Abstract
Background:
Greater hospital volume has been associated with lower mortality after colorectal cancer surgery. The contribution of surgeon volume to processes and outcomes of care is less well understood. We assessed the relation of surgeon and hospital volume to postoperative and overall mortality, colostomy rates, and use of adjuvant radiation therapy.
Methods:
From the California Cancer Registry, we studied 28,644 patients who underwent surgical resection of stage I to III colorectal cancer during 1996 to 1999 and were followed up to 6 years after surgery to assess 30-day postoperative mortality, overall long-term mortality, permanent colostomy, and use of adjuvant radiation therapy.
Results:
Across decreasing quartiles of hospital and surgeon volume, 30-day postoperative mortality ranged from 2.7% to 4.2% (P<0.001). Adjusting for age, stage, comorbidity, and median income among patients with colorectal cancer who survived at least 30 days, patients in the lowest quartile of surgeon volume had a higher adjusted overall mortality rate than those in the highest quartile (hazard ratio, 1.16; 95% confidence interval, 1.09–1.24), as did patients in the lowest quartile of hospital volume relative to those treated in the highest quartile (hazard ratio, 1.11; 95% confidence interval, 1.05–1.19). For rectal cancer, adjusted colostomy rates were significantly higher for low-volume surgeons, and the use of adjuvant radiation therapy was significantly lower for low-volume hospitals.
Conclusions:
Greater surgeon and hospital volumes were associated with improved outcomes for patients undergoing surgery for colorectal cancer. Further study of processes that led to these differences may improve the quality of colorectal cancer care.
Relative contributions of surgeon and hospital volume to processes and outcomes of colorectal cancer care are poorly understood. A total of 28,644 patients who had stage I to III colorectal cancer during 1996 to 1999 were followed up 6 years. Greater surgeon and hospital volume were associated with improved outcomes for patients undergoing surgery for colorectal cancer.
Multiple studies have shown an association between hospital volume and surgical outcomes for cancer care.1,2 High-volume hospitals have lower operative mortality compared with low-volume hospitals, especially for high-risk cancer operations. In one recent study using a national cohort of Medicare beneficiaries in 1998 and 1999, Birkmeyer et al found that surgeon volume was inversely related to operative mortality for several high-risk cardiovascular and cancer procedures.3 Most of these operations are performed by surgeons with additional training or require complex perioperative care, and much of the effect of hospital volume was attributable to surgeon volume.
In contrast, surgery for colorectal cancer does not typically require additional training or highly specialized hospital resources for perioperative care. Prior studies have examined the association of hospital or surgeon volume with outcomes of colorectal cancer surgery,4–17 and have been summarized in systematic reviews.18,19 Few studies have examined the relative contributions of surgeon and hospital volume to survival beyond the initial 30 days and their impact on important processes of care such as sphincter-sparing surgery and adjuvant therapy. Harmon et al,20 Hannan et al,21 and Ko et al22 found that higher surgeon volume and hospital volume were associated with lower in-hospital mortality. Using Surveillance, Epidemiology and End Results (SEER)-Medicare data for the elderly, Schrag et al found that higher surgeon and hospital volume independently predicted lower 30-day and 2-year mortality.23 In a broader population-based study, including patients of all ages from the California Cancer Registry, Hodgson et al found that rectal cancer patients who received care in high-volume hospitals had lower colostomy rates and better survival.24
To assess the relative contributions of surgeon and hospital volume to colorectal cancer outcomes, including both postoperative and overall mortality, we analyzed these outcomes using data from the California Cancer Registry. We also analyzed colostomy rates for patients with stage I to III rectal cancer and use of adjuvant radiation therapy for patients with stage II or III rectal cancer by surgeon and hospital volume. Our objective was to inform the ongoing debate regarding selective referral of colorectal cancer patients to high-volume hospitals or high-volume surgeons to improve outcomes.25
METHODS
Data Sources and Study Cohort
We identified the study cohort from the California Cancer Registry (CCR), the world's largest population-based registry for a geographically contiguous area. The Institutional Review Boards of the California Department of Health Services, Public Health Institute, Northern California Cancer Center, and Harvard Medical School approved the study. Because our study used existing data with encrypted identifiers, written informed consent from subjects was not required.
The study cohort was derived from the 34,520 patients newly diagnosed with stage I, II, or III colorectal cancer in California from January 1996 through December 1999 who underwent surgical resection of their primary cancer. The surgeon performing the most extensive type of surgery was specified in registry records for 27,997 (81.1%) of these 34,520 patients. We identified the attending physicians of 2356 of the remaining 6523 (18.9%) patients as the surgeon because they were listed as general or colorectal surgeons in the state medical licensing database or were designated as the surgeon for 12 or more patients in our cohort. The remaining 4167 patients were excluded. We also excluded 5 patients whose hospital was not recorded, yielding a study cohort of 30,348 (87.9%) patients.
The CCR provided data on patients’ age, sex, race/ethnicity, insurance coverage, tumor site, stage of disease, and number of lymph nodes examined. Codes for extent of disease were converted to stage as specified by the American Joint Committee on Cancer using an algorithm developed by the SEER program.26,27 CCR staff geocoded patients’ addresses to Census block groups. From the 1990 U.S. Census, we obtained median household income in block groups (rounded to the nearest $5000 to preserve confidentiality); 628 patients were missing income. To ascertain patients’ vital status, registry records were matched with the California Death Statistical Master File, National Death Index, and other databases as previously described.24
To determine whether patients received a colostomy and obtain data on comorbid illnesses and type of surgery, CCR staff linked registry data to hospital discharge abstracts maintained by the California Office of Statewide Health Planning and Development, using a probabilistic matching algorithm based on patients’ Social Security number, date of birth, sex, and Zip code.28 Patients who received a colostomy were identified using procedure codes derived from the International Classification of Diseases, 9th Revision (ICD-9-CM) (Appendix). A patient was categorized as having a colostomy if any ostomy procedure was coded in a hospital discharge up to 2 months after diagnosis. A colostomy was defined as permanent if no discharge abstract had an ICD-9-CM procedure code indicating reversal (Appendix) within 1 year of diagnosis.
To maximize ascertainment of relevant comorbid conditions,29 we identified all hospital discharges that occurred within 22 months before or 2 months after patients’ diagnosis. From the principal diagnosis and up to 24 secondary diagnoses (excluding cancer-related diagnoses) on discharge abstracts, we classified comorbidity using the Deyo adaptation of the Charlson scale for use with hospital discharge abstracts.30,31 We excluded 1704 patients without any linked discharge abstracts, resulting in a final cohort of 28,644 patients who underwent major surgical resection with data available on their surgeon, hospital, and demographic and clinical characteristics.
Statistical Analysis
Our primary analyses focused on the relation of surgeon volume and hospital volume to 30-day postoperative mortality and to 6-year overall survival among patients who survived at least 30 days after surgery. We also analyzed colostomy rates for patients with stage I, II, or III rectal cancer, and use of radiation therapy for patients with stage II or III rectal cancer. Hospital volume and surgeon volume were calculated as the total number of surgical resections of colorectal cancer at each hospital and by each surgeon, respectively, over the 4-year period. We categorized hospitals and surgeons into volume quartiles so each quartile contained approximately equal numbers of patients. For bivariate tests, χ2 tests were used for categorical variables and analyses of variance were used for age. Survival by quartile of hospital and surgeon volume was estimated with the Kaplan-Meier method and compared with the log-rank test.
In the multivariable analyses, we adjusted for patients’ age (in 5-year intervals), race/ethnicity (white, black, Hispanic, Asian, unknown), sex, median household income, and Charlson-Deyo comorbidity index. To examine the adjusted associations of surgeon and hospital volume with 30-day mortality, we used logistic regression, adjusting standard errors to control for clustering of patients by hospitals. We used similar models to assess the adjusted associations of surgeon volume and hospital volume with permanent colostomies for patients with stage I to III rectal cancer and use of adjuvant radiation therapy for patients with stage II to III rectal cancer.
In a secondary multivariable analysis of 30-day postoperative mortality, we included a variable from hospital discharge abstracts that distinguished scheduled (>24 hours prior to admission) and unscheduled admissions, as the latter group likely includes patients who presented for urgent or emergent surgery with colorectal obstruction, perforation, or hemorrhage. A prior audit found that the designation of “elective” admissions in California discharge abstracts was accurate in about 75% of cases.32 We also assessed the effect of surgeon and hospital volume on adjusted 30-day mortality separately for patients with colon cancer (N = 21,592) and rectal cancer (N = 7052), after recalculating the volume quartiles separately in each group.
Among patients who survived 30 days following surgery, we used Cox proportional hazards models to analyze overall survival, adjusting for patient characteristics and using the robust sandwich estimate of the covariance matrix to control for clustering by hospital.33 This conditional survival measure removes the effect of postoperative deaths on overall survival. All analyses were performed with SAS software. Results are presented with two-tailed P values or 95% confidence intervals.
RESULTS
Patient Characteristics
Demographic and clinical characteristics of the 28,644 patients who underwent surgery for colorectal cancer during 1996 to 1999 are shown in Table 1 by quartiles of hospital and surgeon volume, including 2993 surgeons who performed colorectal cancer surgery in 397 hospitals. Individual surgeons performed 1 to 163 operations over 4 years (median, 3; interquartile range, 1–13). The number of operations at individual hospitals ranged from 1 to 425 over 4 years (median, 41; interquartile range, 11–112).
TABLE 1. Characteristics of Patients Undergoing Colorectal Cancer Surgery in California by Surgeon Volume and Hospital Volume (1996–1999)
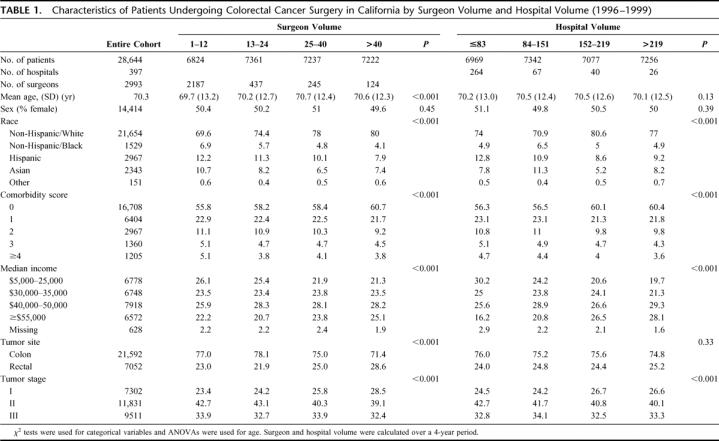
Patients were much more likely to undergo surgery by a low-volume surgeon if they went to a low-volume hospital (36.9%) than if they went to a high-volume hospital (18.9%). Patients were also more likely to undergo surgery by a high-volume surgeon if they went to a high-volume hospital (37.4%) than if they went to a low-volume hospital (6.6%). The 30% of surgeons with patients at more than one hospital in our cohort performed an average of 75% of their operations at their primary surgical hospital.
Patients of minority race/ethnicity or with greater comorbidity were generally more likely to be treated by lower-volume surgeons and at lower-volume hospitals. The number of lymph nodes examined for staging of surgical specimens was slightly but significantly larger in the highest-volume quartile of hospitals (median, 10; interquartile range, 6–16) than in each of the 3 lower-volume quartiles (median, 9; interquartile range, 5–14) (P < 0.001). The number of lymph nodes examined was more similar across quartiles of surgeon volume (median, 9; interquartile range, 5–14 or 5–15).
Postoperative Mortality
There was a significant inverse association between hospital volume and 30-day mortality (Table 2): 2.7% among patients undergoing surgery in the highest-volume hospitals, increasing to 4.2% in the lowest-volume hospitals (P < 0.001). After adjusting for surgeon volume and other potential confounders, low hospital volume (<84 operations over 4 years) remained a statistically significant predictor of 30-day mortality (odds ratio [OR], 1.28; 95% confidence interval [CI], 1.01–1.62) compared with the highest-volume quartile (Table 2).
TABLE 2. Thirty-Day and Overall Mortality After Colorectal Cancer Surgery in California (1996–1999)
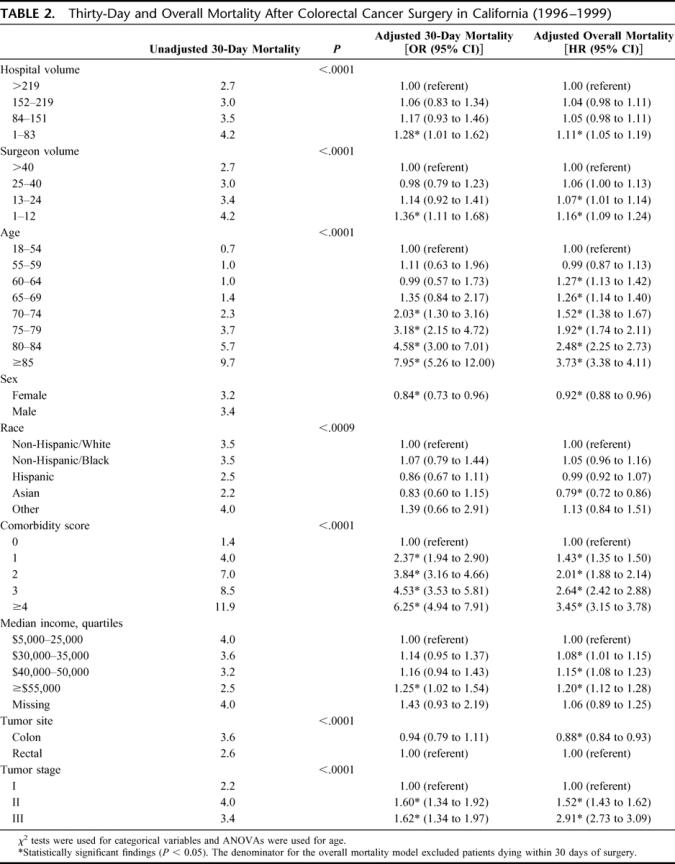
Similarly, surgeon volume was inversely associated with 30-day mortality (Table 2): 2.7% among patients undergoing surgery by the highest-volume surgeons and increasing to 4.2% with the lowest-volume surgeons (P < 0.001). Low surgeon volume (<13 operations over 4 years) remained a statistically significant predictor of 30-day mortality in the multivariable analysis (OR, 1.36; 95% CI, 1.11–1.68) compared with the highest-volume quartile (Table 2). Other factors statistically associated with greater 30-day mortality in the adjusted analyses were male sex, older age, increasing comorbidity index, low income, and stage II and III disease, but not patient race or ethnicity. Interactions of the lowest quartiles for both hospital and surgeon volume, or the lower 2 quartiles for both, had no significant effects in the 30-day mortality models.
Unscheduled admissions (31.1% of the entire cohort) occurred less commonly across increasing quartiles of surgeon volume (39.7%, 32.1%, 29.0%, and 24.1%) and hospital volume (35.6%, 35.2%, 29.1%, and 24.4%). In secondary analyses, such admissions were a highly significant predictor of 30-day mortality (adjusted OR, 2.54; 95% CI, 2.20–2.94). Adjusting for this variable reduced the magnitude of excess adjusted mortality in the lowest volume quartiles by about one third, and the adjusted effects of surgeon volume (OR, 1.20; 95% CI, 0.98–1.48) and hospital volume (OR, 1.25; 95% CI, 0.99–1.57) were of borderline statistical significance.
In stratified analyses, adjusted 30-day mortality remained substantially higher for colon cancer in the lowest surgeon-volume quartile (OR, 1.50; 95% CI, 1.19–1.89) and was of borderline significance in the lowest hospital-volume quartile (OR, 1.26; 95% CI, 0.97–1.64). For rectal cancer, however, adjusted 30-day mortality was not elevated in the lowest surgeon volume quartile (OR, 1.04; 95% CI, 0.64–1.69), but was significantly increased in the lowest hospital volume quartile (OR, 1.77; 95% CI, 1.11–2.80).
Overall Survival
Five-year survival after surgery ranged from 58.9% for patients treated at high-volume hospitals to 51.3% for those treated at low-volume hospitals (P < 0.001) (Fig. 1A). Similarly, 5-year survival ranged from 59.0% for patients treated by high-volume surgeons to 52.9% for those treated by low-volume surgeons (P < 0.001) (Fig. 1B).
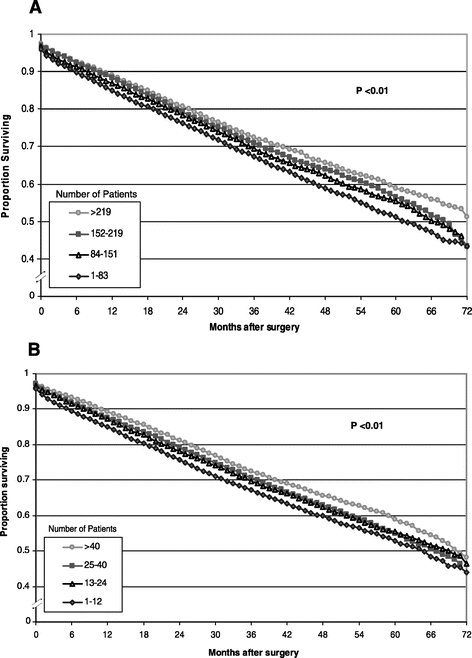
FIGURE 1. Survival of patients in California after surgery for colorectal cancer (1996–1999) by hospital volume (A) and surgeon volume (B). Survival by quartile of hospital and surgeon volume was estimated with the Kaplan-Meier method and log-rank test.
In the Cox proportional hazards model for patients who survived at least 30 days after surgery, the adjusted risk of death increased steadily as either hospital volume or surgeon volume decreased (Table 2). Patients in the lowest quartile of hospital volume had a significantly higher adjusted overall mortality rate than those treated in the highest-volume hospitals (hazard ratio [HR], 1.11; 95% CI, 1.05–1.19). Patients in the lowest quartile of surgeon volume also had a significantly higher adjusted overall mortality rate than those treated by high-volume surgeons (HR, 1.16; 95% CI, 1.09–1.24), as did those in the second lowest quartile (HR, 1.07, 95% CI,1.01–1.14). Other factors independently associated with a higher overall mortality were male sex, older age, greater comorbidity, lower income, and more advanced stage. Asian patients had lower adjusted overall mortality than white patients.
Colostomy Rates for Stage I to III Rectal Cancer
In adjusted analyses, patients of low-volume surgeons were more likely to receive permanent colostomies than patients of high-volume surgeons, but hospital volume had no independent effect on colostomy rates (Table 3). Women were less likely than men to undergo colostomies. Hispanic patients were more likely and Asian patients were less likely to undergo colostomies than non-Hispanic white patients. Patients with stage II or III rectal cancer and lower income were more likely to undergo colostomies.
TABLE 3. Adjusted Analyses of Permanent Colostomy and Radiation Therapy for Rectal Cancer in California (1996–1999)

Use of Adjuvant Radiation Therapy for Stage II to III Rectal Cancer
Radiation therapy was significantly less likely to be administered to patients in the lowest-volume compared with patients in the highest-volume hospitals (Table 3). In contrast, surgeon volume was not significantly associated with adjusted rates of radiation therapy. Patients of greater age and higher Charlson comorbidity score were less likely to receive radiation therapy. Women were less likely than men to undergo radiation therapy for stage II/III rectal cancer. Patients with stage III rectal cancer were more likely to undergo radiation therapy than those with stage II disease.
DISCUSSION
In this large population-based cohort of patients with colorectal cancer, 30-day mortality and overall mortality were similarly higher among patients treated by low-volume surgeons and those treated at low-volume hospitals. This study builds on the few prior studies that have simultaneously assessed effects of hospital and surgical volume on outcomes.14,20,23 The unadjusted difference of 1.5% (2.7% vs. 4.2%) in 30-day mortality between the highest and lowest hospital volume quartiles is somewhat smaller than in other population-based studies of patients with colon cancer.2,8 The 30-day mortality difference between the highest and lowest surgeon volume quartiles is comparable to previous studies.20,21,23 In contrast to studies using SEER-Medicare data, our study included patients under age 65, making it more widely generalizable.
The parallel effects of hospital and surgeon volume on 30-day mortality may be mediated by preoperative, intraoperative, and postoperative decision-making by the surgeons and by the hospitals’ resources. Preoperative factors may include more thorough cardiac evaluations,34 use of perioperative beta-blockers for patients at risk,35 and adequate bowel preparation.36 Intraoperative processes may include more complete local resections with lower subsequent recurrence rates,37 intraoperative air testing of colorectal anastomoses to detect leaks,38 and more thorough or accurate pathologic assessments of surgical specimens.32 Postoperative factors may include appropriate management of postoperative complications such as pneumonia or sepsis. For the minority of colorectal cancer patients who require postoperative intensive care, intensivist-led intensive care units that improve outcomes39 may be more commonly available in high-volume hospitals or more accessible to high-volume surgeons.
This study used conditional survival as a novel and informative measure of long-term survival. By excluding patients who died within 30 days of surgery, this analysis distinguished the effects of surgeon and hospital volume upon long-term survival from short-term effects. Conditional survival among patients who survive at least 30 days postoperatively may be related to the overall quality of cancer care, including the use of adjuvant therapy for appropriate patients.40–43 Their clinical and pathologic staging may be more thorough and accurate, as suggested by the larger number of lymph nodes examined at high-volume hospitals in our study. Other characteristics of higher-volume hospitals that may account for this effect may include the closer affiliation with radiation therapy facilities or a broader range of specialists and technologic resources. Unmeasured factors such as social support or the care of comorbid illnesses may also play a role. In a randomized clinical trial with standardized use of chemotherapy, for example, patients with stage II or III colon cancer who underwent surgery at low-volume hospitals had higher long-term mortality without any evidence of more frequent cancer recurrence or higher cancer-specific mortality, suggesting more severe comorbid conditions or less effective treatment of these comorbid conditions.44
Limitations of our study included the lack of information on the specific attributes of surgeons and hospitals that explained volume-outcome effects. The CCR does not collect data on the distance of tumors from the anal verge or patients’ history of fecal incontinence (factors that may influence the choice of abdominoperineal resection or low anterior resection), so we were unable to assess the appropriateness of treatment choice for rectal cancer by surgeon or hospital volume. Although we examined long-term overall survival and found significant effects of both surgeon volume and hospital volume, data were not available on cancer recurrences or cause of death, so we were unable to analyze cancer-specific survival. We also could not exclude the possibility of unmeasured selection effects, which may have caused patients with fewer resources and less social support to obtain care from low-volume providers. The higher rate of unscheduled admissions among low-volume surgeons and hospitals and the associated worse outcomes may be an indicator of such selection effects. Therefore, the causes and impact of unscheduled surgical admissions warrants further evaluation in future studies of the outcomes of colorectal cancer surgery.
Our results have important policy implications with respect to referring patients to high-volume surgeons or hospitals and targeting interventions to improve the quality of colorectal cancer care. The national impact of our findings can be extrapolated to the 105,000 patients diagnosed each year with stage I, II, or III colorectal cancer. If patients in the lowest quartiles of surgeon or hospital volume experienced the lower mortality of patients in the highest volume quartiles, approximately 400 excess deaths in the 30-day postoperative period might be avoided each year in the United States.
The American College of Surgeons is currently debating standards for maintenance of certification for surgeons. Our analysis suggests that patients would benefit from treatment by surgeons who perform at least 13 colorectal operations over 4 years and in hospitals with at least 84 of these operations over this time period. Furthermore, sphincter-sparing surgery (largely related to surgical technique) was associated almost exclusively with surgeon volume, whereas adjuvant radiation therapy (potentially involving shared decisions by several specialists) was primarily associated with hospital volume. Profiling individual surgeons’ outcomes may help to identify surgeons with better outcomes and thus improve the overall quality of care. As the American College of Surgeons National Surgical Quality Improvement Project matures, robust risk-adjusted outcomes data may guide patients’ and payors’ selection of surgeons and hospitals.
Further research is needed to understand the processes of care that lead to improved outcomes by high-volume hospitals and surgeons so these processes can be provided to all patients undergoing colorectal cancer surgery.45 Conducting rigorous observational studies of processes of care and linking those processes to important clinical outcomes may substantially improve the care of patients with colorectal cancer.
ACKNOWLEDGMENTS
The authors thank Mark E. Allen, MS, for facilitating access to California Cancer Registry data.
APPENDIX
ICD-9 Procedure Codes
Creation of stoma: 46.01 (exteriorization of small intestine), 46.02, 46.03 (exteriorization of large intestine), 48.5 (abdominoperineal resection of rectum), 48.62 (anterior resection of rectum with synchronous colostomy), 49.6 (excision of anus).
Stoma reversal: codes occurring after creation of a stoma and within 1 year of diagnosis: 45.90-.95 and 46.50-.52 (intestinal anastomosis, including 45.90-45.
REFERENCES
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 2.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1741. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
- 4.Tang D, Bowman H, Gordon T, et al. Case volume and outcomes in resection for Colorectal carcinoma. Surg Forum. 1998;49:559–561. [Google Scholar]
- 5.Hermanek P, Hohenberger W. The importance of volume in colorectal cancer surgery. Eur J Surg Oncol. 1996;22:213–215. [DOI] [PubMed] [Google Scholar]
- 6.McArdle CS, Hole D. Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. BMJ. 1991;302:1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg. 1998;227:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. [DOI] [PubMed] [Google Scholar]
- 9.Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001;93:501–515. [DOI] [PubMed] [Google Scholar]
- 10.Simons AJ, Ker R, Groshen S, et al. Variations in treatment of rectal cancer: the influence of hospital type and caseload. Dis Colon Rectum. 1997;40:641–646. [DOI] [PubMed] [Google Scholar]
- 11.Marusch F, Koch A, Schmidt U, et al. Hospital caseload and the results achieved in patients with rectal cancer. Br J Surg. 2001;88:1397–1402. [DOI] [PubMed] [Google Scholar]
- 12.Simunovic M, To T, Baxter N, et al. Hospital procedure volume and teaching status do not influence treatment and outcome measures of rectal cancer surgery in a large general population. J Gastrointest Surg. 2000;4:324–330. [DOI] [PubMed] [Google Scholar]
- 13.Parry JM, Collins S, Mathers J, et al. Influence of volume of work on the outcome of treatment for patients with colorectal cancer. Br J Surg. 1999;86:475–481. [DOI] [PubMed] [Google Scholar]
- 14.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kee F, Wilson RH, Harper C, et al. Influence of hospital and clinician workload on survival from colorectal cancer: cohort study. BMJ. 1999;318:1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm T, Johansson H, Cedermark B, et al. Influence of hospital- and surgeon-related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. Br J Surg. 1997;84:657–663. [PubMed] [Google Scholar]
- 17.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18:2327–2340. [DOI] [PubMed] [Google Scholar]
- 18.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. [DOI] [PubMed] [Google Scholar]
- 19.Gandjour A, Bannenberg A, Lauterbach KW. Threshold volumes associated with higher survival in health care: a systematic review. Med Care. 2003;41:1129–1141. [DOI] [PubMed] [Google Scholar]
- 20.Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg. 1999;230:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannan EL, Radzyner M, Rubin D, et al. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002;131:6–15. [DOI] [PubMed] [Google Scholar]
- 22.Ko CY, Chang JT, Chaudhry S, et al. Are high-volume surgeons and hospitals the most important predictors of inhospital outcome for colon cancer resection? Surgery. 2002;132:268–273. [DOI] [PubMed] [Google Scholar]
- 23.Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol. 2003;83:68–78; discussion 78–79. [DOI] [PubMed]
- 24.Hodgson DC, Zhang W, Zaslavsky AM, et al. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst. 2003;95:708–716. [DOI] [PubMed] [Google Scholar]
- 25.Birkmeyer JD, Skinner JS, Wennberg DE. Will volume-based referral strategies reduce costs or just save lives? Health Aff (Millwood). 2002;21:234–241. [DOI] [PubMed] [Google Scholar]
- 26.Beahrs O, Henson DE, Hutter RV, Kennedy BJ. Manual for Staging of Cancer, 3rd ed. Philadelphia: Lippincott, 1988. [Google Scholar]
- 27.Seifert J. SEER Program: Comparative Staging Guide for Cancer, version 1. 1. Bethesda, MD: National Cancer Institute, 1993. [Google Scholar]
- 28.Automatch 4. 0. Silver Springs, MD: Matchware Technologies, Inc., 1996. [Google Scholar]
- 29.Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson comorbidity adjustment in Medicare claims. Med Care. 1999;37:1128–1139. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 31.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 32.Meux EF, Stith SA, Zach A. Report of Results from the OSHPD Reabstracting Project. Sacramento, CA, Office of Statewide Health Planning and Development, 1990. [Google Scholar]
- 33.Zhalin D, Wei L. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 34.Fleisher LA, Eagle KA. Clinical practice: lowering cardiac risk in noncardiac surgery. N Engl J Med. 2001;345:1677–1682. [DOI] [PubMed] [Google Scholar]
- 35.Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery: Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335:1713–1720. [DOI] [PubMed] [Google Scholar]
- 36.Nichols RI, Choe EU, Weldon CB. Mechanical and antibacterial bowel preparation in colon and rectal surgery. Chemotherapy. 2005;51(Suppl):115–121. [DOI] [PubMed] [Google Scholar]
- 37.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of hospital procedure volume on surgical operation and long-term outcomes in high-risk curatively resected rectal cancer: findings from the Intergroup 0114 Study. J Clin Oncol. 2004;22:166–174. [DOI] [PubMed] [Google Scholar]
- 38.Beard JD, Nicholson ML, Sayers RD, et al. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg. 1990;77:1095–1097. [DOI] [PubMed] [Google Scholar]
- 39.Pronovost PJ, Angus DC, Dorman T, et al. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. [DOI] [PubMed] [Google Scholar]
- 40.Balslev I, Pedersen M, Teglbjaerg PS, et al. Postoperative radiotherapy in Dukes’ B and C carcinoma of the rectum and rectosigmoid: a randomized multicenter study. Cancer. 1986;58:22–28. [DOI] [PubMed] [Google Scholar]
- 41.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. [DOI] [PubMed] [Google Scholar]
- 42.Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma: Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–1472. [DOI] [PubMed] [Google Scholar]
- 43.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. [DOI] [PubMed] [Google Scholar]
- 44.Meyerhardt JA, Catalano PJ, Schrag D, et al. Association of hospital procedure volume and outcomes in patients with colon cancer at high risk for recurrence. Ann Intern Med. 2003;139:649–657. [DOI] [PubMed] [Google Scholar]
- 45.Ayanian JZ, Chrischilles EA, Wallace RB, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. [DOI] [PubMed] [Google Scholar]
Footnotes
Supported by Grant Nos. R01 HS09869 and U01 CA93324 from the Agency for Healthcare Research and Quality and the National Cancer Institute. Dr. Rogers was also supported by the Center for Excellence in Minority Health and Health Disparities, Harvard Medical School. The collection of cancer data used in this study was supported by the California Department of Health Services through the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute's SEER Program, and the Centers for Disease Control and Prevention's National Program of Cancer Registries.
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention is not intended nor should be inferred.
Reprints: John Z. Ayanian, MD, MPP, Department of Health Care Policy, Harvard Medical School, 180 Longwood Avenue, Boston, MA 02115. E-mail: ayanian@hcp.med.harvard.edu.


