Short abstract
Surveillance for infection by endoscopy for variant CJD and other human prion diseases
The agents responsible for transmissible spongiform encephalopathies or prion diseases target the central nervous system, but their unique nature and pathophysiology has meant that prion diseases have made an impact in disciplines as diverse as dentistry and transfusion medicine, in large measure because of their resistance to decontamination by conventional means.1
In 1996, a new variant form of Creutzfeldt–Jakob disease (CJD) was described in the UK that preferentially affects young adults, resulting in a disease that often presents with psychiatric symptoms progressing to ataxia, dementia and terminating in akinetic mutism.2 The cause of this disease was proposed to be oral exposure to the bovine encephalopathy (BSE) agent, and subsequent studies have only served to strengthen this link, with no credible alternative explanation having been proposed.3,4,5,6 All human prion diseases are progressive and uniformly fatal neurodegenerative conditions, and, in the case of the acquired forms (iatrogenic CJD and kuru), associated with potentially very long incubation periods.7 Host genetics substantially influence these diseases: familial prion diseases are closely associated with mutations in the prion protein gene (PRNP), and the methionine /valine polymorphism at PRNP codon 129 appears to influence susceptibility, incubation period and in some respects disease phenotype.7
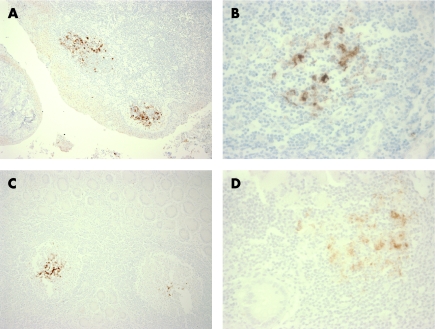
Our understanding of the pathogenesis of prion diseases has been greatly aided by the development of methods to detect and localise abnormal forms of the prion protein (PrPSc), which is the major, if not the sole, component of the infectious agent.7 Variations of standard immunohistochemical methods and Western blotting that use protease digestion and other discriminant steps to distinguish between the ubiquitous normal form of the prion protein (PrPC) and PrPSc have been used. With such tools, the distribution of this protease‐resistant PrP (PrPres) in vCJD has been found to be much broader than in other forms of CJD, involving the lymphoreticular system including the tonsil, appendix, lymph nodes, components of the peripheral nervous system and all the gut‐associated lymphoid tissue (fig 1).8,9 Animal models of prion diseases suggest that lymphoreticular tissues are involved early in the incubation period when the infection is asymptomatic, and examination of archival appendicectomy tissues from patients who went on to develop variant CJD showed that lymphoreticular involvement can predate clinical symptoms by years.10
Figure 1 Widespread distribution of abnormal prion protein in lymphoid follicles throughout the digestive tract (A), tonsil (B), ileum (C), appendix (D) and large intestine. All stained with the anti‐prion protein antibody 12F10 (positive labelling is brown).
Known unknowns
A number of uncertainties rapidly became associated with variant CJD: What proportion of the UK population had been exposed to BSE? Would all exposures result in neurological disease, and if so what would the incubation period be? Were those infected with BSE a risk to others via iatrogenic transmission, and if so by which routes and how might one assess the risk? What measures might reasonably be put in place to counteract these risks?
The numbers of cases of variant CJD have not reached the heights predicted by some, and new cases have been in decline since a peak in 1999 (www.cjd.ed.ac.uk/figures.htm), but there have been unwelcome developments regarding the potential for secondary infection. A retrospective analysis of tonsil and appendix tissue from the general population suggests that a prevalence infection is higher than would be predicted from the disease incidence data alone.11 On the basis of such studies of peripheral involvement, infection is now considered not to be limited to those of the MM PRNP codon 129 genetic subgroup of the population.12,13 Moreover, it now seems likely that blood transfusion from donors in the preclinical phase of variant CJD is infectious.12,14 Taken together, these data suggest a worrying scenario in which a considerable number of people in the normal UK population may be infected with BSE and unknowingly pose a risk to others through blood transfusion, organ donation, surgery or even dentistry. Given the likely incubation periods involved, even significant numbers of such cases might not be seen for many years to come.
One of the difficulties associated with formulating proportionate risk reduction measures involves the translation of experimental data, such as that presented by Wadsworth et al,15(see page 90) to the clinical situation involving the relationship between markers such as PrPres and the actual parameter of interest, in this case human infectivity. These can become compounded by other differences between the situation facing patients and clinicians and those from which the data have been derived. Wadsworth et al should be congratulated for tackling this problem head on, in the context of possible infectivity in the rectum of patients with variant CJD.15 The authors have taken autopsy specimens of brain and rectum from a patient with variant CJD, measured the PrPres load by Western blotting and attempted transmission via the intracerebral route to two lines of transgenic mice carrying the human prion protein gene. Although these mice lack a species barrier with respect to the prion protein sequence and overexpress the gene product, when challenged with variant CJD brain they do not develop a clinical spongiform encephalopathy. Instead, they seem to propagate detectable levels of PrPres in the absence of frank disease (a so‐called subclinical infection). Owing to this, an end‐point titration of infectivity is not possible. What the data do however show is that PrPres is detectable in the brains of recipient animals that were challenged with a variant CJD rectal inoculum that contains 10−4.7 of the amount of PrPres found in the corresponding brain. Admittedly, to detect this low level, a PrPres concentration step was required. The article shows that this specimen of variant CJD rectum has sufficient infectivity to precipitate molecular pathological changes in over half of the recipient animals, assuming that the authors have not simply recovered their initial inoculum. In this respect, the specimens, both brain and rectum, have been shown to be infectious, but to show a quantitative linear relationship between PrPres and infectivity more than two data points will be required and a model susceptible to disease would be desirable. It will be interesting to see whether tissue specimens with PrPres loads intermediate between brain and rectum, such as tonsil, spleen and lymph node, do indeed indicate a quantitative relationship between PrPres load and infectivity, but substantial additional work will be required to justify this claim.
How concerned should we be by these findings? The link between PrPres accumulation and secondary lymphoid follicles has been known for some time, including those in gut‐associated lymphoid tissues.8,9,10,16,17,18 The finding of very low levels of PrPres in rectal specimens, where lymphoid tissue is relatively sparse compared with the distal ileum, might therefore be predicted.8,18 That PrPres found in variant CJD lymphoid tissues (including tonsil and spleen) can transmit disease to wild‐type mice is also well recognised.19 What the article by Wadsworth et al tells us is that the risk of iatrogenic transmission from rectal tissue is more than theoretical.
However, the conditions under which the experiments were performed and those which pertain in clinical practice differ. The experimental challenge was intracerebral, which is impossible to conceive of in terms of exposure resulting from reuse of a contaminated endoscope in humans. The experimental conditions used (including overexpression of the PRNP) favoured successful transmission. Although there is a body of work on oral exposure to the scrapie and BSE agents, the point of entry through the gut wall is as yet ill defined, although it is a topic of considerable recent investigation.20,21
Unknown unknowns?
The foregoing investigation notwithstanding, it is worth pointing out that the study of prion diseases has confounded expectation on previous occasions. For some, BSE posed little or no threat to the human population. Similarly, thorough and repeated evaluations of the risk posed by blood transfusion concluded that the risk of transmission by this route was negligible.22 This last example is perhaps instructive and involved a threefold response to what was considered at the time a hypothetical risk: firstly, the introduction of precautionary measures to reduce the risk23,24; Secondly, the funding of extensive and long‐term animal studies to assess the likelihood of transmission by this route25,26,27; and lastly, an active national surveillance system working in concert with the blood transfusion service to trace and link any potentially affected blood donors and recipients (www.cjd.ed.ac.uk/TMER).
The present
How should these findings influence current measures to reduce the risk of variant CJD transmission by gastrointestinal endoscopy in UK, both from symptomatic patients and from those identified as “at increased risk” of variant CJD? The numbers in the latter category vastly outnumber the former, and include several thousands of people who may have been exposed to variant CJD infectivity by contaminated instruments, blood components and plasma products. The risk of transmission is not confined to the use of the endoscope itself, as accessories such as biopsy forceps may come into close contact with lymphoid follicles in the gut submucosa that may harbour prion infectivity. Guidance on the measures to reduce the risks of prion transmission via endoscopes was revised in 2005 and is available on the Department of Health website at: www.advisorybodies.doh.gov.uk/acdp/tseguidance/Index.htm (Annex F). Since this guidance assumed that gastrointestinal lymphoid tissues were of “medium risk” in terms of prion infectivity (the brain is “high risk”), the results from the article by Wadsworth et al do not justify any additional precautions.15
As endoscopes cannot be autoclaved for reuse, procedures that are deemed likely to be invasive and potentially contaminate endoscopes with prion infectivity (thus requiring quarantine of the endoscope) include endoscopy and biopsy, or endoscopy with any use of diathermy or argon plasma coagulation. Procedures deemed non‐invasive (thus not requiring quarantine of the endoscope) include endoscopy without biopsy, or with brush cytology, or endoscopy with injection of varices (without diathermy), or with mucosal clipping or banding of varices. In general, these steps should not compromise patient care; dedicated endoscopes are available from the National CJD Surveillance Unit for use on patients with vCJD who require insertion of a polyethylene glycol feeding tube.
The future
Although prion agents are notoriously difficult to inactivate by conventional methods that are effective for bacteria and viruses, recent research has identified several novel approaches to removing proteins and prions from the surfaces of metal instruments that might be applicable to endoscopes. These include innovative processes such as the use of gas plasmas and various combinations of detergents and enzymes,28,29,30 These seem particularly interesting, as reductions in prion infectivity on metal surfaces have been measured after their use. However, it remains to be shown whether any of these techniques are readily applicable to endoscopes and whether the promising results in experimental laboratories can be maintained on use in a clinical setting. Further studies on the safety, toxicity and long‐term effects of these methods will also be required before any of them can reach general clinical use. However, the prospects of developing an “anti‐prion” process applicable to endoscopes seem much more promising now than even a few years ago.
Although no evidence of transmission of variant CJD infectivity by endoscopy (or any other medical or surgical device) has yet been identified, continuing surveillance is required for variant CJD and other human prion diseases in the UK, particularly in view of the lengthy incubation periods that can be associated with transmission of these diseases.7
Footnotes
Funding: The National CJD Surveillance Unit is funded by the UK Department of Health and the Scottish Executive.
Competing interests: None.
References
- 1.Taylor D M. Inactivation of the unconventional agents of scrapie, bovine spongiform encephalopathy and Creutzfeldt‐Jakob disease. J Hosp Infect 199118141–146. [DOI] [PubMed] [Google Scholar]
- 2.Will R G, Ironside J W, Zeidler M.et al A new variant of Creutzfeldt‐Jakob disease in the UK. Lancet 1996347921–925. [DOI] [PubMed] [Google Scholar]
- 3.Bruce M E, McConnell I, Will R G.et al Detection of variant Creutzfeldt‐Jakob disease infectivity in extraneural tissues. Lancet 2001358208–209. [DOI] [PubMed] [Google Scholar]
- 4.Collinge J, Sidle K C, Meads J.et al Molecular analysis of prion strains variation and the aetiology of ‘new variant' CJD. Nature 1996383685–690. [DOI] [PubMed] [Google Scholar]
- 5.Scott M R, Will R, Ironside J.et al Compelling transgenic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci USA 19999615137–15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward H J T, Everington D, Couson S N.et al Risk factors for variant Creutzfeldt‐Jakob disease: a case control study. Ann Neurol 200659111–120. [DOI] [PubMed] [Google Scholar]
- 7.Prusiner S B. Prions. Proc Natl Acad Sci USA 19989513363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadsworth J D F, Joiner S, Hill A F.et al Tissue distribution of protease‐resistant prion protein in variant Creutzfeldt‐Jakob disease using a highly sensitive immunoblotting assay. Lancet 2001358171–180. [DOI] [PubMed] [Google Scholar]
- 9.Head M W, Ritchie D, Smith N.et al Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt‐Jakob disease: an immunohistochemical, qautitative and biochemical study. Am J Pathol 2004164143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton D A, Ghani A C, Conyers L.et al Accumulation of prion protein in tonsil and appendix: review of tissue samples. BMJ 2002325633–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilton D A, Ghani A C, Conyers L.et al Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol 2004203733–739. [DOI] [PubMed] [Google Scholar]
- 12.Peden A H, Head M W, Ritchie D R.et al Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 2004364527–529. [DOI] [PubMed] [Google Scholar]
- 13.Ironside J W, Bishop M T, Connolly K.et al Variant Creutzfeldt‐Jakob disease: prion protein genotype analysis of positive appendix samples from a retrospective prevalence study. BMJ 20063321186–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llewelyn C A, Hewitt P E, Knight R S G.et al Possible transmission of variant Creutzfeldt‐Jakob disease by blood transmission. Lancet 2004363417–421. [DOI] [PubMed] [Google Scholar]
- 15.Wadsworth J D F, Joiner S, Fox K.et al Prion infectivity in vCJD rectum. Gut 20075690–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill A F, Zeidler M, Ironside J W.et al Diagnosis of new variant Creutzfeldt‐Jakob disease by tonsil biopsy. Lancet 199734999–100. [DOI] [PubMed] [Google Scholar]
- 17.Hill A F, Butterworth R J, Joiner S.et al Investigating variant Creutzfeldt‐Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 1999353183–189. [DOI] [PubMed] [Google Scholar]
- 18.Joiner S, Lineham J M, Brandner S.et al High levels of disease related prion protein in the ileum in variant Creutzfeldt‐Jakob disease. Gut 2005541506–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce M E, Will R G, Ironside J W.et al Transmissions to mice indicate that ‘new variant' CJD is caused by the BSE agent. Nature 1997389498–501. [DOI] [PubMed] [Google Scholar]
- 20.Jeffrey M, Gonzalez L, Espenes A.et al Transportation of prion protein across the intestinal mucosa of scrapie‐susceptible and scrapie‐resistant sheep. J Pathol 20062094–14. [DOI] [PubMed] [Google Scholar]
- 21.St Rose SG, Hunter N, Matthews L, et al. Comparative evidence for a link between Peyer's patch development and susceptibility to transmissible spongiform encephalopathies. BMC Infect Dis 200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown P, Cervenakova L, McShane P.et al Further studies of blood infectivity in an experimental model of transmissible spongiform encephalopathy, with an explanation of why blood components do not transmit Creutzfeldt‐Jakob disease. Transfusion 1999391169–1178. [DOI] [PubMed] [Google Scholar]
- 23.Eglin R P, Murphy W G. Beyond leukodepletion: removing infectious prions by filtration. Transfusion 2005451836–1838. [DOI] [PubMed] [Google Scholar]
- 24.Ludlam C A, Turner M L. Managing the risk of transmission of variant Creutzfeldt‐Jakob disease by blood products. Br J Haematol 200513213–24. [DOI] [PubMed] [Google Scholar]
- 25.Houston F, Foster J D, Chong A.et al Transmission of BSE by blood transfusion. Lancet 2000356999–1000. [DOI] [PubMed] [Google Scholar]
- 26.Hunter N, Foster J, Chong A.et al Transmission of prion diseases by blood transfusion. J Gen Virol 2002832897–2905. [DOI] [PubMed] [Google Scholar]
- 27.Siso S, Gonzalez L, Houston F.et al The neuropathological phenotype of experimental ovine BSE is maintained after blood transfusion. Blood . 2006;108745–748. [DOI] [PubMed]
- 28.Baxter H C, Campbell G A, Whittaker A G.et al Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio‐frequency gas‐plasma treatment. J Gen Virol 2005862393–2399. [DOI] [PubMed] [Google Scholar]
- 29.Fichet G, Comoy E, Duval C.et al Novel methods for disinfection of prion‐contaminated medical devices. Lancet 2004364521–526. [DOI] [PubMed] [Google Scholar]
- 30.Pertez D, Supattapone S, Giles K.et al Inactivation of prions by sodium dodecyl sulfate. J Virol 200680322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]