Abstract
Background
Colonic lamina propria fibroblasts (CLPFs) play an important role in the pathogenesis of fibrosis and strictures in Crohn's disease.
Aim
To identify colonic epithelial cell (CEC)‐derived factors that activate CLPFs.
Methods
Primary human CECs and CLPFs were isolated from control mucosa and interleukin 8 (IL8) of CLPF cultures was quantified by ELISA. Activation of nuclear factor κB (NF‐κB) was shown, and translocation of NF‐κB was inhibited by a dominant‐negative IκB‐expressing adenovirus. The major CLPF‐activating and IL8 inducing protein was purified using fast‐performance liquid chromatography (HiPrep 16/60 Sephacryl S‐200 High Resolution Column) and sodium dodecyl sulphate gel electrophoresis.
Results
A considerable increase in IL8 secretion by CLPFs cultured in CEC‐conditioned media compared with that in unconditioned media (155.00 (10.00) pg/µg v 1.434 (0.695) pg/µg) was found. The effect of CEC‐conditioned media on CLPF IL8 secretion was NF‐κB dependent. A protein or DNA array confirmed the involvement of NF‐κB and activator protein‐1. Purification of a candidate band isolated with the use of sodium dodecyl sulphate‐polyacrylamide gel electrophoresis and subsequent sequencing showed soluble galectin‐3 to be a strong CLPF‐activating factor. Depletion of galectin‐3 from conditioned media by immunoprecipitation abolished the CLPF stimulatory effect.
Conclusions
Using a classical biochemical approach, soluble galectin‐3 was identified as a strong activator of CLPFs produced by CEC. Galectin‐3 induced NF‐κB activation and IL8 secretion in these cells and may be a target for future therapeutic approaches to reduce or avoid stricture formation.
Tissue fibroblasts have an important role in wound healing and tissue remodelling. After injury, tissue repair takes place with inflammation and new tissue and scar formation.1 Migration of fibroblasts into and through the extracellular matrix appears to be essential during the initial phase of wound healing.2 The transient appearance of myofibroblasts is a feature of normal wound healing, but the persistence of myofibroblasts is associated with excessive collagen deposition and is a hallmark in the pathophysiology of tissue fibrosis.2,3
In addition to tissue repair, fibroblasts have a role in inflammation. After stimulation, they produce cytokines such as interleukin (IL) 1, IL6, IL10 or tumour necrosis factor α (TNFα), growth factors such as transforming growth factor‐ (TGF)β, platelet‐derived growth factor, stem cell factor, hepatocyte growth factor and keratinocyte growth factor, as well as chemotactic factors such as IL8 or monocyte chemoattractant protein‐1.4,5
Fibrotic strictures of the small intestine, migration and proliferation of smooth muscle cells and increased production of extracellular matrix are frequently found in the course of Crohn's disease. 6 The deposition of fibrillar collagen can be detected in the lamina propria, muscularis mucosa, submucosa, muscularis propria and serosa. Increased levels of collagen type I, III, IV and V have been shown.7 Muscularis overgrowth and fibrosis are important contributors to the development of strictures.8
Currently, there are no effective preventive or medical treatments of strictures in Crohn's disease. Surgery remains the major treatment in intestinal fibrosis. Therefore, a better understanding of the pathophysiology of fibrosis and responsible mediators is needed to develop new therapeutic strategies. Colonic lamina propria fibroblasts (CLPFs) are subepithelial fibroblasts located at the interface between the colonic epithelial cells and the mucosal lamina propria in the colon. CLPFs enhance barrier function, modulate chloride secretion and regulate important epithelial‐cell functions via distinct secretory products.9 Treatment of CLPFs with 50 ng/ml TGF‐β for 6 days increases the amount of α‐actin‐positive cells from 8% to almost 100% and reduces their migratory potential.10
In recent studies we found that CLPF‐conditioned media induce CLPF migration in the modified Boyden chamber.11 Further, we showed that fibronectin is mainly responsible for the autocrine induction of CLPF migration essentially being required for the induction of CLPF migration.12
In the past few years, data have accumulated showing that intestinal epithelial cells (IECs) have an active role in the intestinal immune system.13,14 IECs can respond to damage or bacterial invasion by secreting cytokines.15 Cytokines reported to be secreted by IECs, such as IL1, IL6 and IL8, are important inflammatory mediators.16,17 Intestinal epithelial cells from normal mucosa express and secrete IL1ra and low amounts of IL8, but no IL1 or IL6.18 In inflamed mucosa, the secretion of proinflammatory cytokines such as IL8 and IL6 is strongly increased. In contrast with several studies on IEC and CLPF functions, only a few investigators have focused on interactions between intestinal fibroblasts and IEC.19,20,21
Given that the (1) pathophysiology of stricture formation in the intestinal bowel wall is not well understood, (2) fibroblasts have an essential role in this process and (3) that one of the initial steps in the pathophysiology of Crohn's disease is likely to be IEC damage, we aimed to investigate the influence of IEC on CLPF activation. In initial experiments we found that conditioned media from primary colonic epithelial cells (CECs) strongly activated CLPFs after stimulation for 24 h, and induced IL8 secretion by these cells. We therefore sought to identify the factor responsible for this CEC‐induced CLPF activation.
Materials and methods
Isolation and culture of CLPFs
Human CLPFs were isolated and cultured as described previously.11 The mucosa from surgical specimens was cut into 1 mm pieces, whereas biopsy specimens were used directly for the isolation of CLPF. Epithelial cells were separated in Hank's balanced salt solution without Ca2+ and Mg2+ (PAA Laboratories GmbH, Linz, Austria) with 2 mM EDTA (Sigma, Deisenhofen, Germany). The remaining tissue was rinsed and digested for 30 min at 37°C with 1 mg/ml collagenase 1 (Sigma), 0.3 mg/ml DNase I (Boehringer Ingelheim, Ingelheim, Germany) and 2 mg/ml hyaluronidase (Sigma) in phosphate‐buffered saline (PBS; Gibco, Karlsruhe, Germany). The isolated cells were cultured in 25 cm2 cell culture flasks (Costar, Bodenheim, Germany) with Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS; Pan Biotech, Aidenbach, Germany), penicillin 100 IE/ml (PAA Laboratories), streptomycin 100 µg/ml (PAA Laboratories), ciprofloxacin 8 µg/ml (Bayer, Leverkusen, Germany), gentamycin 50 µg/ml (PAA laboratories) and amphotericin B 1 µg/ml (Biochrom, Berlin, Germany). Non‐adherent cells were removed by subsequent changes of medium. The cultured cells were used between passages 3 and 8. The isolation of fibroblasts from biopsy specimens and surgical specimens was approved by the University of Regensburg ethics committee.
Isolation and culture of primary CECs
Colonic tissue was obtained from patients undergoing surgical resection for colorectal carcinoma or diverticulitis. The isolation of CEC was performed as described.22 Table 1 gives the details for the specimens used for cell isolation. The isolation of CEC from biopsy specimens and surgical specimens was approved by the University of Regensburg ethics committee.
Table 1 Characteristics of colonic tissue obtained from patients undergoing surgical resection for colorectal carcinoma or diverticulitis.
| Patient | Localisation | Sex | Age | Diagnosis |
|---|---|---|---|---|
| 1 | Colon | F | 63 | Carcinoma |
| 2 | Colon | M | 60 | Carcinoma |
| 3 | Sigmoid | M | 61 | Diverticulitis |
| 4 | Colon | F | 62 | Carcinoma |
| 5 | Sigmoid | F | 70 | Carcinoma |
| 6 | Sigmoid | M | 65 | Diverticulitis |
| 7 | Sigmoid | F | 44 | Diverticulitis |
| 8 | Rectum | M | 37 | Carcinoma |
| 9 | Sigmoid | M | 50 | Diverticulitis |
| 10 | Colon | M | 54 | Caecum‐carcinoma |
| 11 | Sigmoid | M | 47 | Carcinoma |
| 12 | Colon | F | 76 | Carcinoma |
| 13 | Colon | M | 63 | Carcinoma |
| 14 | Colon | F | 62 | Adenoma |
| 15 | Colon | F | 32 | Carcinoma |
| 16 | Colon | M | 45 | Carcinoma |
| 17 | Rectum | M | 82 | Carcinoma |
| 18 | Colon | F | 43 | Carcinoma |
| 19 | Rectum | M | 56 | Carcinoma |
| 20 | Colon | F | 59 | Carcinoma |
| 21 | Rectum | M | 74 | Carcinoma |
| 22 | Rectum | F | 77 | Carcinoma |
| 23 | Rectum | M | 64 | Carcinoma |
| 24 | Sigma | F | 53 | Diverticulitis |
The specimens were used for the isolation of colonic epithelial cells and for subsequent production of colonic epithelial cell‐conditioned media.
Cell lines
HT‐29 and CaCo‐2 cells were obtained from the European Collection of Cell Culture, Europe and cultured in DMEM (PAA Laboratories) supplemented with 10% FCS, 1% sodium pyruvate (PAA Laboratories), 1% non‐essential amino acids (PAA Laboratories) and 1% penicillin/streptomycin under standard tissue‐culture conditions.
CCD‐18Co cells represent an immortalised fibroblast cell line from human colon (American Type Culture Collection, Manassas, Virginia, USA). Cells were cultured at 37°C in a 10% CO2 atmosphere in DMEM supplemented with 20% FCS, 1% sodium pyruvate, 1% non‐essential amino acids and 1% penicillin/streptomycin.
Conditioned medium from CECs
CECs were isolated and cultured on collagen A (Biochrom)‐coated Millicell CM filters (Millipore Corporation, Bedford, Massachusetts, USA) in CEC medium (penicillin/streptomycin and gentamycin; MEM‐Earle/Biochrom, Berlin, Germany) as described previously.22 After 24 h of incubation, the medium was collected, centrifuged to remove cell debris, and stored at −20°C for up to 3 months. IL8 content of the conditioned media was quantified by ELISA.
Conditioned medium from HT‐29 and CaCo‐2 cells
The medium was removed from a confluent cell monolayer and the cells were washed twice with PBS. Subsequently, cells were cultured with DMEM without FCS for 24 h. The conditioned media were centrifuged and stored at −20°C for up to 3 months.
Cell lysate from HT‐29 cells and CaCo‐2 cells
After removal of culture medium, cells were washed with PBS. Cells were detached from the culture plates by a cell scraper, transferred into a 15‐ml tube and centrifuged. The supernatant was discharged and the pellets treated with ultrasound in a sonicator (Sonix Ultraschallbad, Bandelin, Berlin, Germany) for 10 min on ice. DMEM 1 ml was added and the lysate resuspended with a sterile 23‐G needle (BD Mircolance, Becton Dickinson, Fraga, Spain). After centrifugation (1500 U/min for 5 min; Megafuge 1.0 R, Heraeus Holding GmbH, Hanau, Germany), 200 µl of the supernatant were mixed with 800 µl DMEM and used for cell stimulation.
Denaturation of CEC‐conditioned medium
The CEC‐conditioned medium was heated at 95°C for 10 min, 30 min and 2 h. The denatured medium was used for CLPF stimulation as described earlier.
ELISA
IL8, TGFβ1 and galectin‐3 were quantified by ELISA according to the manufacturer's protocol (Biozol, Eching, Germany; Ray Biotech, Norcross, Germany).
Dominant‐negative IκB adenovirus
The CCD‐18Co cell line was grown on eight‐well chamber slides (Brandt, Wertheim, Germany) until confluence. The medium was removed and the cells were covered with 50 µl MEM medium (Biochrom AG, Berlin, Germany) containing 2% FCS. At a defined multiplicity of infection (MOI; number of infectious particles per cell), 100 µl of the dominant‐negative IκB adenovirus were added to the cells. Cells were incubated for 2 h at 37°C in 10% CO2. Then, the medium was added to a total volume of 200 µl, and cells were cultured for another 2 days. Stimulation was performed with 200 µl of conditioned medium per well for 1 h.
Immunofluorescence
Chamber slides with CLPF grown to confluence were washed twice in PBS for 5 min, air dried, and incubated for 10 min in acetone at −20°C. Subsequently, slides were incubated in 10% FCS medium for 30 min. Then, 50 µl of anti‐p65 antibody (clone 20, BD Biosciences, Heidelberg, Germany) or mouse immunoglobulin G1 antibody (Sigma) were added as isotype control in dilutions of 1:250 and 1:200, respectively. The slides were placed in a humid box for 1 h. After repeated washing, the secondary antibody (goat anti‐mouse Immunoglobulin G1, 1:300; Molecular Probes, Eugene, Oregon, USA) with 0.5% goat serum (Dako Cytomation, Hamburg, Germany) was added for 30 min in the dark. After washing, the fluorescent mounting medium (Dako) was added, and the slides were covered (Deckgläser, Laborcenter, Nürnberg, Germany) and stored at 4°C.
Protein/DNA array
This test is designed to simultaneously determine the up regulation or down regulation of 96 transcription factors in the nuclear extract of cells. The nuclear extracts of CCD‐18Co cells stimulated with CEC‐conditioned and unconditioned media were compared in this assay. A set of biotin‐labelled DNA‐binding oligonucleotides was pre‐incubated with the nuclear extracts to allow the formation of protein/DNA complexes. The complexes were extracted from the free probes and hybridised to the TranSignal array membrane spotted with consensus sequences for the specific transcription factors. Cell culture flasks (5×75 cm2) with CLPFs grown to confluence were stimulated with CEC‐conditioned media. Stimulation was performed for 1 h with 5 ml of conditioned medium and control medium at 37°C. A nuclear extraction kit (BioCat GmbH, Heidelberg, Germany) was used for the isolation of nuclear proteins according to the manufacturer's protocol. Protein concentration was measured using the bicinchoninic acid test (Sigma). The protein/DNA array (BioCat GmbH, Heidelberg, Germany) was used according to the manufacturer's protocol to identify transcription factors involved in the effects of CEC‐conditioned medium.
Protein precipitation
To concentrate the conditioned medium for subsequent fast‐performance liquid chromatography (FPLC) analysis, it was mixed with ammonium sulphate and precipitated for 24 h under rotation at 4°C, followed by centrifugation for 30 min at 6000 g. The protein pellet was resuspended in 1 ml PBS and frozen at −20°C. The pellet still induced IL8 secretion without loss of activity (data not shown).
Fast‐performance liquid chromatography
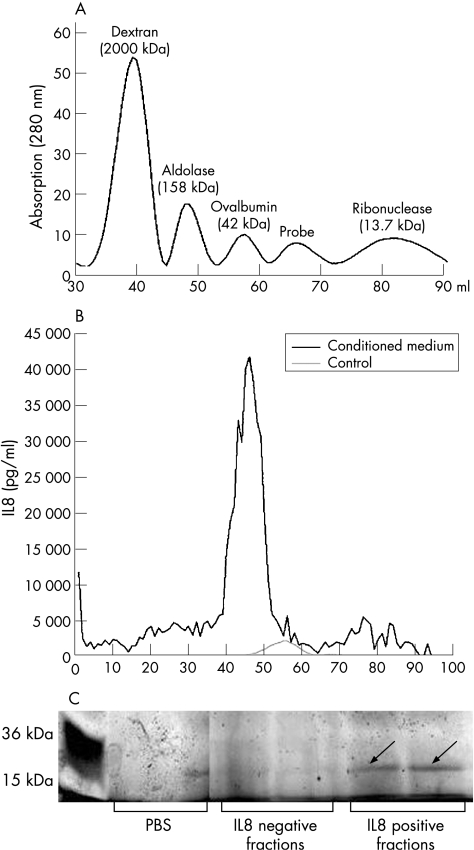
For the separation of the proteins from conditioned media, FPLC was performed with a HiPrep 16/60 Sephacryl S‐200 High Resolution column . The column separates proteins with a molecular weight between 5 and 250 kDa. To define the elution volume, blue dextran, aldolase, ovalbumin, ribonuclease, chymotrypsinogen A and albumin were added. FPLC was performed at 4°C. A volume of 50 ml of CEC‐conditioned media containing CEC isolated from each of 11 surgical specimens was collected, the protein was precipitated, and the protein mix supplied to the column. The total elution volume was 120 ml and the first 20 ml were discarded. The probe volume was 1 ml, fraction size 1 ml and total volume collected 100 ml. Each fraction was tested for the capacity to induce IL8 secretion from CLPF.
Sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE)
The FPLC fractions were used in concentrations of 1:1, 1:5, 1:10, 1:25, 1:50 1:100, 1:250, 1:500 and 1:1000; probe volume was 21 µl, and 4 µl of Laemmli buffer were added to each probe. A 15% TRIS‐HCl gel (1.0 mm; BioRad Laboratories, Hercules, California, USA) was used. The gels were run at 500 V and 25 mA for 1 h and at 35 mA for another hour. After separation, the gels were incubated in 7.5% acetic acid (Merck, Darmstadt, Germany) with addition of a luminescent and fluorescing antibody (SYPRO Red protein gel stain; Molecular Probes) in the dark. After washing, the bands were analysed with a Typhoon Scanner (Typhoon 9200 Molecular Dynamics, Amersham Pharmacia Biosciences, Freiburg, Germany).
Protein identification
The IL8‐inducing FPLC fractions were separated by electrophoresis (SYPRO Red protein gel stain). The bands, which were found only in strong IL8‐inducing fractions, were extracted and used for protein analysis. The protein bands were “in‐gel” digested with trypsin. The fragments were analysed using mass spectrometry (matrix‐assisted laser desorption/ionisation time‐of‐flight (MALDI‐TOF) mass spectrometer). The results were compared using a database (NCBInr) for identification of the protein.
Immunoprecipitation of galectin‐3
We added 50 µl of protein‐G‐sepharose and 20 µl of purified mouse anti‐human galectin‐3 monoclonal antibody (BD Biosciences Pharmingen, Le.Pont de Claix, France) to 1 ml of the CEC‐conditioned medium. For control medium without addition of galectin‐3, antibody containing only protein‐G‐sepharose was used. Tubes were incubated for 2 h at 4°C under rotation. The antibody–antigen complex was precipitated by centrifugation (6000 g, 5 min; Zentrifuge, Eppendorf, Hamburg, Germany). The supernatants were frozen at −20°C. The pellet was washed repeatedly with 500 µl PBS. Immunoprecipitated proteins were separated with SDS‐PAGE. The gel was blotted on a nitrocellulose membrane for 30 min. The membrane was subsequently incubated in blocking buffer containing PBS, 0.2% Triton‐X (Sigma) and 5% milk powder, followed by washing with Triton‐X and PBS. Anti‐galectin‐3 antibody was added in 5 ml of blocking buffer in a concentration of 5 µg/ml for 1 h, followed by washing. Anti‐biotin antibody with streptavidin at concentrations of 1:1000 and 1:4000 were added for 30 min. After washing, ECL‐development was performed (ECL Plus Western Blotting Detection System, Amersham, Illinois, USA). Curix 60(Agfa, Mortsel, Belgium) was used to develop the film.
Stimulation with recombinant galectin‐3
To test the CLPF‐activating potential of galectin‐3, CLPFs were stimulated with recombinant galectin‐3 at different concentrations (10 µg/ml, 0.1 µg/ml or 1 ng/ml). As control, CLPFs were treated with CEC‐conditioned medium, unconditioned medium and galectin‐3‐depleted conditioned medium. After 24 h of incubation, IL8 secretion was measured in the supernatants by ELISA.
Statistics
Statistical analyses were performed using the Student's t test. Differences were considered significant at p<0.05.
Results
CEC‐conditioned medium activates intestinal fibroblasts
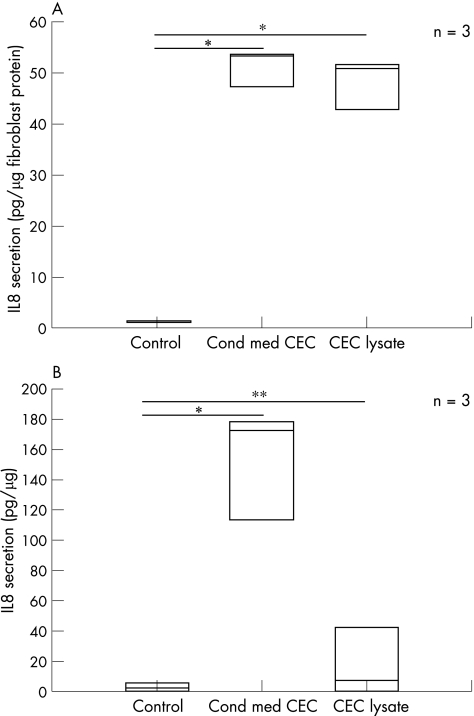
CLPFs and the CCD‐18Co cell line were incubated with unconditioned media, CEC‐conditioned media and a pellet of CECs for 24 h. Only low levels of IL8 and TGF‐β1 were measured in CEC‐conditioned media before incubation with CLPF. Subsequently, IL8 secretion as a readout for cell activation was quantified in the supernatants of the cells by ELISA. Values of cytokines present in the conditioned media were subtracted. Figure 1A, B show the results. Low levels of IL8 secretion were detected in the CCD‐18Co cell line (1.41 (0.17) pg/µg; fig 1A) and in CLPFs (1.43 (2.37) pg/µg; fig 1B) grown under standard conditions with unconditioned medium. Stimulation with cell lysates of CECs induced a 40‐fold increase in IL8 secretion in CCD‐18Co cells (48.56 pg/µg) compared with the unconditioned medium (fig 1A). In CLPFs, CEC lysate induced a 15‐fold increase in IL8 secretion (32.61 pg/µg) compared with the unconditioned medium (fig 1B).
Figure 1 (A) Interleukin 8 (IL8) secretion of CCD‐18Co cells incubated with control, colonic epithelial cell (CEC)‐conditioned medium (Cond med CEC) and CEC lysates for 24 h. IL8 secretion was quantified in the supernatant of these cells by ELISA. Results are given as pg/µg fibroblast protein and as the mean of three different CEC‐conditioned media. In comparison to control medium, CEC‐conditioned medium and CEC lysates strongly induced IL8‐secretion (*<0.01 v control). Data are given as mean (SD). (B) IL8 secretion of primary CLPFs incubated with control media, CEC‐conditioned media and CEC lysates for 24 h. CEC‐conditioned media again induced IL8 secretion (*p<0.01 v control). The levels were 10‐fold higher than IL8 levels in cells incubated with CEC lysates (**p<0.05 v control) and control media. CEC‐conditioned media of three different patients were used. Data are given as mean (SD).
Moreover, a high increase in IL8 secretion in CCD‐18Co cells (51.17 (3.53) pg/µg); fig 1A) and in CLPFs (155 (10) pg/µg fig 1B) was observed after culture of these cells with CEC‐conditioned media. IL8 secretion under these conditions was 50‐fold or 70‐fold (respectively) higher than cells cultured with unconditioned media. When CCD‐18Co cells and CLPFs were incubated with conditioned media and lysates of the intestinal epithelial cell lines HT‐29 and CaCo‐2 (fig 2A, B, slightly different results were obtained: incubation with HT‐29‐conditioned media induced low IL8 secretion in CCD‐18Co cells (7.05 (3.92) pg/µg) and in CLPFs (3.17 (1.48) pg/µg). CaCo‐2 conditioned medium also induced low IL8 secretion in CCD‐18Co cells (9.45 (10.24) pg/µg) and CLPF (1.8 (1.37) pg/µg). The stimulation of CCD‐18Co cells with lysates of HT‐29 cells, however, resulted in higher induction of IL‐8 secretion (93.79 (77.01) pg/µg). Similar results were obtained with CLPFs (11.44 (7.35) pg/µg). Stimulation with lysates of CaCo‐2 cells had no effect on IL8 secretion in both CLPF (8.97 (5.48) pg/µg) and CCD‐18Co cells (21.22 (7.43) pg/µg). Because of a high variability in the IL8 response of CLPF cultures from different donors, the CCD‐18Co cell line was used for further investigations to optimise the reproducibility of the results.
Figure 2 (A) Interleukin 8 (IL8) secretion of CCD‐18Co cells incubated with conditioned medium (cond med) and lysates of HT‐29 and CaCo‐2 cells for 24 h. Unconditioned medium was used as control. Incubation with the pellet of HT‐29 cells strongly induced IL8 secretion. Data represent three different experiments and are given as mean (SD). *p<0.01 versus control; NS, not significant. (B): IL8 secretion of primary colonic lamina propia fibroblasts (CLPFs) incubated with control medium and with conditioned medium and lysates of HT‐29 and CaCo‐2 cells for 24 h. The stimulation with lysates of HT‐29 and CaCo‐2 induced IL8 secretion in CLPF. Data represent three different experiments and are given as mean (SD). NS, not significant versus control.
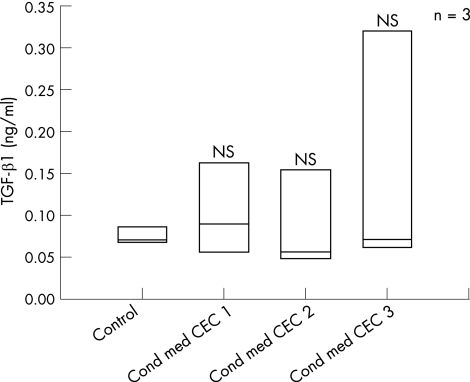
TGF‐β1 secretion was not induced by incubation with CEC‐conditioned media (fig 3).
Figure 3 CCD‐18Co cells were stimulated with unconditioned medium and colonic epithelial cells (CEC)‐conditioned media (cond med). TGF‐β1 was quantified in the supernatant. TGF‐β1 secretion was not significantly higher in cells incubated with CEC‐conditioned media compared with control. NS, not significant versus control.
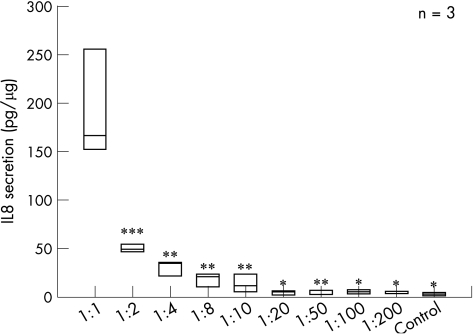
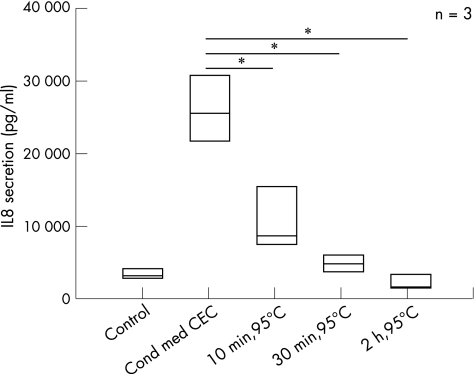
We further investigated whether dilutions of CEC‐conditioned media still had an IL8‐inducing effect on intestinal fibroblasts. CCD‐18Co cells were incubated with undiluted conditioned media and increasing dilutions of these media. IL8 secretion was quantified in culture supernatants (fig 4). CEC‐conditioned media still induced IL8 secretion of CCD‐18Co cells up to a dilution of 1:10 (control, 4.03 (1.73) pg/µg; 1:1 dilution, 192.21.(56.19) pg/µg; 1:10 dilution, 14.77 (9.16) pg/µg). Dilutions between 1:20 and 1:200 did not induce IL8 secretion. These data indicated a dose‐dependent effect of the postulated CEC‐derived CLPF activator. To elucidate whether the CEC‐derived CLPF activator was a protein, CEC‐conditioned media were denatured at 95°C. Denaturation of the CEC‐conditioned media at 95°C was followed by an almost complete loss of IL8‐inducing effect (fig 5). These data indicate that one or more proteins were responsible for the strong IL8‐inducing (CLPF activating) effect of CEC‐conditioned medium.
Figure 4 Interleukin 8 (IL8) induction in CCD‐18Co cells incubated with subsequent dilutions of the colonic epithelial cells (CEC)‐conditioned medium for 24 h. The supernatants were tested for IL8 secretion by ELISA. Dilutions up to 1:10 still induced IL8 secretion. No IL8‐inducing effect was seen in dilutions from 1:20 to 1:200 or in control medium. Data are given as mean (SD). *p<0.01, **p<0.02 and ***p<0.03, all versus 1:1 dilution.
Figure 5 Denaturation of colonic epithelial cells (CEC)‐conditioned media (cond med) at 95°C for 10 min, 30 min and 2 h. The media were used for stimulation of CCD‐18Co cells for 24 h. As controls, cells were stimulated with unconditioned and non‐denatured, CEC‐conditioned media. Denaturation of the medium significantly (p<0.01) reduced interleukin 8 (IL8) secretion compared with CEC‐conditioned media, indicating a protein responsible for CLPF activation. Data are given as mean (SD). *p<0.01 versus Cond med CEC.
CEC‐conditioned medium‐induced activation of CLPF is mediated by NF‐κB
NF‐κB has a central role in the regulation of IL8 transcription. Therefore, we tested whether NF‐κB activation was involved in CEC‐conditioned medium‐induced IL8 secretion by intestinal fibroblasts. CCD‐18Co cells were incubated with CEC‐conditioned media for 1 h. Immunofluorescence with an antibody against p65 of the NF‐κB complex showed high rates of translocation of NF‐κB to the nucleus after incubation with CEC‐conditioned media compared with unconditioned media (data not shown), indicating activation of this transcription factor. In CCD‐18Co cells incubated with unconditioned media, no signal enhancement in the nucleus was observed. These results indicated an involvement of NF‐κB activation and translocation in the IL8‐inducing effect of CEC‐conditioned media.
We confirmed this finding using a protein/DNA array. This test is designed to simultaneously determine the up regulation or down regulation of 96 transcription factors in the nuclear extract of cells. Nuclear extract of CCD‐18Co cells stimulated with CEC‐conditioned and unconditioned media were compared in this assay. High levels of NF‐κB were found in the nuclear extracts of cells treated with CEC‐conditioned medium compared with those incubated with unconditioned medium. In addition to an induction of NF‐κB activation, an increase in nuclear translocation of AP‐1, c‐Myb, AP‐2 and p53 was observed, whereas C/EBP levels were low in stimulated and control cells (data not shown).
To ensure that NF‐κB activation was required for IL8 induction by CEC‐conditioned media, IL8 secretion was quantified in cell cultures transfected with the dominant‐negative IκB adenovirus. Cells were infected with different amounts of adenovirus (0.25×106–0.75×106 PFU/ml) followed by stimulation with CEC‐conditioned media for 1 h. The culture supernatants were tested for IL8 secretion. The nuclear translocation of the NF‐κB complex was confirmed by immunofluorescence. A significant decrease (p<0.05) was observed in IL8 secretion of cells infected with the dominant‐negative IκB adenovirus after stimulation with CEC‐conditioned media compared with control cells. Whereas IL8 secretion of cells cultured in CEC‐conditioned media was 13.37 (4.73) pg/µg, IL8 secretion in adenovirus‐transfected cells (depending on the MOI), was MOI 5, 10.07 (5.50) pg/µg and MOI 15, 1.33 (0.63) pg/µg. Corresponding anti‐p65 immunofluorescence also showed less or no nuclear translocation of the NF‐κB complex in dominant‐negative IκB adenovirus‐transfected cells, whereas a high NF‐κB signal in the nucleus of control cells was detectable (data not shown).
Identification of the CEC‐derived CLPF‐stimulating factor
FPLC was used to isolate the CLPF‐stimulating protein from CEC‐conditioned media. For FPLC analysis, 50 ml of CEC‐conditioned media were collected from epithelial cells derived from 11 patients. The conditioned media were initially precipitated to concentrate the protein. As mentioned above, the conditioned media could be diluted up to 1:10 while still retaining the IL8‐inducing effect. The ability either to dilute or to concentrate the conditioned media without loss of stimulatory effect was mandatory if FPLC separation was to be used for further identification of IL8‐inducing activity. The precipitated proteins retained their ability to induce IL8 secretion (data not shown). A calibration curve was obtained by determining the elution volumes of several standard substances (chromatogram shown in fig 6A). The concentrated CEC‐conditioned medium was supplied to the column. Each of the 100 elution fractions was tested for its IL8‐inducing effect. Figure 6B shows the IL8 levels. The highest potential to induce IL8 secretion from intestinal fibroblasts was found at a molecular mass between 15 and 48 kDa. The FPLC fractions with high IL8‐inducing potential were subjected to SDS‐PAGE. For comparison, elution fractions without IL8‐inducing effect were separated. Figure 6C shows the results. A band that was exclusively found in IL8‐inducing fractions was cut out of the gel and used for protein finger printing. The MALDI‐TOF analysis showed that this protein was soluble galectin‐3.
Figure 6 (A) Standard proteins of known molecular size were separated by fast performance liquid chromatography (FPLC) to determine the elution volume of the HiPrep 16/60 Sephacryl S‐200 High Resolution column. The chromatogram was used to determine the molecular size of the unknown protein in colonic epithelial cells (CEC)‐conditioned media. (B) FPLC was used to further purify the colonic lamina propia fibroblasts (CLPF)‐stimulating factor from CEC‐conditioned medium. A volume of 50 ml of CEC‐conditioned media containing CEC isolated from each of 11 surgical specimens was collected, the protein was precipitated, and the protein mix supplied to the column. Total elution volume was 120 ml; the first 20 ml were discarded. Each fraction was tested for its interleukin 8 (IL8)‐inducing effect on CLPFs. In control (unconditioned) media, no fraction significantly induced IL8 secretion. In conditioned media, fractions 42–52 induced strong IL8 secretion. This area was used for further analysis. (C) The fractions of FPLC chromatography (IL8‐inducing fractions, non‐IL8‐inducing fractions, and phosphate‐buffered saline (PBS) as control; See BluePlus 2 as standard) were used for protein gel analysis. A mixture of bands of different molecular sizes or no bands in the IL‐8‐negative fractions was found. In the IL8‐positive fractions, there was one clear band (arrow) at a molecular size of about 30 kDa that did not appear in the other lanes. This area was separated and analysed by matrix‐assisted laser desorption/ionisation time‐of‐flight analysis.
Confirmation of galectin‐3 as a major CEC‐derived CLPF‐stimulating factor
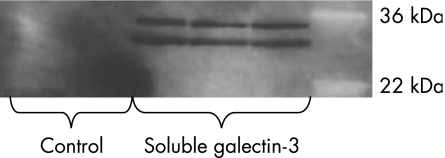
To confirm that conditioned media contain galectin‐3, we performed immunoprecipitation in the conditioned media. The extracted protein was then analysed by gel electrophoresis. We found an intense signal in conditioned media at the correct molecular mass, but there were no bands in the controls (fig 7).
Figure 7 Immunoprecipitation of galectin‐3 from colonic epithelial cells (CEC)‐conditioned media. Protein‐G‐sepharose and anti‐human galectin‐3 antibody were added to the CEC‐conditioned medium and immunoprecipitated. The medium without anti‐galectin‐3 antibody was used as a control. The immunoprecipitated protein was analysed by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis. No bands were detectable in control medium. The CEC‐conditioned media showed one band at the molecular size of galectin‐3 at 30 kDa (additionally, the heavy chain of the galectin‐3 antibody was seen).
Galectin‐3‐depleted media no longer induce CLPF activation and IL8 secretion
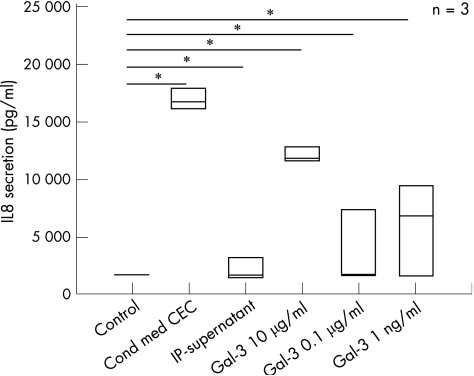
We further investigated whether the IL8‐inducing effect of conditioned media could be reproduced with recombinant galectin‐3. CCD‐18Co cells were incubated with unconditioned media, with CEC‐conditioned media and with recombinant galectin‐3 at different concentrations, as well as with galectin‐3‐depleted (immunoprecipitated) conditioned media. Recombinant galectin‐3 induced strong activation of the cells, comparable to IL8 secretion after culture with CEC‐conditioned media. Galectin‐3‐depleted media no longer induced CLPF activation and IL8 secretion (fig 8). Galectin‐3 concentrations in CEC‐conditioned media were determined by ELISA. They varied between 8 and 26 µg/ml (data not shown) and were usually higher than the highest concentration of recombinant galectin‐3 used in the experiment shown in fig 8. This may explain why the highest galectin‐3 concentration used in this experiment (10 µg/ml) did not stimulate CLPFs to the level of conditioned medium. These data confirmed that galectin‐3 is a strong CLPF activator.
Figure 8 Recombinant galectin‐3 stimulation induced strong colonic lamina propia fibroblasts activation, indicating that the strong interleukin 8 (IL8) ‐inducing effect of colonic epithelial cells (CEC)‐conditioned medium could be reproduced by recombinant galectin‐3. CCD‐18Co cells were stimulated with unconditioned medium, CEC‐conditioned medium, immunoprecipitated medium and recombinant galectin‐3 at different concentrations. Recombinant galectin‐3 induced strong IL8 secretion in the cells, comparable to the levels of CEC‐conditioned media. Galectin‐3‐depleted media only reached IL8 levels of unconditioned media. *p<0.01 versus unconditioned media.
Discussion
Using a classical biochemical approach, we identified galectin‐3 as the major CLPF‐activating factor secreted by CEC. CEC‐conditioned media induced strong activation of CLPF. Dilutions of the CEC‐conditioned media up to 1:10 still induced CLPF activation. Heat denaturation of the conditioned media abolished CLPF stimulation, indicating that one or more proteins were responsible for the activation. Inhibition of the NF‐κB activation with dominant negative IκB adenovirus considerably reduced the IL8‐inducing effect of CEC‐conditioned media. The concentrated CEC‐conditioned media were separated by FPLC chromatography, and the IL8‐inducing fractions were further analysed. Finally, one protein band was sequenced. Galectin‐3 was the only protein in this band. Immunoprecipitation confirmed that galectin‐3 is part of the CEC‐conditioned media and is the major activator of CLPFs. Stimulation of CLPFs with recombinant galectin‐3 resulted in a high increase in CLPF IL8 secretion.
Isolation of CEC from the mucosa and culture of these cells for 24 h on culture inserts may induce a stress reaction, followed by induced transcription of cytokines, acute‐phase proteins and other secretory products. This outcome may explain why CEC‐conditioned media had a more pronounced effect than HT‐29 and CaCo‐2 conditioned media. Nevertheless, conditioned media as well as lysates of HT‐29 and the CaCo‐2 cells induced IL8 secretion from intestinal fibroblasts. The more pronounced effect of the HT‐29 and CaCo‐2 lysate compared with HT‐29 and CaCo‐2 conditioned media may indicate that in these cell lines galectin‐3 is membrane bound, whereas it is mainly secreted by primary CEC. Presently we are investigating whether cell activation of CEC is followed by a shift from membrane‐bound to soluble galectin‐3.
TGF‐β1 was not induced by incubation of CLPF with CEC‐conditioned media. TGF‐β1 is known to be involved in fibrosis as well as in intestinal inflammation.23,24 The role and influence of CEC‐conditioned media on proinflammatory cytokines in fibroblasts of inflammatory bowel disease need further investigation.
Transcription factors of the NF‐κB/Rel family have a central role as regulators in immunological and inflammatory processes. At the same time, they have specific functions in different organs such as the intestine.25,26 After phosphorylation and subsequent ubiquitination and degradation of the NF‐κB inhibitors (the IκB‐family), the NF‐κB complex translocates to the nucleus and interacts with several specific genes involved in inflammation and proliferation. The NF‐κB complex can be activated by TNF, viral products, bacteria, lipopolysaccharides and cytokines.27,28 We showed that the IL8‐inducing effect of CEC‐conditioned media depends on the NF‐κB pathway. CLPF activation was abrogated after inhibition of this pathway by the dominant‐negative IκB adenovirus. IL8 secretion was considerably reduced in cells infected with dominant negative IκB‐adenovirus. Quantification of NF‐κB translocation with a protein/DNA array confirmed these results. Further experiments are required to analyse the involvement of galectin‐3 in other pathways sush as the MAPK pathway.
FPLC‐based analysis resulted in one peak at a molecular size between 15 and 48 kDa that induced high IL8 secretion. MALDI‐TOF analysis identified soluble galectin‐3 as the responsible CLPF‐activating factor. Galectin‐3 is a member of a growing protein family. This family is defined by two aspects; their binding affinity for galactosides and a conserved sequence element in the carbohydrate‐binding site. Every galectin has a domain consisting of a minimum of 130 amino acids, which is responsible for carbohydrate binding and is called the carbohydrate recognition domain (CRD). There are three total subgroups of galectins, including galectin‐3: the prototype group containing a specific domain of CRD (galectin‐1, ‐2, ‐5, ‐7, ‐10, ‐11, ‐13 and ‐14), the tandem repeat group with two CRDs (galectin‐4, ‐6, ‐8, ‐9 and ‐12) and the chimera‐group, with galectin‐3 as the only member with a proline‐rich and glycine‐rich domain of 130 amino acids connected to the CRD domain.29,30 Galectin‐3 is known to be expressed in epithelial cells, in cartilage, in macrophages and in activated T cells.31
Many different functions have been suggested for galectin‐3. The protein has been shown to be involved in apoptosis, where the protein interacts with bcl‐2, a known suppressor of apoptosis with some sequence similarity with galectin‐3.32,33 Further, galectin‐3 seems to have a protective effect against the formation of reactive oxygen products.34 Galectin‐3‐transfected T cells showed higher and faster growth rates compared with controls.32 Galectin‐3 may also be involved in nuclear splicing of pre‐mRNA.35 Also, galectin‐3 plays a role in wound healing, accelerating re‐epithelialisation.37 Galectin‐3 may also act as an inducer of inflammation in some tissues. Galectin‐3‐deficient mice developed less inflammatory cell infiltration in the peritoneal cavity after thioglycollate treatment compared with control mice.37 In response to stress, glioblastoma cells showed increased levels of galectin‐3, whereas a heat shock decreased galectin‐3 levels.39 In addition, galectin‐3 induces monocyte and macrophage migration.39
The protein can be found in the cytoplasm or in the nucleus, as well as attached to the cell membrane. The phosphorylated form is both in the nucleus and in cytoplasm, whereas the non‐phosphorylated form localises exclusively in the nucleus.40 Galectin‐3 can also be secreted. Phosphorylation may be necessary for the anti apoptotic effect and the cell‐cycle arrest.41 Galectin‐3 is part of intracellular vesicles of phagosomes in macrophages and of exosomes of dentritic cells.42 In general, galectins are synthesised in the cytosol and released from the cell by an unorthodox secretory mechanism that uses the endoplasmic reticulum and the Golgi.43 The finding of Kasper et al,44 who provided evidence that galectin‐3 may have a role in pulmonary alveolar epithelial expansion and differentiation during injury and repair, is most important for the interpretation of our results. They suggested that galectin‐3 is involved in the pathophysiology of pulmonary fibrosis. Previously, no study had investigated the function and involvement of galectin‐3 in the pathogenesis of fibrosis and stricture formation in the intestine.
A pathological strong activation and stimulation of fibroblasts could be associated with development of strictures during the course of Crohn's disease. Although the effect of galectin‐3 has been shown only in CLPFs isolated from control patients in this study, it may be speculated that galectin‐3 could be involved in the induction of fibrosis in Crohn's disease. Jensen‐Jarolim et al showed that the titre of auto‐antibodies against galectin‐3 was higher in patients with Crohn's disease than in those with ulcerative colitis or healthy controls and correlated positively with Crohn's disease activity.45,46 However, so far it is not clear whether the galectin‐3‐induced activation of CLPF is more pronounced in Crohn's disease. Further studies are needed to clarify whether there are changes in galectin‐3 expression in Crohn's disease or ulcerative colitis and whether CLPF activation will induce or prevent a fibrogenic CLPF phenotype. In any case, a better understanding of galectin‐3‐mediated effects on intestinal fibroblasts may provide a new opportunity of intervention in the process of intestinal fibrosis.
Acknowledgements
We thank the surgeons and pathologists of the University of Regensburg for providing us with colonic specimens. We are grateful to the patients for contributing the tissue samples.
Abbreviations
CEC - colonic epithelial cell
CLPF - colonic lamina propria fibroblast
CRD - carbohydrate recognition domain
DMEM - Dulbecco's modified Eagle's medium
FCS - fetal calf serum
FPLC - fast‐performance liquid chromatography
IEC - intestinal epithelial cell
IκB - inhibitor protein κB
MALDI‐TOF - matrix‐assisted laser desorption/ionization time‐of‐flight
MOI - multiplicity of infection
NF‐κB - nuclear factor κB
PBS - phosphate buffered saline
SDS‐PAGE - sodium dodecyl sulphate‐polyacrylamide gel electrophoresis
TGF - transforming growth factor
Footnotes
Funding: This work was supported by the Bundesministerium für Bildung und Forschung, BMBF (Kompetenznetz CED) and the Deutsche Forschungsgemeinschaft (SFB 585).
Competing interests: None declared.
The isolation of fibroblasts from biopsies and surgical specimens was approved by the University of Regensburg ethics committee.
References
- 1.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 200169513–521. [PubMed] [Google Scholar]
- 2.Tranquillo R T, Murray J D. Mechanistic model of wound contraction. J Surg Res 199355233–247. [DOI] [PubMed] [Google Scholar]
- 3.Nedelec B, Ghahary A, Scott P G.et al Control of wound contraction. Basic and clinical features. Hand Clin 200016289–302. [PubMed] [Google Scholar]
- 4.Rogler G, Gelbmann C M, Vogl D.et al Differential activation of cytokine secretion in primary human colonic fibroblast/myofibroblast cultures. Scand J Gastroenterol 200136389–398. [DOI] [PubMed] [Google Scholar]
- 5.Pang G, Couch L, Batey R.et al GM‐CSF, IL‐1 alpha, IL‐1 beta, IL‐6, IL‐8, IL‐10, ICAM‐1 and VCAM‐1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL‐1 alpha and TNF‐alpha. Clin Exp Immunol 199496437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham M F, Diegelmann R F, Elson C O.et al Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology 198894257–265. [DOI] [PubMed] [Google Scholar]
- 7.Matthes H, Herbst H, Schuppan D.et al Cellular localization of procollagen gene transcripts in inflammatory bowel diseases. Gastroenterology 1992102431–442. [DOI] [PubMed] [Google Scholar]
- 8.Becker J M. Surgical therapy for ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am 199928371–ix. [DOI] [PubMed] [Google Scholar]
- 9.Beltinger J, McKaig B C, Makh S.et al Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol 1999277(2 Pt 1)C271–C279. [DOI] [PubMed] [Google Scholar]
- 10.Narine K, DeWever O, Cathenis K.et al Transforming growth factor‐beta‐induced transition of fibroblasts: a model for myofibroblast procurement in tissue valve engineering. J Heart Valve Dis 200413281–289. [PubMed] [Google Scholar]
- 11.Leeb S N, Vogl D, Falk W.et al Regulation of migration of human colonic myofibroblasts. Growth Factors 20022081–91. [DOI] [PubMed] [Google Scholar]
- 12.Leeb S N, Vogl D, Grossmann J.et al Autocrine fibronectin‐induced migration of human colonic fibroblasts. Am J Gastroenterol 200499335–340. [DOI] [PubMed] [Google Scholar]
- 13.Spottl T, Hausmann M, Kreutz M.et al Monocyte differentiation in intestine‐like macrophage phenotype induced by epithelial cells. J Leukoc Biol 200170241–251. [PubMed] [Google Scholar]
- 14.Wu K C, Jackson L M, Galvin A M.et al Phenotypic and functional characterisation of myofibroblasts, macrophages, and lymphocytes migrating out of the human gastric lamina propria following the loss of epithelial cells. Gut 199944323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace T D, Bradley S, Buckley N D.et al Interactions of lactic acid bacteria with human intestinal epithelial cells: effects on cytokine production. J Food Prot 200366466–472. [DOI] [PubMed] [Google Scholar]
- 16.Eckmann L, Jung H C, Schurer‐Maly C.et al Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 19931051689–1697. [DOI] [PubMed] [Google Scholar]
- 17.Garat C, Arend W P. Intracellular IL‐1Ra type 1 inhibits IL‐1‐induced IL‐6 and IL‐8 production in Caco‐2 intestinal epithelial cells through inhibition of p38 mitogen‐activated protein kinase and NF‐kappaB pathways. Cytokine 20032331–40. [DOI] [PubMed] [Google Scholar]
- 18.Daig R, Rogler G, Aschenbrenner E.et al Human intestinal epithelial cells secrete interleukin‐1 receptor antagonist and interleukin‐8 but not interleukin‐1 or interleukin‐6. Gut 200046350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goke M, Kanai M, Podolsky D K. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am J Physiol 1998274(5 Pt 1)G809–G18. [DOI] [PubMed] [Google Scholar]
- 20.Perdue M H, McKay D M. Integrative immunophysiology in the intestinal mucosa. Am J Physiol 1994267(2 Pt 1)G151–G65. [DOI] [PubMed] [Google Scholar]
- 21.Halttunen T, Marttinen A, Rantala I.et al Fibroblasts and transforming growth factor beta induce organization and differentiation of T84 human epithelial cells. Gastroenterology 19961111252–1262. [DOI] [PubMed] [Google Scholar]
- 22.Rogler G, Daig R, Aschenbrenner E.et al Establishment of long‐term primary cultures of human small and large intestinal epithelial cells. Lab Invest 199878889–890. [PubMed] [Google Scholar]
- 23.Vallance B A, Gunawan M I, Hewlett B.et al TGF‐beta1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol 2005289G116–G28. [DOI] [PubMed] [Google Scholar]
- 24.Monteleone G, Mann J, Monteleone I.et al A failure of transforming growth factor‐beta1 negative regulation maintains sustained NF‐kappaB activation in gut inflammation. J Biol Chem 20042793925–3932. [DOI] [PubMed] [Google Scholar]
- 25.Neurath M F, Becker C, Barbulescu K. Role of NF‐kappaB in immune and inflammatory responses in the gut. Gut 199843856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelbmann C M, Leeb S N, Vogl D.et al Inducible CD40 expression mediates NFkappaB activation and cytokine secretion in human colonic fibroblasts. Gut 2003521448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiscott J, Kwon H, Genin P. Hostile takeovers: viral appropriation of the NF‐kappaB pathway. J Clin Invest 2001107143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellits K H, Mullen J, Wand M.et al Activation of the transcription factor NF‐kappaB by Campylobacter jejuni. Microbiology 2002148(Pt 9)2753–2763. [DOI] [PubMed] [Google Scholar]
- 29.Seetharaman J, Kanigsberg A, Slaaby R.et al X‐ray crystal structure of the human galectin‐3 carbohydrate recognition domain at 2.1‐A resolution. J Biol Chem 199827313047–13052. [DOI] [PubMed] [Google Scholar]
- 30.Liu F T, Patterson R J, Wang J L. Intracellular functions of galectins. Biochim Biophys Acta 20021572263–273. [DOI] [PubMed] [Google Scholar]
- 31.Joo H G, Goedegebuure P S, Sadanaga N.et al Expression and function of galectin‐3, a beta‐galactoside‐binding protein in activated T lymphocytes. J Leukoc Biol 200169555–564. [PubMed] [Google Scholar]
- 32.Yang R Y, Hsu D K, Liu F T. Expression of galectin‐3 modulates T‐cell growth and apoptosis. Proc Natl Acad Sci USA 1996936737–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshii T, Fukumori T, Honjo Y.et al Galectin‐3 phosphorylation is required for its anti‐apoptotic function and cell cycle arrest. J Biol Chem 20022776852–6857. [DOI] [PubMed] [Google Scholar]
- 34.Matarrese P, Tinari N, Semeraro M L.et al Galectin‐3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett 2000473311–315. [DOI] [PubMed] [Google Scholar]
- 35.Dagher S F, Wang J L, Patterson R J. Identification of galectin‐3 as a factor in pre‐mRNA splicing. Proc Natl Acad Sci USA 1995921213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Z, Said N, Amin S.et al Galectins‐3 and ‐7, but not galectin‐1, play a role in re‐epithelialization of wounds. J Biol Chem 200227742299–42305. [DOI] [PubMed] [Google Scholar]
- 37.Hsu D K, Yang R Y, Pan Z.et al Targeted disruption of the galectin‐3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol 20001561073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumic J, Lauc G, Flogel M. Expression of galectin‐3 in cells exposed to stress‐roles of jun and NF‐kappaB. Cell Physiol Biochem 200010149–158. [DOI] [PubMed] [Google Scholar]
- 39.Sano H, Hsu D K, Yu L.et al Human galectin‐3 is a novel chemoattractant for monocytes and macrophages. J Immunol 20001652156–2164. [DOI] [PubMed] [Google Scholar]
- 40.Cowles E A, Agrwal N, Anderson R L.et al Carbohydrate‐binding protein 35. Isoelectric points of the polypeptide and a phosphorylated derivative. J Biol Chem 199026517706–17712. [PubMed] [Google Scholar]
- 41.Davidson P J, Davis M J, Patterson R J.et al Shuttling of galectin‐3 between the nucleus and cytoplasm. Glycobiology 200212329–337. [DOI] [PubMed] [Google Scholar]
- 42.Kim K, Mayer E P, Nachtigal M. Galectin‐3 expression in macrophages is signaled by Ras/MAP kinase pathway and up‐regulated by modified lipoproteins. Biochim Biophys Acta 2003164113–23. [DOI] [PubMed] [Google Scholar]
- 43.Mehul B, Hughes R C. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci 1997110(Pt 10)1169–1178. [DOI] [PubMed] [Google Scholar]
- 44.Kasper M, Hughes R C. Immunocytochemical evidence for a modulation of galectin 3 (Mac‐2), a carbohydrate binding protein, in pulmonary fibrosis. J Pathol 1996179309–316. [DOI] [PubMed] [Google Scholar]
- 45.Jensen‐Jarolim E, Neumann C, Oberhuber G.et al Anti‐galectin‐3 IgG autoantibodies in patients with Crohn's disease characterized by means of phage display peptide libraries. J Clin Immunol 200121348–356. [DOI] [PubMed] [Google Scholar]
- 46.Jensen‐Jarolim E, Gscheidlinger R, Oberhuber G.et al The constitutive expression of galectin‐3 is downregulated in the intestinal epithelia of Crohn's disease patients, and tumour necrosis factor alpha decreases the level of galectin‐3‐specific mRNA in HCT‐8 cells. Eur J Gastroenterol Hepatol 200214145–152. [DOI] [PubMed] [Google Scholar]