Abstract
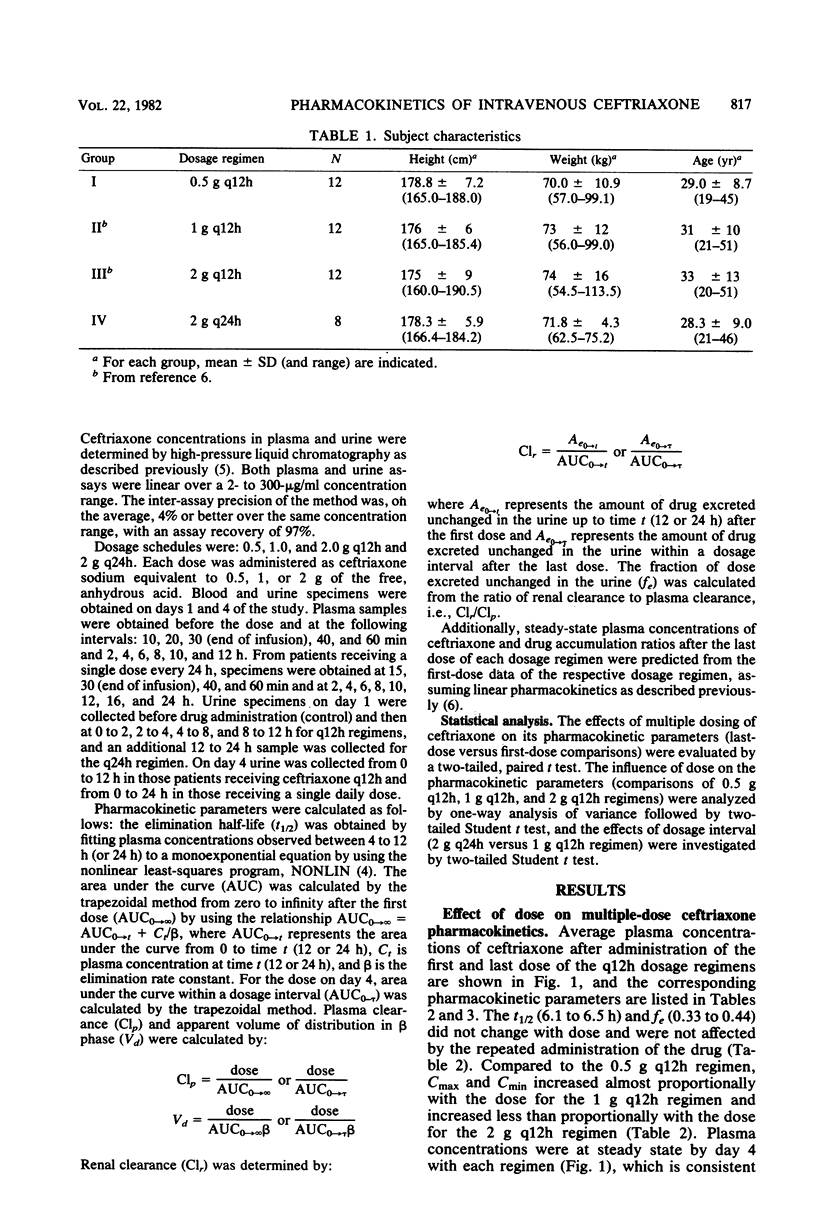
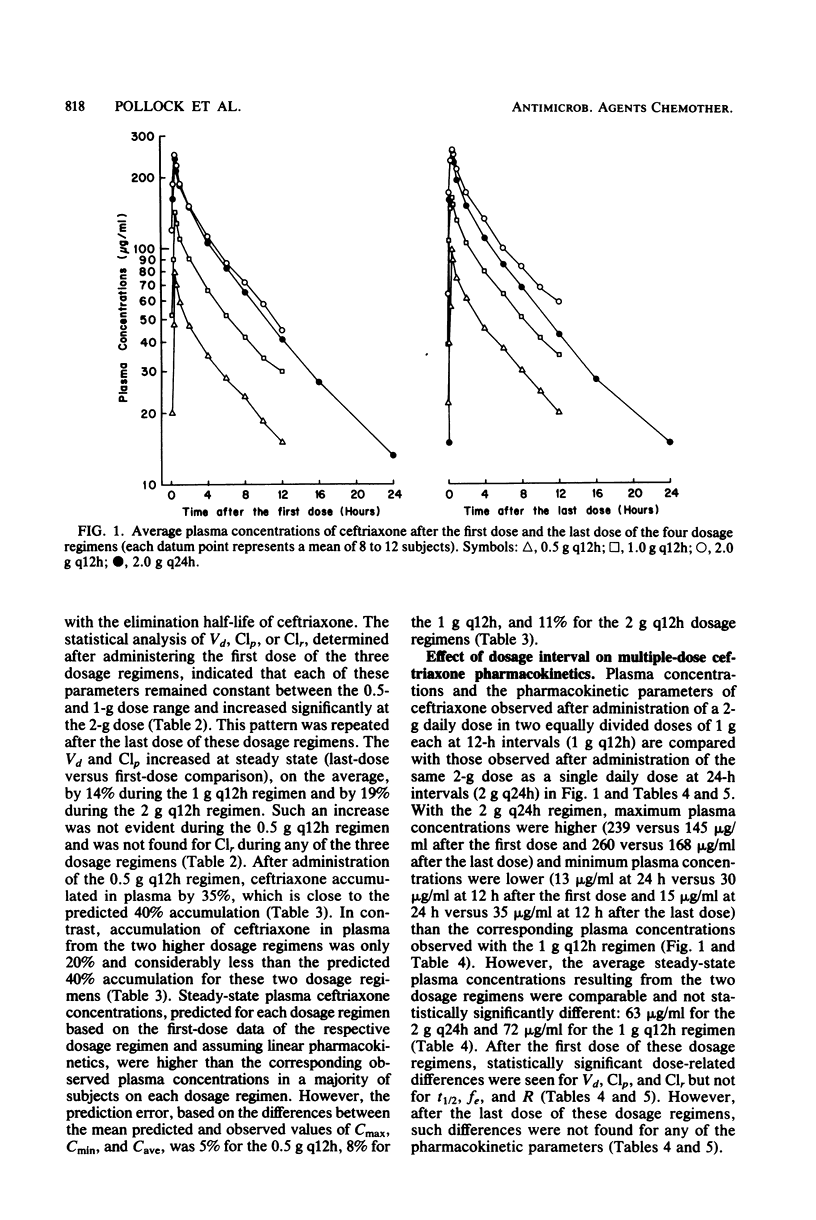
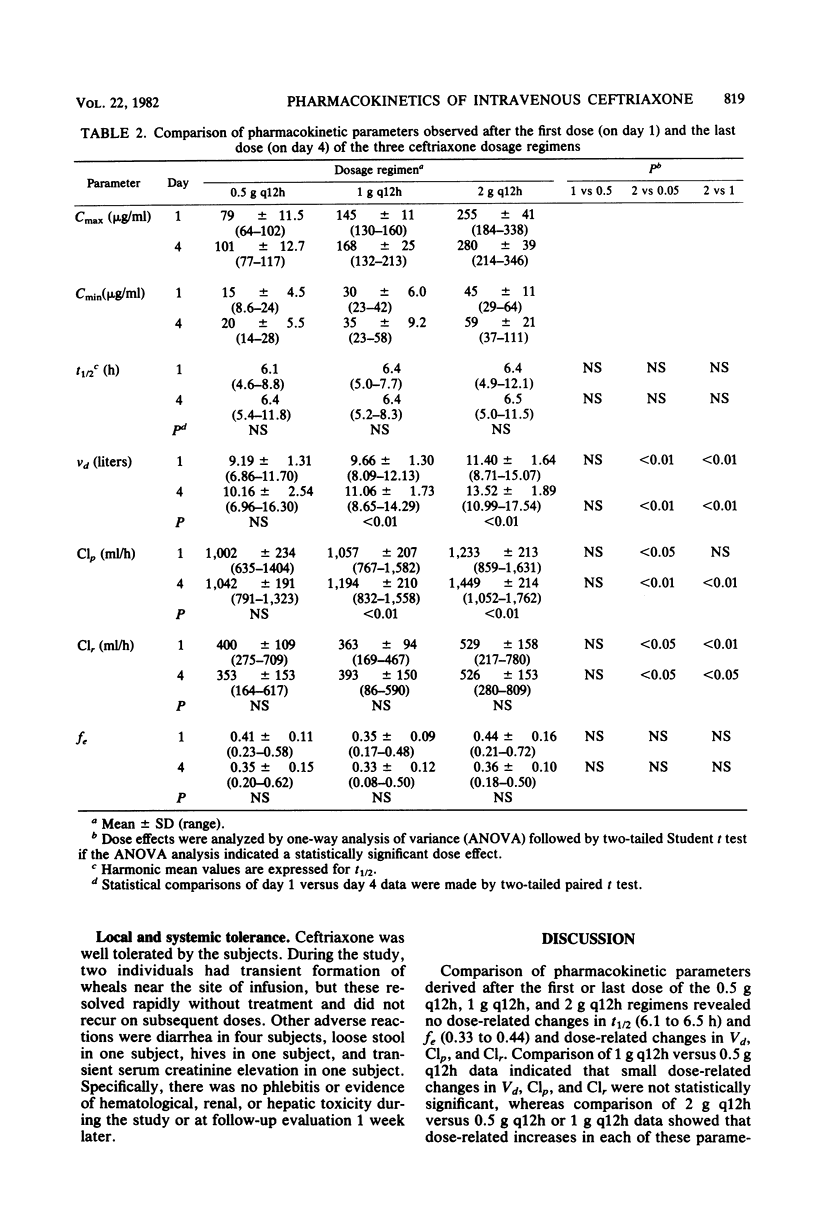
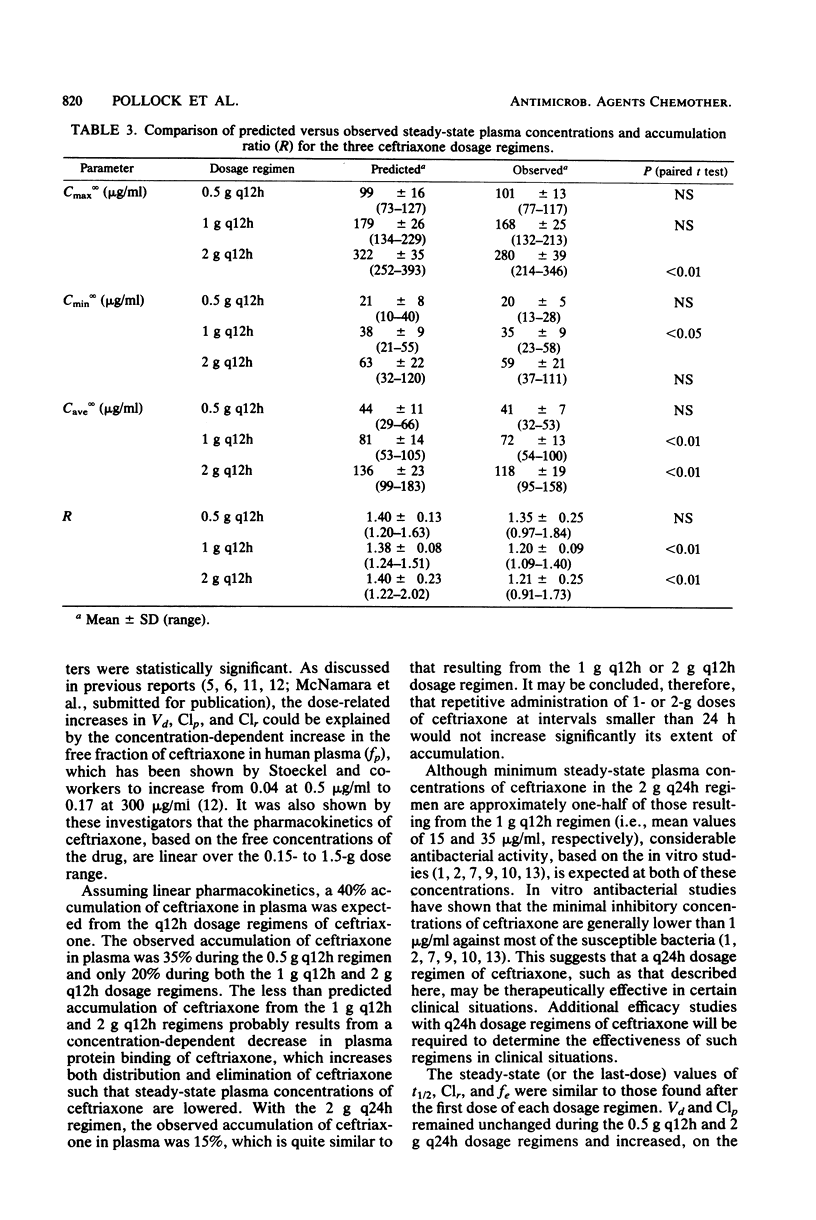
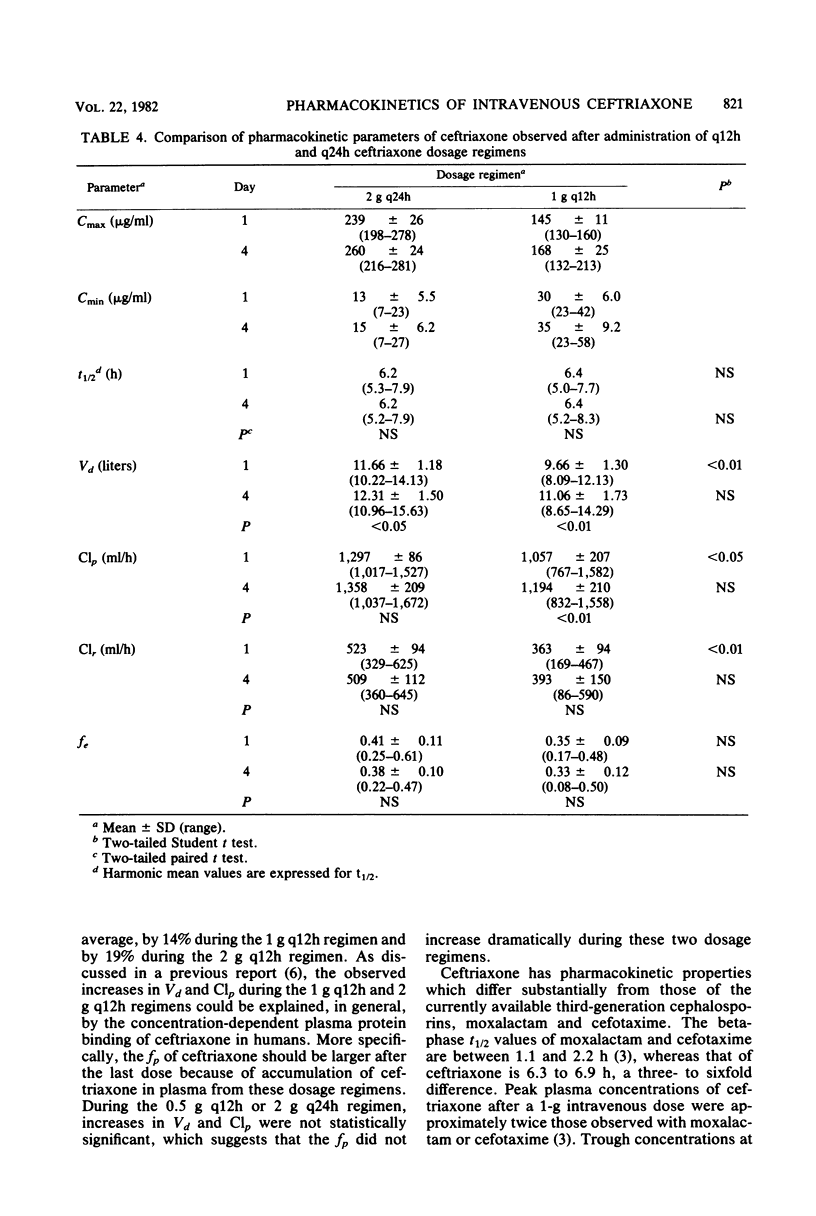
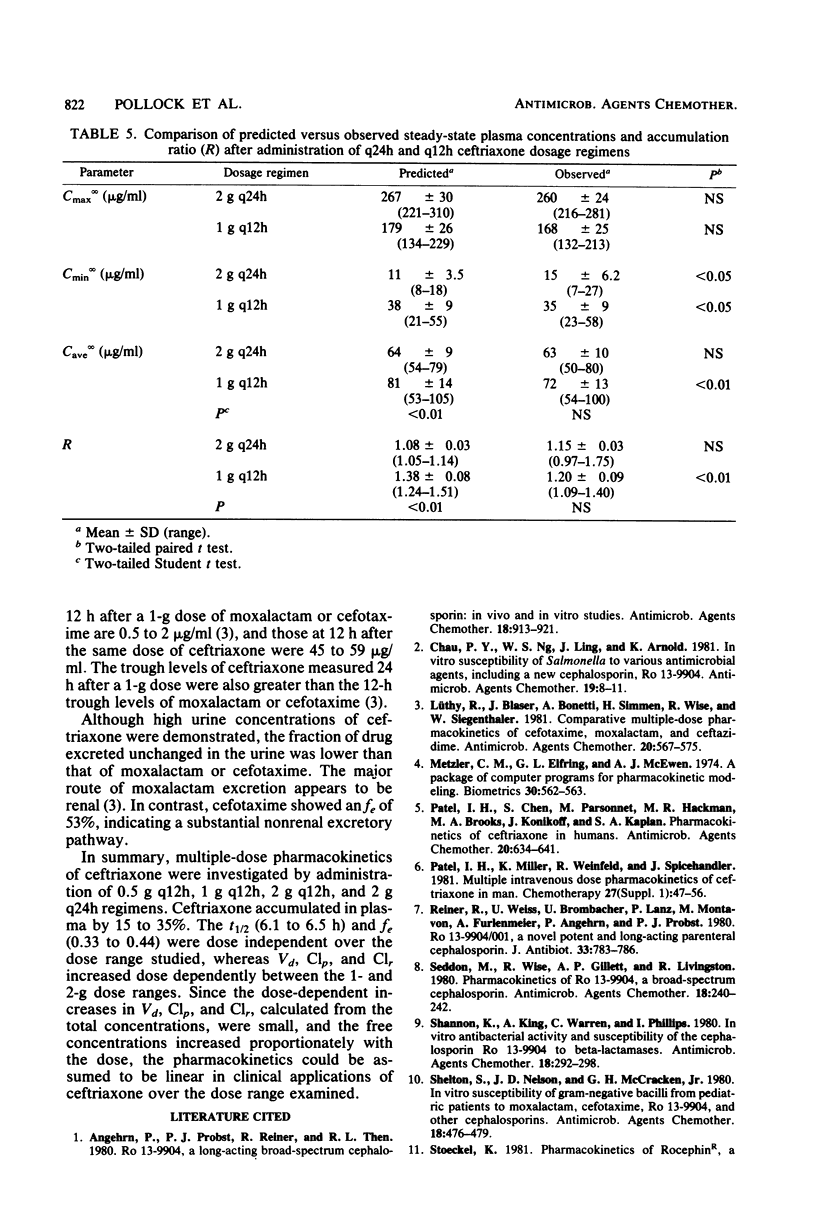
The multiple-dose pharmacokinetics and tolerance of intravenous ceftriaxone were investigated in 44 adults with normal renal function. Doses of 0.5, 1.0, and 2.0 g every 12 h and 2 g every 24 h were administered intravenously at a constant rate over 30 min. Plasma and urine samples were collected after the first (day 1) and last (day 4) dose and assayed for ceftriaxone by high-pressure liquid chromatography. Considering all four doses, mean peak plasma concentrations ranged from 79 to 255 micrograms/ml on day 1 and from 101 to 280 micrograms/ml on day 4. Trough concentrations at 12 h on day 1 were 15 to 45 micrograms/ml and 20 to 59 micrograms/ml on day 4. After a dose regimen of 2 g every 24 h, trough levels were still in the clinically therapeutic range (13 to 15 microgram/ml). The mean beta-phase t1/2 was markedly long (6.3 to 6.9 h) and was independent of dose. The fraction of dose excreted unchanged in the urine (0.33 to 0.44) indicated a substantial nonrenal mechanism of elimination. The plasma clearance ranged between 1,002 and 1,449 ml/h, and renal clearance ranged from 353 to 529 ml/h. The apparent volume of distribution varied from 9.2 to 13.5 liters. The dose-related increases in calculated Vd and Clp could be attributed to concentration-dependent plasma protein binding because of a larger free fraction of drug at higher concentrations. The drug was well tolerated, and no significant clinical or laboratory abnormalities were noted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau P. Y., Ng W. S., Ling J., Arnold K. In vitro susceptibility of Salmonella to various antimicrobial agents, including a new cephalosporin, Ro 13-9904. Antimicrob Agents Chemother. 1981 Jan;19(1):8–11. doi: 10.1128/aac.19.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthy R., Blaser J., Bonetti A., Simmen H., Wise R., Siegenthaler W. Comparative multiple-dose pharmacokinetics of cefotaxime, moxalactam, and ceftazidime. Antimicrob Agents Chemother. 1981 Nov;20(5):567–575. doi: 10.1128/aac.20.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Miller K., Weinfeld R., Spicehandler J. Multiple intravenous dose pharmacokinetics of ceftriaxone in man. Chemotherapy. 1981;27 (Suppl 1):47–56. doi: 10.1159/000238029. [DOI] [PubMed] [Google Scholar]
- Reiner R., Weiss U., Brombacher U., Lanz P., Montavon M., Furlenmeier A., Angehrn P., Probst P. J. Ro 13-9904/001, a novel potent and long-acting parenteral cephalosporin. J Antibiot (Tokyo) 1980 Jul;33(7):783–786. doi: 10.7164/antibiotics.33.783. [DOI] [PubMed] [Google Scholar]
- Seddon M., Wise R., Gillett A. P., Livingston R. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1980 Aug;18(2):240–242. doi: 10.1128/aac.18.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K., King A., Warren C., Phillips I. In vitro antibacterial activity and susceptibility of the cephalosporin Ro 13-9904 to beta-lactamases. Antimicrob Agents Chemother. 1980 Aug;18(2):292–298. doi: 10.1128/aac.18.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton S., Nelson J. D., McCracken G. H., Jr In vitro susceptibility of gram-negative bacilli from pediatric patients to moxalactam, cefotaxime, Ro 13-9904, and other cephalosporins. Antimicrob Agents Chemother. 1980 Sep;18(3):476–479. doi: 10.1128/aac.18.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. In vitro activity of Ro 13-9904, a new beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Feb;19(2):222–225. doi: 10.1128/aac.19.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]



