Identification of mucosal abnormalities is aided by the use of dyes during colonoscopy (chromoendoscopy).1 Two dyes that have found particular favour are methylene blue and indigo carmine.2,3
Methylene blue, which, unlike indigo carmine, is taken up by cells, induces cellular DNA damage in vitro via the generation of singlet oxygen when photoexcited by white light.4 In contrast, indigo carmine appears to be photostable and to possess little potential to damage genetic material in vitro.5,6 A recent clinical study has shown that the extent of DNA damage (particularly oxidative DNA damage) in human oesophageal cells is increased after methylene blue chromoendoscopy.7 Additional iatrogenic oxidative DNA damage to epithelial cells is of particular concern in such precancerous tissue because of the association between oxidative DNA damage, mutagenesis and the development of malignancy.8 We hypothesised that indigo carmine would induce less DNA damage than methylene blue both in vitro in cultured colon cells during simulated chromoendoscopy conditions and in vivo in colonic biopsy samples collected at chromoendoscopy.
We used the alkaline comet assay to determine DNA damage in the cells treated with methylene blue/indigo carmine and white light. This is a sensitive technique for analysing and measuring such damage in mammalian cells, with the percentage of DNA in the comet tail being linearly related to DNA damage.9 The inclusion of the DNA repair enzyme, Fapy‐DNA‐glycosylase (FPG), in the comet assay results in the excision of oxidised guanines to yield additional DNA strand breaks that are detectable in the comet assay.10 This allows an estimation of specific oxidative DNA damage to cells. In all the experiments described below, the cell viability exceeded 70%.
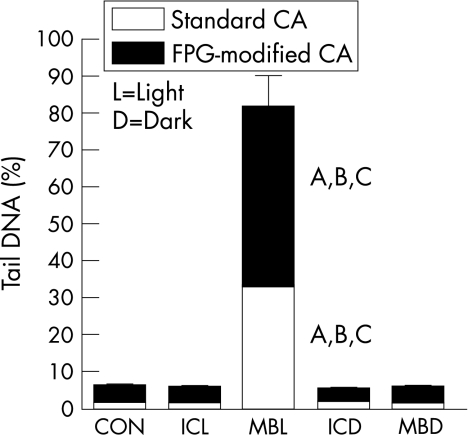
To simulate chromoendoscopy in vitro, 50 µl of either 0.1% methylene blue or 0.1% indigo carmine dye was added to a monolayer of cultured CaCo2 adenocarcinoma cells for 2 min in the presence and absence of cold white light. Only low levels of DNA damage are found in cells in both the alkaline and the FPG‐modified comet assay when the exposure is to white light alone (fig 1). Treatment with indigo carmine either in the light or in the dark, or with methylene blue in the dark did not result in any major change in the extent of DNA damage compared with controls. In contrast, cells treated with methylene blue in the light showed a salient increase in DNA damage compared with controls in both the alkaline and the FPG‐modified comet assay (p<0.001).
Figure 1 CaCo2 cells exposed to 0.1% methylene blue (MB) or 0.1% indigo carmine (IC) either in white light or in the dark for 2 min. Control cells (CON) were treated with white light only (no dye). Data are presented as mean (SE) tail DNA (%) for three experiments. Both the DNA damage from the alkaline comet assay (CA; no fill) and the additional damage with Fapy‐DNA‐glycosylase (FPG; fill) are shown. (A) Significance at p<0.001 relative to control cells in the alkaline or FPG‐modified comet assay. (B) Significance at p<0.001 in methylene blue versus indigo carmine cells under the same experimental conditions (either in the light or in the dark) in the alkaline or the FPG‐modified comet assay. (C) Significance at p<0.001 in methylene blue‐treated cells in the light versus in the dark in the alkaline or FPG‐modified comet assay.
For in vivo experiments, ethical approval and patient consent were obtained to take biopsy samples from colonic mucosa during routine endoscopic examination. Mucosal biopsy samples were taken from the same area of the colon before and after the application of 2 ml of 0.1% methylene blue or indigo carmine dye onto the colonic mucosa.
Patients in the methylene blue chromoendoscopy group, but not those in the indigo carmine group, had significantly greater DNA damage in biopsy samples after dye spraying than before the application of dye in both the alkaline (p = 0.014) and the FPG‐modified comet assay (p = 0.002; table 1).
Table 1 Median DNA damage measured by the standard comet assay and the Fapy‐DNA‐glycosylase‐ modified comet assay before and after methylene blue and indigo carmine dye spraying in the two groups of patients.
| Patient group | n | Median DNA damage (IQR) before spraying (%) | Median DNA damage (IQR) after spraying (%) | p Value (Wilcoxon's test) |
|---|---|---|---|---|
| IC spraying (standard comet assay) | 10 | 7.2 (5.1–15.8) | 5.3 (4.1–11.7) | 0.084 |
| IC spraying (FPG‐modified comet assay) | 10.5 (6.0–32.1) | 12.3 (5.9–17.3) | 0.492 | |
| MB spraying (standard comet assay) | 10 | 5.9 (4.9–8.1) | 12.0 (5.2–19.8) | 0.014 |
| MB spraying (FPG‐modified comet assay) | 9.8 (7.5–14.0) | 34.3 (23.9–58.8) | 0.002 |
FPG, Fapy‐DNA‐glycosylase; IC, indigo carmine; IQR, interquartile range; MB, methylene blue.
Biopsy samples were digested with 100 µl of 0.5% pronase and 100 µl of 0.3% collagenase in 800 µl DMEM at 37°C for 1 h, to create a single‐cell suspension for comet assay analysis.
Of the 10 patients receiving methylene blue chromoendoscopy, eight had higher levels of DNA damage, post‐endoscopy, measured by alkaline comet assay, and all had higher levels of DNA damage, measured by the FPG‐modified comet assay.
Our study highlights the potential for the induction of DNA damage by methylene blue when used as a dye during colonoscopy. It is reasonable to suggest that any iatrogenic DNA damage induced in colonocytes by dye spraying should be avoided where possible, particularly in high‐risk groups such as patients with ulcerative colitis. The efficacy of methylene blue versus indigo carmine during chromoendoscopy has not been formally compared, but if assumed to be equal, indigo carmine rather than methylene blue should be considered for use.
Acknowledgements
We thank Andrew Collins, University of Oslo, for the kind gift of the FPG enzyme, and the endoscopy staff at Leeds General Infirmary for their support.
Footnotes
Funding: This research was supported by a local research grant from the Leeds Teaching Hospitals NHS Trust.
Competing interests: None.
Ethical approval was given by the Harrogate Local Research Ethics Committee to collect biopsy samples from 20 patients undergoing elective sigmoidoscopic and colonoscopic examinations for a variety of clinical indications at the Endoscopy Department at The General Infirmary at Leeds (Ethics reference number 04/Q1107/16).
References
- 1.Bruno M J. Magnification endoscopy, high resolution endoscopy, and chromoscopy; towards a better optical diagnosis. Gut 200352iv7–NaN11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiesslich R, Fritsch J, Holtmann M.et al Methylene blue aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003124880–888. [DOI] [PubMed] [Google Scholar]
- 3.Rutter M D, Saunders B P, Schofield G.et al Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut 200453256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boiteux S, Gajewski E, Laval J.et al Substrate specificity of the Escherichia coli FPG protein (formamidopyrimidine‐DNA glycosylase): excision of purine lesions in DNA produced by ionising radiation or photosensitisation. Biochemistry 199231106–110. [DOI] [PubMed] [Google Scholar]
- 5.Abbruzzetti S, Viappiani C, Murgida D H.et al Non‐toxic water‐soluble photocalorimetric reference compounds for UV and visible excitation. Chem Phys Lett 1999304167–172. [Google Scholar]
- 6.Rhee Y, Termini J, Valentine M. Oxidative base damage in DNA detected by reverse transciptase. Nucl Acids Res 1995233275–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olliver J R, Wild C P, Sahay P.et al Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus. Lancet 2003362373–374. [DOI] [PubMed] [Google Scholar]
- 8.Volkovova K, Dunsinska M, Collins A R. From oxidative DNA damage to molecular epidemiology. J Appl Biomed 200531–5. [Google Scholar]
- 9.Wong V W C, Szeto Y T, Collins A R.et al The comet assay: a biomonitoring tool for nutraceutical research. Curr Top Nutraceutical Res 200531–14. [Google Scholar]
- 10.Collins A R. Measurement of oxidative DNA damage using the Comet Assay. In: Lunce J, Griffiths HR, eds. Measuring in vivo oxidative damage. Chichester: Wiley, 200081–94.