinomas, predominantly in women.34
Chemotherapy was provided to 13 patients (39%). Six different regimens were used, including octreotide in combination with alpha-interferon or dacarbazine, and a combination of streptozotocin with 5-fluorouracil or doxorubicin and gemcitabine, and we are not able to draw any conclusions from these data regarding survival and benefit for the patients.
In the current series, overall postoperative mortality was acceptable at 4.8%. Major liver resection incurs a risk of postoperative liver dysfunction and infection, and there is a lack of objective evidence relating residual liver volume to these complications. In a recent study, Schindl et al35 found that the incidence of severe hepatic dysfunction and infection following liver resection increased significantly with smaller % relative residual liver volume (% RLV). A critical % RLV of 26.6% was identified as associated with severe hepatic dysfunction (P < 0.0001); % RLV was calculated as the relation of residual to total functional liver volume. Additionally, body mass index, operating time, and intraoperative blood loss were significant prognostic indicators for severe hepatic dysfunction. It was not possible to predict the individual risk of postoperative infection precisely by % RLV. Que et al36 reviewed the data for 212 patients with partial hepatectomy for metastatic neuroendocrine tumors, including hemihepatectomy in 96 patients, wedge resection in 34 patients, extended lobar resections in 30 patients, and enucleation in 2 patients. The overall morbidity rate was 14%, and the operative mortality rate after partial hepatectomy for metastatic carcinoid disease was 2.3%. Complications directly related to cytoreductive hepatic surgery for carcinoid tumors included postoperative liver failure in only 2 patients.
Postoperative morbidity remains high (45%), although most of the complications were self-limited and not life-threatening. Patients with PETs, especially those with MEN-1, generally have soft, normal pancreatic glands, making the pancreaticoenteric anastomoses challenging. Analogous to mortality, a rise of postoperative complications from first to third to fifth reoperation have been noted (Table 2).
So far, only 2 studies have directly evaluated the effect of reoperation after recurrence or persistent disease in patients with mainly sporadic ZES25 or sporadic organic hyperinsulinism,26 respectively. In the study of Jaskowiak et al, 120 patients with ZES underwent operation for gastrinoma resection. Seventy-eight patients had recurrent or persistent ZES after operation; 17 (15 with sporadic disease) patients with imageable disease underwent reoperation and 30% of these patients remained disease free.25 This is comparable to the present study, where a biochemical cure in 38% ZES patients was achieved after reoperation, even with the much longer follow-up in the present study (142 vs. 30 months). Simon et al reoperated 6 patients (5 with sporadic disease) with persistent or recurrent organic hyperinsulinism and were able to cure all but 1 patient with MEN-1.26 Although the experienced endocrine surgeon is able to detect and resect 90% to 95% of all PETs by IOUS and bidigital palpation of the pancreas,9,37 sometimes pancreatic exploration is unsuccessful in locating a tumor. This may occur especially in small insulinomas and gastrinomas. In this study, this happened in 6 patients, with a higher rate being operated in a less experienced institution (Table 3). However, in a second operation, we were able to identify 5 of the 6 tumors, and all 5 patients were biochemically cured postoperatively and in a long-term follow-up. Whereas the 3 patients with the benign insulinoma are probably cured for life, the ZES patients are still at high risk of developing liver metastases. Therefore, we recommend sending patients with PETs to tertiary referral centers.
Because of the existing germline mutation, recurrence of PETs is a common event in patients with MEN-1.2,19 In this group, PETs are of outstanding interest because malignant PETs represent the most common cause of death in the MEN-1 syndrome.38,39 After primary surgery, a life-long cure is uncommon and recurrence should be anticipated.40 Recently, we and others have shown that early and aggressive surgery of PETs in MEN-1 prevents the development of distant metastases which are the unequivocal life-threatening determinant.19,41 In this study, no patient with MEN-1 died because of PETs. The survival was much better than in patients with sporadic disease, even due to the low number of MEN-1 patients, this was statistically not significant (Fig. 4) Furthermore, our aggressive surgical approach prevented developing distant metastases in all 9 patients. However, new mainly nonfunctioning PETs arose frequently in the pancreatic remnant during long-term follow-up.
CONCLUSION
We have shown that an aggressive surgical approach leads to long-term survival in patients with malignant PETs. Although long-term cure can only be achieved in a proportion of patients with malignant PETs, significant long-term palliation can be achieved. This aggressive surgical approach can be recommended, keeping in mind that additional prospective, randomized long-term follow-up studies are needed to establish its value in patients with PETs.
Discussions
Dr. Laureano Fernández-Cruz: The group led by Matthias Rothmund should be congratulated for their efforts to evaluate the outcome of reoperations in patients with pancreaticoduodenal neuroendocrine tumors. The majority of your study population of endocrine tumors comprised mainly gastrinomas and some were MEN-1 tumors.
The initial procedure comprised 27 pancreatic resections, among them 15 distal pancreatectomies, 4 pancreaticoduodenectomies, and 3 tumor enucleations. The frequently reported dominant location of neuroendocrine pancreatic tumors is in the pancreatic head. However, half of the patients underwent distal pancreatectomy as the initial operation. Would you comment on that?
In the total group, 15 operations were performed. 56% were resections for liver metastases, 38% pancreaticoduodenectomies, and 6% resections of lymph node metastases. The indications were of a failed primary operation without identifying a primary tumor and newly developed pancreatic endocrine tumor or local and distal metastases.
After a medical follow-up of 124 months, 82% of patients are still alive. Of 27 patients with malignancy, 33% were free of disease, 45% alive with disease, and 22% are deceased.
You have achieved excellent results with acceptable morbidity and mortality. We know that with extensive resections, tumor recurrence, and progression occur frequently and, ultimately, continue until death in most patients with metastatic islet cell tumors. What are the strategies to identify all metastatic disease during preoperative and perioperative assessment in relation to the underlying tumor biology?
The last question: what are the clinical markers that could predict outcome in patients with metastatic islet cell carcinoma and to improve decision-making for patients who are candidates for surgical, medical, or combination therapy?
Dr. Volker Fendrich: Thank you very much. These are very interesting comments, and I am very happy to hopefully answer these questions to your full satisfaction.
The distal pancreatic resections are mainly because, in the MEN-1 patients, we adopt a strategy using the so-called Norman Thompson procedure. That means that we perform a distal pancreatic resections plus enucleation of tumors in the pancreatic head, to avoid a total pancreatectomy. We have demonstrated this strategy when our previous results were presented at the ESA meeting in Stockholm 2005. This is the only explanation for such a high number of distal pancreatic resections in this group of patients in whom tumors are more commonly found in the pancreatic head.
Regarding the preoperative and intraoperative strategy to detect metastases, we do not have any particular management programs. Preoperatively, we undertake a CT scan and a somatostatin receptor scintigraphy. Intraoperatively, we perform an intraoperative ultrasound of the entire pancreas and, of course, the liver.
Clinical markers for islet cell tumors are an important issue. Unfortunately, we only have data of the proliferation marker Ki-67 for 10 of our 33 patients. Unfortunately, the numbers in our series are too low to draw a valid conclusion. We could go back and do the stainings for all the patients; we should definitely do this.
Dr. Krister Höckerstedt: I think there was one specific question about clinical markers for islet cell tumors.
Dr. Volker Fendrich: Actually, there is, as I know, no marker that is highly specific for islet cell tumors. We are trying to find a new marker that is specific for metastases, and often the loss of E-cadherin is one of these markers. New markers have been described in previous years for other epithelial tumors, which have been shown to predict the development of metastases. We are trying to confirm this for neuroendocrine tumors in a study in our laboratory. Hopefully, we will find something.
Dr. Peter Neuhaus: Dr. Fendrich, I offer my congratulations for this really large analysis of your patients in Marburg. My question is related to the indication for liver transplantation. You already mentioned Ki-67. We know of the retrospective series from Lehmann in Heidelberg on liver transplantation and from another recently published paper from the Mayo Clinic. At the moment, it is not quite clear to me when liver transplantation is really indicated, especially for nonfunctioning tumors. Then I would like to know for which patient liver transplantation is prohibited. When I talked to Dr. Plöckinger in Berlin—you quoted her, too—she said Ki-67 is the marker, which shows whether you should or should not consider transplantation. But when I look at the recent Mayo Clinic publication, it seems to me that this is not proven.
Also, I would like you to comment on new possibilities of immunosuppression, where there is no acceleration of tumor growth any more, for instance, experimentally with sirolimus. Could that change the picture? This is a really urgent issue, since we see a number of patients where the indication for liver transplantation is questionable.
Dr. Volker Fendrich: Thank you very much, Professor Neuhaus. Fortunately, I have read your review about liver transplantation for neuroendocrine tumors, which was published in 2005. You reviewed 120 to 130 patients who underwent liver transplantation for neuroendocrine tumors. The results are not very satisfactory and, as you said referring to Ms Plöckinger, the cutoff level of E-cadherin (the loss of E-cadherin) and the high proliferation of Ki-67 should prohibit liver transplantation. We don't do liver transplantation in Marburg.
But to your next question, sirolimus or rapamycin is administered to patients after renal transplantation and, I think, is a very interesting and promising drug. It was shown that it diminishes the effects of the higher tumor rate after transplantation resulting from immunosuppression. This is because it has direct antitumoral effects, and is in use in patients with advanced breast cancer and with advanced renal carcinoma. I believe that there is a new derivate available (CCI-779), which has a lower toxicity and is better tolerated by patients. Perhaps this would be an interesting approach for these patients.
Dr. Daniel Jaeck: Congratulations on your excellent presentation. I would like to ask you about the case where you didn't find the gastrinoma. Did you use an intraoperative test such as the test described by Imamura (Ann Surg. 1989;210:710–718), with selective injection of secretin, to try to localize the gastrinoma.
And my second question is concerning the MEN-1 gastrinoma. It is still not clear to me whether you recommend pancreaticoduodenectomy or the conservative procedure described by Norman Thompson?
Dr. Volker Fendrich: Thank you very much for the comments. I would like to start with your last question.
We had, in the early years, started to operate on MEN-1 patients with Zollinger Ellison syndrome using the Norman Thomson approach. In the last 6 years, we have undertaken a pylorus-preserving pancreaticoduodenectomy (PPPD) if the tumor or source of the gastrin was shown to be in the pancreatic head by the Imamura procedure. In our MEN-1 group, 7 of 11 patients operated for ZES are biochemically cured after a median follow-up of 123 months (range, 38–213 months). Four of these 7 patients underwent PPPD either as initial or reoperative procedure. Of course, we cannot prove so far that the patient will benefit in the long term because it's an operation where you take away the whole pancreatic head. If the patient then develops new tumors in the pancreatic tail, you are close to a total pancreatectomy.
To your first question (for the patient in whom the gastrinoma is still not located): The first operation was performed in another hospital where they resected a lymph node metastases. For the second operation in our hospital, we didn't perform an intraoperative Imamura test.
Dr. Pierre Clavien: Congratulations, Dr. Fendrich, for this impressive series and very nice presentation. I have one short question. You comment that you undertake surgery if you can remove 90% of the overall tumor load. This figure is always the subject of some debate: what percentage is right? What about 70% or 80%? Could you tell us what is the basis for 90% in Marburg.
Dr. Volker Fendrich: Actually, recommendations from studies like this!
Footnotes
Reprints: Volker Fendrich, MD, Department of Surgery, Philipps University Marburg, Baldingerstraβe, D-35043 Marburg, Germany. E-mail: fendrich@med.uni-marburg.de.
REFERENCES
- 1.Öberg K, Eriksson B. Endocrine tumors of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–781. [DOI] [PubMed] [Google Scholar]
- 2.Akerstrom G, Hessman O, Hellman P, et al. Pancreatic tumours as part of the MEN-1 syndrome. Best Pract Res Clin Gastroenterol. 2005;19:819–830. [DOI] [PubMed] [Google Scholar]
- 3.Solcia E, Kloeppel G, Sobin LH. World Health Organization: International Histological Classification of Tumours: Histological Typing of Endocrine Tumours. Berlin: Springer, 2000. [Google Scholar]
- 4.Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadiot G, Vuagnat A, Doukhan I, et al. Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Groupe d'Etude des Neoplasies Endocriniennes Multiples (GENEM and groupe de Recherche et d'Etude du Syndrome de Zollinger-Ellison (GRESZE). Gastroenterology. 1999;116:286–293. [DOI] [PubMed] [Google Scholar]
- 6.Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–630. [DOI] [PubMed] [Google Scholar]
- 7.Norton JA, Kivlen M, Li M, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–866. [DOI] [PubMed] [Google Scholar]
- 8.Sarmiento JM, Que FG, Grant CS, et al. Concurrent resections of pancreatic islet cell cancers with synchronous hepatic metastases: outcomes of an aggressive approach. Surgery. 2002;132:976–983. [DOI] [PubMed] [Google Scholar]
- 9.Fendrich V, Bartsch DK, Langer P, et al. Diagnosis and surgical treatment of insulinoma: experiences in 40 cases. Dtsch Med Wochenschr. 2004;129:941–946. [DOI] [PubMed] [Google Scholar]
- 10.Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783–798. [DOI] [PubMed] [Google Scholar]
- 11.Fendrich V, Bartsch DK, Langer P, et al. Zollinger-Ellison syndrome. Chirurg. 2005;76:217–226. [DOI] [PubMed] [Google Scholar]
- 12.Norton JA. Surgery and prognosis of duodenal gastrinoma as a duodenal neuroendocrine tumor. Best Pract Res Clin Gastroenterol. 2005;19:699–704. [DOI] [PubMed] [Google Scholar]
- 13.Bartsch DK, Schilling T, Ramaswamy A, et al. Management of nonfunctioning islet cell carcinomas. World J Surg. 2000;24:1418–1424. [DOI] [PubMed] [Google Scholar]
- 14.Dralle H, Krohn SL, Karges W, et al. Surgery of resectable nonfunctioning neuroendocrine pancreatic tumors. World J Surg. 2004;28:1248–1260. [DOI] [PubMed] [Google Scholar]
- 15.Plöckinger U, Wiedenmann B. Management of metastatic endocrine tumors. Best Pract Res Clin Gastroenterol. 2005;19:553–576. [DOI] [PubMed] [Google Scholar]
- 16.Norton JA. Surgical treatment of neuroendocrine metastases. Best Pract Res Clin Gastroenterol. 2005;19:577–583. [DOI] [PubMed] [Google Scholar]
- 17.Fraker DL, Norton JA, Alexander R, et al. Surgery for Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kouvaraki MA, Solorzano CC, Shapiro SE, et al. Surgical treatment of non-functioning pancreatic islet cell tumors. J Surg Oncol. 2005;89:170–185. [DOI] [PubMed] [Google Scholar]
- 19.Bartsch DK, Fendrich V, Langer P, et al. Outcome of duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg. 2005;242:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease: results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med. 1998;243:495–500. [DOI] [PubMed] [Google Scholar]
- 21.Hellman P, Andersson M, Rastad J, et al. Surgical strategy for large or malignant endocrine pancreatic tumors. World J Surg. 2000;24:1353–1360. [DOI] [PubMed] [Google Scholar]
- 22.Dousset B, Saint-Marc O, Pitre J, et al. Metastatic endocrine tumors: medical treatment, surgical resection, or liver transplantation. World J Surg. 1996;20:908–914. [DOI] [PubMed] [Google Scholar]
- 23.Chu QD, Hill HC, Douglass HO Jr, et al. Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol. 2002;9:855–862. [DOI] [PubMed] [Google Scholar]
- 24.Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–1065. [DOI] [PubMed] [Google Scholar]
- 25.Jaskowiak NT, Fraker DL, Alexander HR, et al. Is reoperation for gastrinoma excision indicated in Zollinger-Ellison syndrome? Surgery. 1996;120:1055–1063. [DOI] [PubMed] [Google Scholar]
- 26.Simon D, Starke A, Goretzki PE, et al. Reoperative surgery for organic hyperinsulinism: indications and operative strategy. World J Surg. 1998;22:666–672. [DOI] [PubMed] [Google Scholar]
- 27.Que FG, Nagorny DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169:36–42. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88–92. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–435. [DOI] [PubMed] [Google Scholar]
- 30.Nave H, Mossinger E, Feist H, et al. Surgery as primary treatment in patients with liver metastases from carcinoid tumors: a retrospective, unicentric study over 13 years. Surgery. 2001;129:170–175. [DOI] [PubMed] [Google Scholar]
- 31.Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. [DOI] [PubMed] [Google Scholar]
- 32.Moertel CG. An odyssey in the land of small tumors. J Clin Oncol. 1987;5:1503–1522. [DOI] [PubMed] [Google Scholar]
- 33.Thompson GB, van Heerden JA, Grant CS, et al. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988;104:1011–1017. [PubMed] [Google Scholar]
- 34.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindl MJ, Redhead DN, Fearon KC, et al. Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Que FG, Sarmiento JM, Nagorney DM. Hepatic surgery for metastatic gastrointestinal neuroendocrine tumors. Cancer Control. 2002;9:67–79. [DOI] [PubMed] [Google Scholar]
- 37.Rothmund M, Angelini L, Brunt LM, et al. Surgery for benign insulinoma: an international review. World J Surg. 1990;14:393–399. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson S, Teh BT, Davey KR, et al. Cause of death in multiple endocrine neoplasia type 1. Arch Surg. 1993;128:683–690. [DOI] [PubMed] [Google Scholar]
- 39.Doherty GM, Olson JA, Frisella MM, et al. Lethality of multiple endocrine neoplasia type I. World J Surg. 1998;22:581–587. [DOI] [PubMed] [Google Scholar]
- 40.Hausman MS Jr, Thompson NW, Gauger PG, et al. The surgical management of MEN-1 pancreatoduodenal neuroendocrine disease. Surgery. 2004;136:1205–1211. [DOI] [PubMed] [Google Scholar]
- 41.Skogseid BS, Eriksson B, Lundqvist G, et al. Multiple endocrine neoplasia type 1: a 10-year prospective screening study in four kindreds. J Clin Endocrinol Metab. 1991;73:281–287. [DOI] [PubMed] [Google Scholar]


