Abstract
Background
Previously, proteomic methods were applied to characterise differentially expressed proteins in microdissected pancreatic ductal adenocarcinoma cells.
Aims
To report that CapG and a related protein, gelsolin, which have established roles in cell motility, are overexpressed in metastatic pancreatic cancer; and to describe their pattern of expression in pancreatic cancer tissue and their effect on cell motility in pancreatic cancer cell lines.
Methods
CapG was identified by mass spectrometry and immunoblotting. CapG and gelsolin expression was assessed by immunohistochemical analysis on a pancreatic cancer tissue microarray and correlated with clinical and pathological parameters. CapG and gelsolin levels were reduced using RNA interface in Suit‐2, Panc‐1 and MiaPaCa‐2 cells. Cell motility was assessed using modified Boyden chamber or wound‐healing assays.
Results
Multiple isoforms of CapG were detected in pancreatic cancer tissue and cell lines. Immunohistochemical analysis of benign (n = 44 patients) and malignant (n = 69) pancreatic ductal cells showed significantly higher CapG staining intensity in nuclear (p<0.001) and cytoplasmic (p<0.001) compartments of malignant cells. Similarly, gelsolin immunostaining of benign (n = 24 patients) and malignant (n = 68 patients) pancreatic ductal cells showed higher expression in both compartments (both p<0.001). High nuclear CapG was associated with increased tumour size (p = 0.001). High nuclear gelsolin was associated with reduced survival (p = 0.01). Reduction of CapG or gelsolin expression in cell lines by RNAi was accompanied by significantly impaired motility.
Conclusions
Up regulation of these actin‐capping proteins in pancreatic cancer and their ability to modulate cell motility in vitro suggest their potentially important role in pancreatic cancer cell motility and consequently dissemination.
Pancreatic ductal adenocarcinoma is a leading cause of cancer‐related deaths. It was responsible for an estimated 213 000 deaths worldwide in 2000.1 The disease is characterised by rapid tumour spread, and the overall median survival is <6 months.2 Local invasion and metastasis are partly responsible for the dismal prognosis.
Cell migration is a prerequisite for tumour cell invasion and metastasis. It is believed that cancer cells migrate using mechanisms similar to those used by normal cells for processes that involve regulated and often extensive movement, such as wound healing and immune‐cell trafficking.3 The processes underlying pancreatic cancer cell invasion and metastasis are poorly understood.4 We and several other groups have used genomic and proteomic methods to study gene and protein expression in invasive pancreatic cancer. Our recent work combined microdissection and two‐dimensional gel electrophoresis to identify proteins that are differentially expressed in malignant compared with benign, pancreatic ductal cells.5 One of the proteins that we found to be overexpressed in malignant cells is the actin‐binding protein, CapG.
CapG is a member of the gelsolin superfamily of proteins, which regulates actin filament length by capping or severing filaments.6 Gelsolin, the first identified member of the family,7 has a six‐domain structure. In the presence of calcium, it binds to and severs actin filaments, after which it remains as a cap on the fast‐growing, barbed end of the cut filament. Release and uncapping occurs when gelsolin binds phosphatidylinositol lipids.6 The basic gelsolin domain structure and mechanism are maintained in other members of the family, except that some, such as CapG, have three instead of six repeating domains. CapG caps actin filaments in the presence of Ca2+, but does not sever filaments.8 The actin‐binding activity of CapG is controlled by micromolar calcium (Ca2+) and is reversible by reducing the Ca2+ concentration.
Several studies have shown a role for CapG and gelsolin in regulating cell motility.9,10,11,12,13,14,15 Moderate overexpression of CapG in fibroblasts resulted in increased motility in wound‐healing assays and in translocation through microporous membranes.9 Similarly, overexpression of CapG in endothelial cells led to increased motility.10 Neither CapG nor gelsolin are essential for normal development, at least in the mouse, for which CapG‐null and CapG/gelsolin double‐null strains have been produced. Both developed normally and had no gross abnormalities.11 However, all of these mice exhibited changes in cellular processes, involving cell motility. The absence of CapG led to impaired macrophage motile function. Both spontaneous and induced macrophage membrane ruffling was diminished in CapG‐null cells compared with wild‐type macrophages.11 Moreover, bone marrow‐derived dendritic cells and neutrophils from CapG‐null mice were shown to have impaired ruffling responses and decreased migration speeds, respectively.12 Furthermore, CapG‐null mice were dramatically more susceptible to the intracellular bacterium Listeria monocytogenes compared with wild‐type animals; and this may be attributed to defects in specific motility mechanisms, the exact nature of which remains to be identified. Gelsolin overexpression in cultured fibroblasts also resulted in increased motility,14 whereas in gelsolin−/− mice the motility of osteoclasts was decreased.13 Studies of neuronal growth cones in these mice also showed delayed retraction of lamellipodia and filopodia.15 Thus, although CapG‐null and gelsolin‐null phenotypes are not identical, both of them have cell motility defects.
Gelsolin expression has been studied in a variety of other cancers.16,17,18,19,20 This is not the case for CapG, for which relatively little is known about its association with cancer. To our knowledge, nothing has been reported about gelsolin or CapG protein levels in pancreatic adenocarcinoma.
We report here the detection of CapG overexpression in a proteomic study of microdissected pancreatic cancer specimens and the validation of its overexpression in malignant pancreatic cancer cells by immunohistochemical analysis. In addition, we show the overexpression of gelsolin in pancreatic cancer cells and demonstrate that reducing the levels of either of these proteins decreases the motility of pancreatic cancer cell lines. The marked up regulation of motility‐modulating actin‐capping proteins in pancreatic cancer cells may have important consequences for the motility and consequently the dissemination of these cells.
Materials and methods
Mass spectrometry
We described previously5 the detection of differentially expressed proteins from microdissected pancreatic specimens by two‐dimensional gel electrophoresis. Protein spots, excised from Coomassie Blue‐stained gels, were trypsin digested, as described previously.5 Mass spectra were obtained on a matrix‐assisted laser desorption/ionisation‐time‐of‐flight (MALDI‐TOF) mass spectrometer (Micromass, Manchester, UK) and searched against human sequences in the NCBI non‐redundant database, using Mascot software (Matrix Science, Boston, USA, http://www.matrixscience.com).
Two‐dimensional electrophoresis and immunoblotting
Proteins from 30, 7‐μm‐thick sections of a frozen pancreatic tumour sample were solubilised in lysis buffer (7 M urea, 2 M thiourea, 4% 3‐[(3‐cholamidopropyl)dimethylammonio]‐1‐propane sulphonate, 40 mM Tris base and 1% dithiothreitol). The viscosity of the protein lysate was reduced by syringing through a 21‐G needle and insoluble material removed by centrifugation at 14 000 g for 30 min at room temperature. Proteins were focused on pH 3–10 non‐linear strips, 13 cm in length, as described,5 and then separated on 12% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis gels. Gels were stained with colloidal Coomassie Blue, or electroblotted on nitrocellulose membrane and immunostained for CapG, using a rabbit anti‐CapG antibody. Visualisation was performed using an anti‐rabbit horseradish peroxidase‐conjugated secondary antibody and electrochemiluminescence, using Western Lightning Chemiluminescence Reagent Plus (Perkin‐Elmer Life Sciences, Beaconsfield, Bucks, UK).
Generation and purification of recombinant CapG
A recombinant thioredoxin–CapG fusion protein was produced using the pBAD/TOPO thiofusion kit (Invitrogen, Paisley, UK), following the manufacturer's instructions. The full coding sequence of CapG was amplified using the primer pair 5′‐atgtacacagccattccccagagtggc‐3′ and 5′‐tcttttccagtccttgaaaaattgcttgaag‐3′—the second of these replaces the stop codon with an arginine‐encoding triplet. This was cloned into the pBAD/TOPO thiofusion vector and transformed into Escherichia coli. Optimal expression of the fusion protein was achieved after incubation of cultures with 0.002% (w/v) L(+)‐arabinose (Sigma, Poole, UK) for 20 h. The fusion protein, which is (His)6‐tagged, was purified using nickel‐loaded HiTrap chelating HP columns (Amersham Biosciences, Buckinghamshire, UK), and eluted in a buffer of 0.02 M sodium phosphate, 0.5 M NaCl, 0.5 M imidazole and 8 M urea (pH 7.4). Purity of recombinant protein was estimated at >90% on a Coomassie Blue‐stained sodium dodecyl sulphate‐polyacrylamide gel electrophoresis gel (data not shown).
Immunohistochemistry
Immunohistochemical detection of CapG and gelsolin was carried out on a pancreatic cancer tissue microarray, containing matched duplicate non‐malignant (normal ducts) and malignant (tumour) cores from 69 patients treated at the Royal Liverpool University Hospital, Liverpool, UK, between 1994 and 2003. Non‐malignant cores from 24 and 44 of the 69 patients contained sufficient benign ductal cells for evaluation of gelsolin and CapG, respectively. Immunohistochemical analysis was carried out as described previously.5 Briefly, 5‐μm‐thick microarray sections were deparaffinised in xylene and then rehydrated through alcohol to distilled water. Antigen retrieval was carried out by pressure cooking the slides in 10 mM EDTA (pH 7.0) for 3 min. Immunohistochemical staining was carried out using an automatic staining system (Autostainer; DakoCytomation, Ely, Cambridgeshire, UK). Slides were incubated for 40 min with rabbit anti‐CapG antibody (using an optimal dilution of 1:700) or a monoclonal gelsolin antibody GS‐2C4 (Sigma) titrated at the optimal dilution of 1:40 000, then rinsed in Tris‐buffered saline‐Tween 20 (pH 7.6) and the antibody localisation visualised by incubating sections with a horseradish peroxidase‐conjugated labelled polymer for 30 min, followed by treatment with diaminobenzidine for 20 min (ChemMate EnVision, DakoCytomation Cambridgeshire, UK). Slides were counterstained with haematoxylin, dehydrated with 100% ethanol, cleared in xylene and mounted with DPX mountant (VWR International, Poole, UK). As a control for antibody specificity, rabbit anti‐CapG polyclonal antibody was mixed with an excess of the purified recombinant thioredoxin–CapG fusion protein and incubated overnight at 4°C before application to tissue sections as described above. Buffer constituents in both control and blocked antibody preparations were identical.
Scoring and statistical analysis of immunohistochemically stained tissue arrays
Scoring of the pancreatic cancer tissue microarray cores was performed by a specialist histopathologist. The information recorded included the subcellular location of CapG or gelsolin staining (nuclear or cytoplasmic), the intensity of staining (graded 0, negative; 1, weak; 2, moderate; and 3, strong) and the percentage of cells showing positive immunoreactivity (0, no staining; 1, <20%; 2, 20–50%; and 3, >50% of cells). The total score for each compartment was obtained as the product of intensity and extent of staining. Weak or negative cases were defined as having a score of <2; strong cases had a score of ⩾2.
Clinicopathological parameters such as patients' sex, age at surgery, tumour grade, lymph node status, resection margin status, presence of vascular invasion, presence of perineural invasion and tumour size (categorised as <20 mm and ⩾20 mm) were extracted from histopathological reports. To obtain associations between CapG or gelsolin expression and clinicopathological parameters, data were cross tabulated and Fisher's two‐sided exact test or χ2 test (for tumour grade) was applied. Analysis of the relationship between tumour size and CapG staining was also carried out using the Mann–Whitney U test. CapG and gelsolin immunohistochemical scores of benign and malignant cells were also compared using the Mann–Whitney U test. To evaluate the effect of CapG or gelsolin expression on patient survival, life tables were constructed from survival data and Kaplan–Meier curves plotted. Overall survival was measured from date of initial surgery to date of death, counting death from any cause as the end point, or the last date of information as the end point if no death was documented. The date of death was available for all patients, with the exception of four who were still alive at the time of analysis. For Kaplan–Meier curves, comparisons between groups were performed using the log rank test. All statistical analyses were carried out using Statview V.5.01. Results were considered significant for values of p<0.05.
siRNA knockdown of CapG and gelsolin expression
The human pancreatic adenocarcinoma cell lines Panc‐1, MiaPaCa‐2 and Suit‐2 were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l‐glutamine, 2500 IU/ml penicillin and 5 μg/ml streptomycin (all from Sigma) at 37°C in a humidified atmosphere of 5% CO2. For siRNA treatment, cells were plated in six‐well plates at either 2×105 cells per well (Panc‐1 and Suit‐2) or 4×105 cells/well (MiaPaCa‐2) 24 h before transfection. Before transfection, the medium was replaced with 2.6 ml of antibiotic‐free medium. Cells were transfected with siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) and Optimem I (Invitrogen) according to the manufacturer's instructions. Final siRNA concentrations (in a total of 3 ml of medium) were 10 nmol/l in experiments using CapG‐targeting siRNA molecules and 20 nmol/l in those using gelsolin‐targeting siRNA molecules. The plates were returned to the incubator until motility assays or wound healing assays were carried out. Generally, in the case of CapG knockdown, these were carried out 72 h after transfection and in the case of gelsolin at 48 h after transfection. When Suit‐2 cells were used in CapG knockdown experiments, cells were transferred from six‐well dishes into 75 cm2 flasks, at 48 h after transfection, and plated for motility assays after a further 48 h. The number of viable cells present after treatment with control siRNAs, RISC‐free siRNA and targeting SiRNAs was determined for all cell lines by counting cells incubated with trypan blue at 48 h (gelsolin) and 72 h (CapG) after transfection.
Two siRNA molecule (Dharmacon, Illinois, Chicago, USA) sequences GGAGGAGCCUGCUGAGAUG and GAUAUCUGAACUGCUUU (corresponding to positions 642–661 and 831–849, respectively, within the coding sequence of CapG, Genbank accession number NM_001747) were used to knock down CapG. Two siRNA molecule (Dharmacon) sequences GAACGGAAAUCUGCAGUAUUU and GAACUCCAACGAUGCCUUUUU (corresponding to positions 359–377 and 1811–1831, respectively, within the coding sequence of gelsolin, Genbank accession number NM_000177) were used to knock down gelsolin. Three control siRNAs were used—a non‐functional “RISC‐free” siRNA (sicontrol RISC‐free siRNA 1, Dharmacon) and two non‐targeting siRNAs designated control 1 (sicontrol non‐targeting siRNA 1 from Dharmacon) and control 2 (GGACGCAUCCUUCUUAA, a gift from Dr M Boyd, University of Liverpool, Liverpool, UK). To monitor the knockdown, cell lysates were prepared by extraction into 100 mM TRIS‐HCl (pH 6.8) containing 2% w/v sodium dodecyl sulphate and a protease inhibitor cocktail (Complete, Mini, EDTA‐free protease inhibitors; Roche Applied Science, Reading, UK). Immunoblotting was used to assess CapG and gelsolin levels, using a chicken (IgY) anti‐human CapG antibody (GenWay Biotech, San Diego, California, USA) and a monoclonal gelsolin antibody GS‐2C4 (Sigma), respectively.
In vitro cell motility assay
Motility assays were carried out in 24‐well format cell culture inserts (BD Biosciences, Oxford, UK), with 8 μm pores. At the appropriate time after transfection with siRNA or control RNA, cells were harvested and plated in complete medium on top of the culture insert at 5×104 cells/insert (MiaPaCa‐2 and Suit‐2) or 1×104 cells/insert (Panc‐1) in 0.5 ml. The lower chamber contained 0.75 ml complete medium. Inserts were incubated at 37°C, 5% CO2 for 18 h. Non‐invading cells were removed with a cotton swab soaked in medium. Cells that had moved through the pores (to the lower surface of the filters) were fixed and stained using the Diff‐Quik staining kit (Dade Behring, Düdigen FR, Switzerland) and counted on a Leica CME microscope at 40× total magnification. Three inserts were counted for each treatment in each experiment and experiments were carried out at least three times.
In vitro wound‐healing assay
Panc‐1 and Suit‐2 cells were treated with siRNA as described above. After incubation for 72 (CapG) or 48 (Gelsolin) h, the cells were removed by trypsinisation, counted and plated at 4×105 cells/ml in 12‐well dishes. Cells were incubated overnight yielding confluent monolayers for wounding. Wounds were made using a pipette tip and photographs taken immediately (time zero) and 12 or 16 h after wounding for Suit‐2 and Panc‐1 cells, respectively. The distance migrated by the cell monolayer to close the wounded area during this time period was measured. Results were expressed as a migration index—that is, the distance migrated by siRNA treated (control or targeted) relative to the distance migrated by RISC‐free control RNA treated cells. Experiments were carried out in triplicate and repeated at least five times.
3‐(4, 5‐Dimethythiazol‐2‐yl‐2,5‐diphenyltetrazolium bromide) proliferation assay
While plating for motility, cells were also plated onto 96‐well plates at 5×103 cells per well (MiaPaCa‐2 and Suit‐2) or 1×104 cells per well (Panc‐1), in triplicate. After 18 h, 15 μl of 3‐(4, 5‐dimethythiazol‐2‐yl‐2,5‐diphenyltetrazolium bromide) (MTT) solution (5 mg/ml of 3,(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide in PBS; Sigma) was added to each well and plates incubated for 3 h at 37°C. The medium was then removed, 100 μl dimethyl sulphoxide added and incubated at room temperature for 30 min, and the optical density at 550 nm determined using an Anthos 2001 plate reader (Anthos Labtec Instruments, Salzberg, Austria). The number of cells plated for each cell line was chosen, on the basis of earlier optimisation experiments, such that absorbance readings were in the linear range with respect to cell number. Results were expressed as MTT reading after siRNA treatment or MTT reading after RISC‐free control RNA treatment. Experiments were carried out in triplicate and repeated between three and nine times.
Results
Identification of CapG as a protein overexpressed in microdissected malignant pancreatic cell extracts
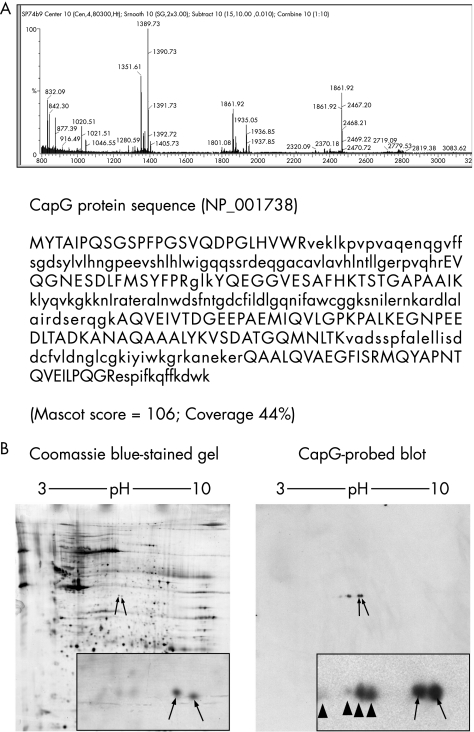
In a previous proteomics study,5 we reported an increase in the intensity of a feature in two‐dimensional protein profiles derived from laser‐capture microdissected malignant pancreatic cancer cells. This was observed in various forms such as a single spot or as a pair of closely located spots (labelled spot(s) 2 in Shekouh et al5). Using MALDI‐TOF mass spectrometry, we have now shown that both protein spots contain the same protein, CapG (NCBI accession number NP_001738). Figure 1A shows an example of the MALDI‐TOF mass spectrometry spectrum and peptide coverage map for the right‐hand side of the two spots. Similar results were found for the left spot (data not shown). To further confirm our mass spectrometry identification, we carried out western analysis on proteins (separated by two‐dimensional gel electrophoresis) from a tumour known to express both spots. Comparison of a Coomassie blue‐stained gel (fig 1B, left panel) and a CapG‐probed immunoblot (fig 1B, right panel) shows that the two spots identified by mass spectrometry as CapG (arrowed on the Coomassie blue stained gel) comigrated with those detected by western blotting with the CapG‐specific antibody. Additionally, we also observed several spots on the immunoblot with isoelectric points lower than the main spots (arrowheads, fig 1B). The combination of mass spectrometry and immunoblotting confirms, to a high degree of confidence, the spots identified as CapG. Previous studies have shown that at least some of the differences in electrophoretic mobility can be attributed to differential phosphorylation.
Figure 1 CapG was identified by mass spectrometry and confirmed by two‐dimensional immunoblotting. (A) Mass spectrum of a trypsin‐digested spot from a two‐dimensional gel of proteins from a malignant pancreatic specimen. The matching peptides, in upper case, are indicated, along with coverage and Mascot score. (B) Coomassie blue‐stained gel from malignant pancreas, with spots identified by mass spectrometry as CapG indicated by arrows. A corresponding two‐dimensional blot from the same protein extract, immunostained for CapG with a polyclonal rabbit antibody, showing the principal spots (arrows), along with less intense spots to the left of these (arrowheads).
Immunohistochemistry confirmed overexpression of CapG in malignant pancreatic ductal adenocarcinoma cells
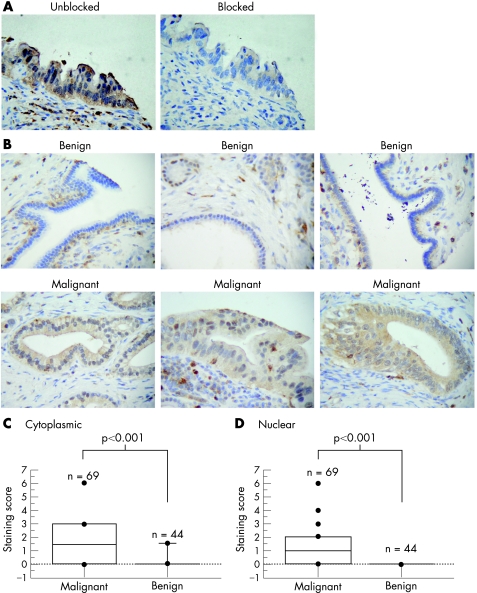
Immunohistochemical analysis for CapG was carried out on a pancreatic cancer tissue microarray using the same rabbit polyclonal anti‐CapG antibody as used for two‐dimensional immunoblotting. In general, immunostaining of CapG in malignant and benign ductal cells was relatively weak. To confirm that this staining was not because of a non‐specific component in our antibody source, we produced a recombinant thioredoxin–CapG fusion protein for use as a blocking protein. Preincubating the blocking protein with CapG antibody removed the CapG signal from immunoblots (not shown). Similarly, applying this to immunohistochemical analysis resulted in almost total loss of staining in all immunopositive cells (fig 2A).
Figure 2 CapG is overexpressed in malignant cells, but not in benign ducts. (A) Immunohistochemical (IHC) images of sequential sections from a malignant pancreatic specimen, showing loss of staining after blocking of CapG antibody with a recombinant thioredoxin–CapG fusion protein. (B) IHC images of sections containing benign (upper row) and malignant (lower row) ductal cells, stained for CapG. (C, D) Plots showing staining scores for cytoplasmic (C) and nuclear (D) CapG on tissue microarray sections for both malignant and benign ductal specimens. The p values shown are those obtained for comparisons between staining score distributions in malignant and benign cells using the Mann–Whitney U test.
Figure 2B shows examples of CapG immunohistochemical staining for tissues containing benign ducts (upper row of images) and malignant cells (lower row). Intense staining was found in cells, morphologically similar to macrophages, in which CapG is known to be highly expressed. A pancreatic cancer tissue microarray was scored for CapG staining in both cytoplasmic and nuclear compartments of benign and malignant ductal cells, enabling analysis of benign ducts from 44 patients and malignant cells from 69 patients. Statistical analysis of cytoplasmic CapG staining scores confirmed increased staining in malignant cells compared with benign ductal cells (fig 2C,D; Mann–Whitney U test, p<0.001). The median score in the cytoplasmic compartment of malignant specimens was 1.5 (interquartile range (IQR) 0–3), compared with 0 (IQR 0–0) in benign ducts (p<0.001, Mann–Whitney U test). Similarly, the median nuclear score in malignant ducts was 1 (IQR 0–2), whereas no nuclear staining was observed in benign cells.
RNA interference‐mediated reduction in CapG expression resulted in impaired cell motility
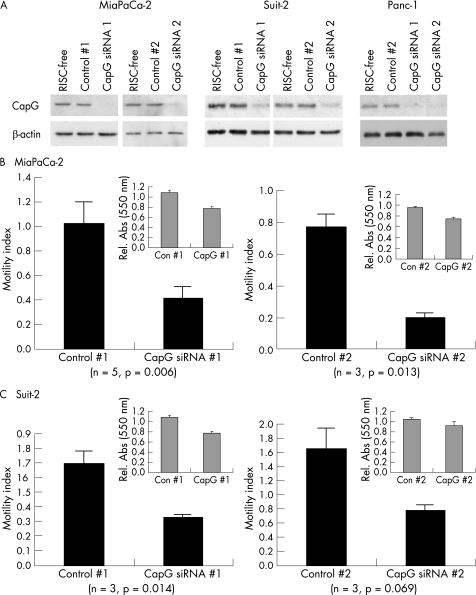
RNA interference (RNAi) was effective in reducing CapG protein levels in three pancreatic cancer cell lines, MiaPaCa‐2, Suit‐2 and Panc‐1 (fig 3A). CapG levels were generally observed to diminish between 48 and 72 h after transfection with CapG‐targeting siRNAs (all three cell lines), and remained low for at least 120 h (not shown). Two assays were used to assess the effect of CapG knockdown on cell motility; a migration assay using Boyden chambers and an in vitro wound‐healing assay. In the case of the migration assay, cell numbers translocating across microporous membranes after treatment with RISC‐free, non‐targeting or CapG‐targeting siRNAs were obtained. The results (fig 3B, C) are reported as motility indexes, which represent the number of cells translocating across the membranes, expressed as a proportion of the number translocating in the RISC‐free control. In MiaPaCa‐2 cells, knockdown of CapG with both CapG‐targeting siRNAs significantly reduced cell motility, by an average of 59% (CapG siRNA 1) or 73% (CapG siRNA 2) compared with their respective non‐specific controls (fig 3B). The metabolic activity of cells during the time period of translocation assays was assessed by MTT and results are shown as insets. In MiaPaCa‐2 cells, marked reductions in MTT readings of 29% for CapG siRNA 1 and 21% for CapG siRNA 2 over respective controls were observed. This may have contributed to the observed decreases in motility, although it is unlikely, certainly in the case of CapG siRNA 2, to be wholly responsible. CapG siRNA #1 caused a 55% decrease in motility in Suit‐2 cells (fig 3C, left panel). The corresponding decrease in MTT activity was 8%. CapG siRNA #2, while showing 50% decreased motility relative to control #2, was not significantly lower (p = 0.069). Moreover, control siRNA 2 also had an unexpected positive effect on cell motility in Suit‐2, with an average motility index of 1.65. A 12% decrease in MTT activity for CapG siRNA 2 over control 2 was observed in these cells. For Panc‐1 cells, no difference in motility was observed after transfection with either of the CapG‐targeting siRNAs (n = 9 attempts in three independent experiments; data not shown). These cells showed a significant increase of 30% for CapG siRNA 1 and a significant decrease of 25% in MTT readings for CapG siRNA 2 compared with respective controls (data not shown). For a more direct evaluation of the effects of CapG knockdown on cell viability, cells were counted 72 h after transfection with CapG targeting and control siRNAs. Significant differences in cell number were not observed after CapG knockdown in any of the three cell lines evaluated (n = 4–14 independent experiments).
Figure 3 Knockdown of CapG by RNA interference inhibits cell migration. (A) Immunoblotting carried out using 1.5 μg protein per lane from indicated cell lines 72 h after transfection with 10 nM of the indicated siRNAs. Data are shown for two cell lines; MiaPaCa‐2 (B) and Suit‐2 (C). Bars represent the motility index of each treatment, expressed as a value relative to the number of cells translocating in an RISC‐free control. Error bars represent SEs, and the number of experiments is indicated on each graph. p values are for Student's paired t tests, one‐tailed. MTT data are shown in insets. Error bars represent SEs.
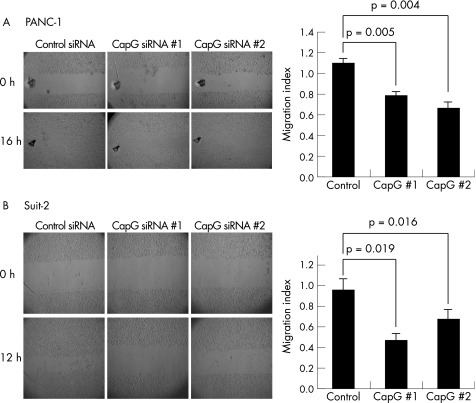
In wound‐healing assays, the distance moved by a wounded cell monolayer on plastic after treatment with RISC‐free, non‐targeting control siRNA or CapG‐targeting siRNA was obtained. The results (fig 4) are reported as migration indices, which represent the distance moved by the non‐targeting control siRNA or CapG‐targeting siRNA‐treated cells to close the wound, expressed as a proportion of the distance moved by the RISC‐free control treated cells. MiaPaCa‐2 cells, regardless of CapG levels (control or knockdown), migrated very small distances and were unable to achieve wound closure. Thus, they were considered unsuitable for this assay. In Panc‐1 cells, CapG siRNA 1 and CapG siRNA 2 significantly decreased migration by 25% and 30%, respectively, when compared with non‐targeting controls (p = 0.005 and p = 0.004; fig 4A). This was in direct contrast with results obtained for this cell line in the translocation assay, described above. In Suit‐2 cells (fig 4B), knockdown of CapG using CapG siRNA 1 and CapG siRNA 2 decreased migration by an average of 50% and 30%, respectively, when compared with treatment with non‐targeting control siRNA (p = 0.019 and 0.016).
Figure 4 Knockdown of CapG by RNA interference inhibits wound healing. Data and representative images are shown for two cell lines: Panc‐1 (A) and Suit‐2 (B). Bars represent the migration index of each treatment, expressed as a value relative to the distance moved by the cell monolayer in a RISC‐free control. Error bars represent the SE for n = 5 (Panc‐1) and n = 6 (Suit‐2) experiments carried out in triplicate. The p values were obtained using the Student's paired t‐test, two‐tailed.
CapG overexpression is associated with increased tumour size
We sought to determine whether overexpression of CapG in patients was associated with clinicopathological parameters. For the purposes of analysis, immunostaining scores were classified as weak (score <2) or strong (⩾2). Using this classification, 24 of 69 (35%) patients exhibited strong immunoreactivity in the cytoplasmic compartment and 20 of 69 (29%) had strong immunoreactivity in the nuclear compartment. Nine (13%) patients had both strong nuclear and strong cytoplasmic staining. Data were available for all 69 patients, with the exception of perineural invasion (n = 67), vascular invasion (n = 52) and resection margin status (n = 66). A significant association was identified between tumour size and nuclear CapG staining intensity (Fisher's exact test, p = 0.001). This was based on a classification of tumour size into groups of <20 mm and ⩾20 mm. Of 20 patients who had high levels of nuclear CapG staining, 19 (95%) had tumours ⩾20 mm. By contrast, of the remaining 49 patients with weak nuclear CapG staining, only 55% had tumours ⩾20 mm. No association was observed for cytoplasmic CapG and tumour size (p = 0.23). No significant associations between CapG expression and other parameters examined (table 1) were observed. Similarly, no significant association was observed for survival and CapG staining intensity, for either nuclear or cytoplasmic compartments. Only five patients were alive at the time of the study. Median survival for patients with weak cytoplasmic staining was 13 months compared with 14.82 months for patients with strong cytoplasmic staining (p = 0.61, log rank test). For patients with weak nuclear CapG staining, median survival was 13 months compared with 15.45 months for patients with strong nuclear staining (p = 0.89, log rank test).
Table 1 Statistical analysis of cytoplasmic and nuclear CapG staining with clinical and pathological parameters.
| Parameter | All cases (n = 69) | Cytoplasmic CapG | Nuclear CapG | ||||
|---|---|---|---|---|---|---|---|
| Strong | Weak | p Value | Strong | Weak | p Value | ||
| Sex | |||||||
| Male | 40 | 14 | 26 | >0.99 | 13 | 27 | 0.59 |
| Female | 29 | 10 | 19 | 7 | 22 | ||
| Age at surgery (years) | |||||||
| <60 | 19 | 9 | 10 | 0.25 | 4 | 15 | 0.55 |
| >60 | 50 | 15 | 35 | 16 | 34 | ||
| Tumour grade | |||||||
| Poorly differentiated | 26 | 8 | 18 | 9 | 17 | ||
| Moderately differentiated | 36 | 13 | 23 | 0.81 | 11 | 25 | 0.81 |
| Well differentiated | 7 | 3 | 4 | 0 | 7 | ||
| Nodal metastases | |||||||
| Present | 55 | 22 | 33 | 0.11 | 16 | 39 | >0.999 |
| Not present | 14 | 2 | 12 | 4 | 10 | ||
| Involved resection margin | |||||||
| Yes | 41 | 16 | 25 | 11 | 30 | ||
| No | 25 | 7 | 18 | 0.43 | 6 | 19 | >0.99 |
| Not recorded | 3 | 1 | 2 | 3 | 0 | ||
| Vascular invasion | |||||||
| Present | 33 | 15 | 18 | 6 | 27 | ||
| Not present | 19 | 5 | 14 | 0.23 | 8 | 11 | 0.10 |
| Not recorded | 17 | 4 | 13 | 6 | 11 | ||
| Perineural invasion | |||||||
| Present | 64 | 24 | 40 | 18 | 46 | ||
| Not present | 3 | 0 | 3 | 0.54 | 0 | 3 | 0.55 |
| Not recorded | 2 | 0 | 2 | 2 | 0 | ||
| Tumour size | |||||||
| <20 mm | 23 | 6 | 17 | 0.42 | 1 | 22 | 0.001 |
| ⩾20 mm | 46 | 18 | 28 | 19 | 27 | ||
Significance figures are p values for Fisher's exact test, or for χ2 test in the case of tumour grade only.
Gelsolin is also overexpressed in pancreatic cancer specimens
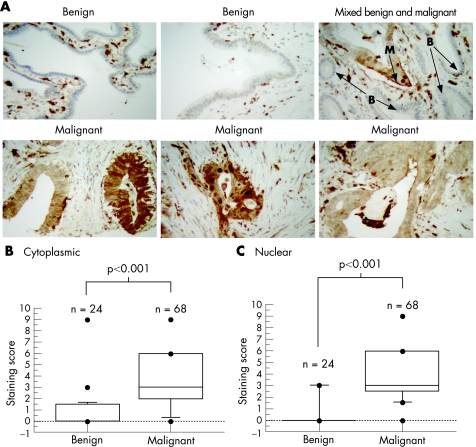
After titration of a mouse monoclonal gelsolin antibody in a variety of tissues (data not shown), immunostaining of the pancreatic cancer tissue microarray was carried out, enabling analysis of benign ducts from 24 patients and malignant cells from 68 patients. Unlike CapG immunostaining, which was relatively weak, gelsolin staining was readily detectable, although staining was heterogeneous in some tumour cases. Intense staining was found in inflammatory cells and also in tumour cells (fig 5A). The overall intensity of gelsolin staining was higher in malignant than in benign ducts (fig 5B, C). The median score in the cytoplasmic compartment of malignant specimens was 3 (IQR 2–6), compared with 0 (IQR 0–1.5) in benign ducts (p<0.001, Mann–Whitney U test). Similarly, the median nuclear score in malignant ducts was 3 (IQR 2.5–6) compared with 0 (IQR 0–0) in benign ducts (p<0.001).
Figure 5 Gelsolin is overexpressed in malignant cells, but not in benign ducts. (A) Immunohistochemistry images of sections containing benign and malignant samples as indicated. B, benign; M, malignant. (B, C) Plots showing staining scores for cytoplasmic (B) and nuclear (C) gelsolin on tissue microarray sections for both malignant and benign ductal specimens. The p values shown are for comparisons between staining score distributions in malignant and benign cells, using the Mann–Whitney U test.
RNAi‐mediated reduction in gelsolin expression resulted in impaired cell motility
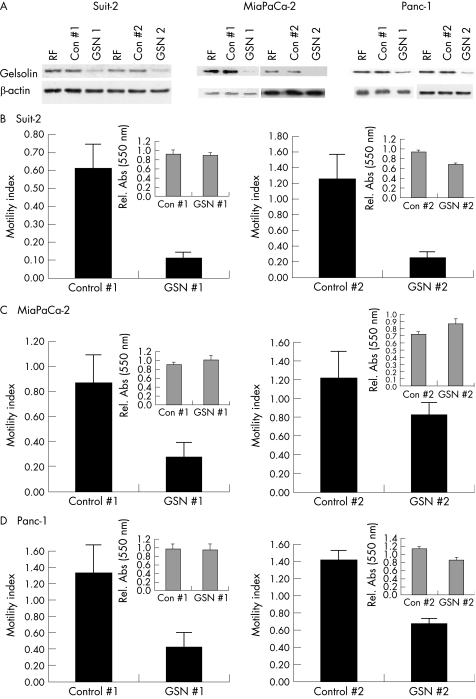
Having observed high levels of expression of gelsolin in pancreatic cancer specimens, we wished to determine whether this protein might contribute to the motility of pancreatic cancer cells in vitro. RNA interference was used to reduce gelsolin protein levels in Suit‐2, Panc‐1 and MiaPaCa‐2 cells with two gelsolin‐targeting siRNAs, GSN 1 and GSN 2 (fig 6A). In translocation assays using Boyden‐style chambers, GSN 1 and GSN 2 siRNAs significantly reduced cell motility in Suit‐2 cells by an average of 82% and 76%, respectively (p = 0.024 and 0.015, respectively) when compared with respective control siRNAs (fig 6B). In MiaPaCa‐2 cells, GSN siRNA 1 reduced motility by 69% (p = 0.001, fig 6C; left panel) and in Panc‐1 cells by 84% (p = 0.039; fig 6D left panel). GSN 2 siRNA caused a 52% reduction in Panc‐1 cells, although an increase in motility was noted with control 2. In MiaPaCa‐2 cells, GSN 2 caused a modest but insignificant inhibition of cell translocation (p = 0.08) when compared with control 2 (fig 6C; right panel). MTT assays carried out over the same time periods are presented as insets. GSN # 1 had no significant effects on MTT readings compared with control 1 in any cell line. By contrast, both Suit‐2 and Panc‐1 cells showed significant decreases of 25% in MTT readings after GSN 2 treatment compared with control 2. In MiaPaCa‐2 cells, a significant increase of 19% in MTT reading was observed after GSN 2 treatment compared with control 2.
Figure 6 Knockdown of gelsolin by RNA interference inhibits cell migration. (A) Immunoblots, using 3 μg protein per lane, carried out on cell extracts prepared 48 h after transfection with 20 nM of the indicated siRNAs. (B–D) Data are shown for Suit‐2 (B), MiaPaCa‐2 (C) and Panc‐1 (D) cells. Bars represent the motility index of each treatment, expressed as a value relative to the number of translocating cells in the RISC‐free control. Error bars represent the standard errors. MTT data for each GSN‐targeting siRNA molecule compared with its respective control are shown in insets. Experiments were carried out in triplicate at least nine times; the bars on each graph represent standard errors.
The numbers of viable cells were determined 48 h after treatment with gelsolin‐targeting siRNAs compared with controls in MiaPaCa‐2, Suit‐2 cells and Panc‐1 cells (n = 9, 17 and 17 independent experiments, respectively). Differences in cell number were not observed after transfection of cells with GSN 2. However, transfection of Suit‐2 cells with GSN 1 resulted in an 11% decrease in cell number compared with control 1 (p = 0.058).
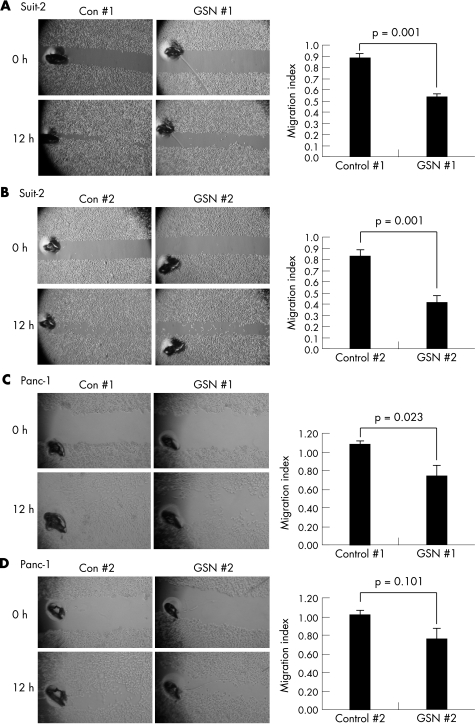
In wound‐healing assays, both GSN 1 and GSN 2 siRNAs significantly impaired the ability of Suit‐2 cells to migrate into the wounded monolayer by 51.6% (p⩽0.001) and 55.7% (p = 0.001), respectively, when compared with non‐targeting controls (fig 7A, B). In Panc‐1 cells, GSN 1 impaired cell migration by an average of 25% when compared with its respective non‐targeting control (p = 0.023; fig 7C). Treatment of Panc‐1 cells with GSN 2 reduced migration by 22% compared with control siRNA, although this was not significant (p = 0.101; fig 7D).
Figure 7 Knockdown of gelsolin by RNA interference inhibits wound healing. Wound‐healing data are shown for Suit‐2 (A, B) and Panc‐1 cells (C, D). Bars represent the migration index of each treatment, expressed as a value relative to the distance traveled by the cell monolayer in a RISC‐free control. Error bars represent the standard errors for six experiments carried out in triplicate. p Values are for two‐tailed Student's paired t test.
High levels of nuclear gelsolin were associated with reduced patient survival
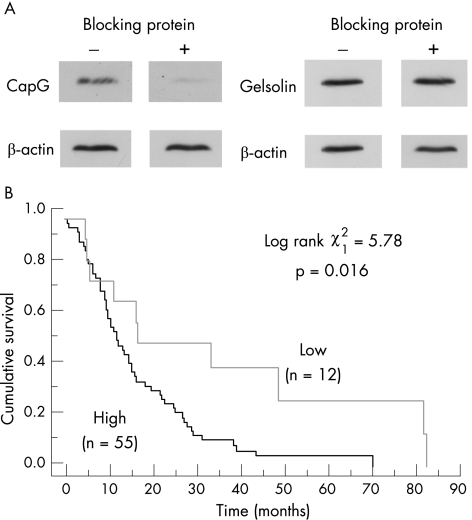
As in the case of CapG staining, for the purposes of analysis, immunostaining scores were classified as weak (score <2) or strong (⩾2). Using this classification, 54 of 68 (79%) patients exhibited strong immunoreactivity in the cytoplasmic compartment and 53 of 68 (78%) had strong immunoreactivity in the nuclear compartment. Forty nine (72%) patients had both strong nuclear and strong cytoplasmic staining. No significant associations were found between cytoplasmic or nuclear gelsolin expression levels and the parameters of sex, age at surgery, tumour size, nodal status, vascular or perineural invasion, or resection margin status. However, when high cytoplasmic gelsolin or CapG (staining score >2 in either case) were compared, a significant association was observed (Fisher's exact test, p = 0.021). Virtually all patients positive for cytoplasmic CapG (22/23 (95%)) were also positive for cytoplasmic gelsolin. Similarly, 12 of 13 (92%) patients with weak cytoplasmic gelsolin staining also had weak cytoplasmic staining CapG (table 2). It seems therefore that the overexpression of CapG is accompanied by the overexpression of gelsolin. To account for the possibility that the gelsolin antibody had some reactivity against CapG, we preincubated gelsolin antibody with recombinant CapG and showed that there was no reduction in gelsolin signal observed in an immunoblot (fig 8A). In addition, two‐dimensional immunoblots showed no cross reactivity between CapG antibody and gelsolin (eg, fig 1) or between gelsolin antibody and CapG (data not shown).
Table 2 Statistical analysis of cytoplasmic and nuclear CapG staining with cytoplasmic and nuclear gelsolin staining.
| Parameter | All cases (n = 61) | Cytoplasmic gelsolin | Nuclear gelsolin | ||||
|---|---|---|---|---|---|---|---|
| Strong (n = 48) | Weak (n = 13) | p Value | Strong (n = 50) | Weak (n = 11) | p Value | ||
| Strong cytoplasmic CapG | 23 | 22 | 1 | 0.021 | 21 | 2 | 0.182 |
| Weak cytoplasmic CapG | 38 | 26 | 12 | 29 | 9 | ||
| Strong nuclear CapG | 16 | 12 | 4 | 0.727 | 13 | 3 | >0.999 |
| Weak nuclear CapG | 45 | 36 | 9 | 37 | 8 | ||
Significance figures are p values for Fisher's exact test.
Figure 8 High gelsolin levels are associated with reduced survival. (A) Immunoblots showing CapG and gelsolin signals from Suit‐2 cell extracts, with and without preincubation of the antibodies with a recombinant thioredoxin–CapG fusion protein (rCapG). CapG antibody (IgY, 1:2000 dilution) and gelsolin antibody (mouse monoclonal, 1:2000 dilution) were incubated with (+) or without (−) rCapG. β‐Actin serves as a loading control. (B) Kaplan–Meier survival curves for 67 patients with pancreatic cancer, with low (staining score <2) or high expression (staining score ⩾2) of nuclear gelsolin. Test statistics: log rank test (1 df, p = 0.016).
Kaplan–Meier analysis showed that patients with low nuclear gelsolin had improved survival over patients with high gelsolin (log rank χ21 = 5.78; p = 0.016; fig 8B). Moreover, univariate analysis showed nuclear gelsolin to be independent of other prognostic indicators such as nuclear S100A6 expression,21 lymph node status or resection margin status. Low cytoplasmic gelsolin also tended to be associated with improved survival, although this did not reach statistical significance (log rank χ21 = 3.91; p = 0.055).
Discussion
Pancreatic ductal adenocarcinoma is an extremely aggressive disease, with most patients having metastases or extensive local invasion by the time of diagnosis. Little is known about the potential role of actin cytoskeleton reorganisation in the process of pancreatic cancer cell motility or dissemination. In this study, we describe the overexpression, in pancreatic cancer specimens, of two actin‐capping proteins of the gelsolin superfamily. CapG expression was generally uniform in tumour specimens, whereas gelsolin expression was heterogeneous in some tumour cases. Our interest was originally focused on CapG, which we observed in a proteomics study5 to be expressed to abnormally high levels in microdissected cancer cells compared with microdissected benign ductal cells. Although we subsequently showed gelsolin also to be overexpressed, this was not observed in the proteomics study, largely because protein spots containing gelsolin were not detected in that study. This may imply that gelsolin is expressed in lower levels than CapG as the proteomics study allowed detection of relatively abundant proteins only. Alternatively, it may reflect properties of the protein that render it less amenable to extraction or two‐dimensional gel separation than CapG.
Very little is known about CapG in relation to cancer. Protein overexpression has been reported in only one other cancer type‐ocular melanoma,22 although its functional consequences were not examined. Two independent gene array studies reported increased RNA transcripts for CapG in pancreatic cancer23,24 although protein levels were not determined in these studies.
Given the evidence that CapG has a role in normal physiological cell movement,9,10,11,12 we examined whether it could contribute to the motility of pancreatic cancer cells. Our RNA knockdown experiments showed that all three pancreatic cancer cell lines examined were sensitive to CapG depletion, showing substantial reductions in cell motility. Interestingly, the form of cell motility affected by CapG depletion was cell specific. Loss of CapG had no effect on the ability of Panc‐1 cells to migrate in translocation assays, but severely affected the rate at which they achieved wound closure. Suit‐2 cells, by contrast, were sensitive to CapG depletion in both assays, and MiaPaCa‐2 cells, although exhibiting impaired motility in translocation assays after CapG knockdown, were unable to migrate to any considerable extent in wound‐healing assays regardless of CapG status. Thus, CapG may contribute to motility in these cells in different ways and other factors are likely to be important. Unlike CapG, the expression levels of gelsolin have been studied in a variety of other cancers. In some, including bladder,16 prostate17 and lung,18 gelsolin is down regulated, whereas in others—for example, urothelial carcinomas19 and a subset of non‐small cell lung cancer20—overexpression was reported. Our finding that gelsolin is overexpressed in a high proportion of pancreatic cancers led us to investigate whether it too could contribute to the motility of pancreatic cancer cells. Depletion of gelsolin in Suit‐2 cells caused a marked reduction in motility, observed in both translocation and wound‐healing experiments. Reductions in motility were also observed in both Panc‐1 and MiaPaCa‐2 cells, although they were less pronounced. Taken as a whole, our motility data for CapG and gelsolin are suggestive of potential roles for these proteins in pancreatic cancer cell motility and consequently dissemination. However, further experiments, ideally using animal models, will be required to formally deal with whether either of these proteins or indeed other gelsolin superfamily members are important for the process of pancreatic cancer cell dissemination.
De Corte et al25,26 have, in separate studies, shown that overexpression of nuclear CapG or gelsolin promotes invasion in in vitro systems. We found no statistically significant correlations between high CapG or gelsolin expression and putative parameters of invasion. However, this is not altogether surprising. We know that almost all patients with pancreatic cancer have advanced disease, characterised by metastases at the time of diagnosis. Only 3 of 69 patients in this study lacked demonstrable perineural invasion in the tissue blocks examined. Thus, the contribution of these proteins to invasion in the earlier stages of disease cannot be measured by examining associations in patients with advanced disease, and clearly other models are required. Nonetheless, we did observe an inverse association between gelsolin levels and survival, which was independent of other known prognostic parameters. In non‐small cell lung cancers high gelsolin levels correlated with lymphatic invasion,20 and were associated with poor survival.27 Interestingly, there was considerable overlap between the cytoplasmic expression of CapG and gelsolin. It suggests that both actin‐capping proteins are overexpressed in a subset of pancreatic cancers, although whether they act in concert or regulate each other's expression or activity remains to be determined.
Although this study has focused on the motility‐associated functions of CapG and gelsolin, it is entirely possible that these proteins have other distinct roles in this cancer. CapG is a nuclear protein28 and was readily detected in the nuclei of pancreatic cancer cells. This may be due to its lack of a nuclear export sequence.29 Phosphorylated forms of CapG have been suggested to be preferentially associated with the nucleus.30 Our observation, by two‐dimensional western, of a trail of spots with isoelectric points lower than those of the two principal CapG spots is compatible with the presence of phosphorylated forms of CapG in pancreatic cancer. The role of nuclear CapG is largely unknown. We observed an association between nuclear, but not cytoplasmic CapG and larger tumour sizes. Although larger tumour sizes do not necessarily reflect enhanced growth rates, the question of whether CapG promotes tumour growth merits specific investigation. Although we did not observe significant reductions in cell number after CapG knockdown, MTT readings, particularly in MiaPaCa‐2 cells, were reduced in response to lower CapG levels. Gelsolin also has other functions6—for example, a role in apoptosis, which may have potentially important implications for pancreatic cancer cells. Our study was limited to two members of the gelsolin superfamily of actin‐capping proteins. However, other gelsolin superfamily members may be important. Recently, Prasad et al31 have reported up regulation of villin transcripts in PanIN lesions.
In summary, we provide the first study in which CapG and gelsolin protein levels have been described in pancreatic cancer. Our observations that depletion of CapG or gelsolin in pancreatic cancer cell lines diminishes their motility suggests the possible involvement of this family of proteins in the motility and consequently the dissemination of pancreatic cancer cells. Moreover, the association with gelsolin and poor survival indicate that this actin‐capping protein may also contribute to the aggressive nature of this cancer. Further studies into the role of this family of actin‐capping proteins in pancreatic cancer are merited.
Acknowledgements
We thank John P Neoptolemos for invaluable discussion and support throughout.
Abbreviations
IQR - interquartile range
MALDI‐TOF - matrix‐assisted laser desorption/ionisation‐time‐of–flight
MTT - 3‐(4, 5‐dimethythiazol‐2‐yl‐2, 5‐diphenyltetrazolium bromide)
Footnotes
Funding: This work is supported by grants from Cancer Research UK, North West Cancer Research Fund, The Medical Research Council and the National Institute of Health Burn Centre grant P05GM21681.
Competing interests: None.
Ethical approval: The study was conducted with ethical approval from Cheshire and Merseyside Health Authority (Hamilton House, 24 Pall Mall, Liverpool L3 6AL, UK), LREC ref 03/02/316A.
References
- 1.Parkin D M, Bray F I, Devesa S S. Cancer burden in the year 2000. The global picture. Eur J Cancer 200137(Suppl 8)S4–66. [DOI] [PubMed] [Google Scholar]
- 2.Sener S F, Fremgen A, Menck H R.et al Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg 19991891–7. [DOI] [PubMed] [Google Scholar]
- 3.Friedl P, Wolf K. Tumour‐cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 20033362–374. [DOI] [PubMed] [Google Scholar]
- 4.Keleg S, Buchler P, Ludwig R.et al Invasion and metastasis in pancreatic cancer. Mol Cancer 2003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekouh A R, Thompson C C, Prime W.et al Application of laser capture microdissection combined with two‐dimensional electrophoresis for the discovery of differentially regulated proteins in pancreatic ductal adenocarcinoma. Proteomics 200331988–2001. [DOI] [PubMed] [Google Scholar]
- 6.Silacci P, Mazzolai L, Gauci C.et al Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci 2004612614–2623. [DOI] [PubMed] [Google Scholar]
- 7.Yin H L, Stossel T P. Control of cytoplasmic actin gel‐sol transformation by gelsolin, a calcium‐dependent regulatory protein. Nature 1979281583–586. [DOI] [PubMed] [Google Scholar]
- 8.Yu F X, Johnston P A, Sudhof T C.et al gCap39, a calcium ion‐ and polyphosphoinositide‐regulated actin capping protein. Science 19902501413–1415. [DOI] [PubMed] [Google Scholar]
- 9.Sun H Q, Kwiatkowska K, Wooten D C.et al Effects of CapG overexpression on agonist‐induced motility and second messenger generation. J Cell Biol 1995129147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellieux C, Desgeorges A, Pigeon C H.et al Cap G, a gelsolin family protein modulating protective effects of unidirectional shear stress. J Biol Chem 200327829136–29144. [DOI] [PubMed] [Google Scholar]
- 11.Witke W, Li W, Kwiatkowski D J.et al Comparisons of CapG and gelsolin‐null macrophages: demonstration of a unique role for CapG in receptor‐mediated ruffling, phagocytosis, and vesicle rocketing. J Cell Biol 2001154775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh S S, Litherland S A, Clare‐Salzler M J.et al CapG(−/−) mice have specific host defense defects that render them more susceptible than CapG(+/+) mice to Listeria monocytogenes infection but not to Salmonella enterica serovar Typhimurium infection. Infect Immun 2003716582–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chellaiah M, Kizer N, Silva M.et al Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J Cell Biol 2000148665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham C C, Stossel T P, Kwiatkowski D J. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science 19912511233–1236. [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Witke W, Kwiatkowski D J.et al Delayed retraction of filopodia in gelsolin null mice. J Cell Biol 19971381279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Mullauer L, Ogiso Y.et al Gelsolin: a candidate for suppressor of human bladder cancer. Cancer Res 1995553228–3232. [PubMed] [Google Scholar]
- 17.Lee H K, Driscoll D, Asch H.et al Downregulated gelsolin expression in hyperplastic and neoplastic lesions of the prostate. Prostate 19994014–19. [DOI] [PubMed] [Google Scholar]
- 18.Dosaka‐Akita H, Hommura F, Fujita H.et al Frequent loss of gelsolin expression in non‐small cell lung cancers of heavy smokers. Cancer Res 199858322–327. [PubMed] [Google Scholar]
- 19.Rao J, Seligson D, Visapaa H.et al Tissue microarray analysis of cytoskeletal actin‐associated biomarkers gelsolin and E‐cadherin in urothelial carcinoma. Cancer 2002951247–1257. [DOI] [PubMed] [Google Scholar]
- 20.Shieh D B, Godleski J, Herndon JE I I.et al Cell motility as a prognostic factor in Stage I non small cell lung carcinoma: the role of gelsolin expression. Cancer 19998547–57. [DOI] [PubMed] [Google Scholar]
- 21.Vimalachandran D, Greenhalf W, Thompson C.et al High nuclear S100A6 (Calcyclin) is significantly associated with poor survival in pancreatic cancer patients. Cancer Res 2005653218–3225. [DOI] [PubMed] [Google Scholar]
- 22.Van Ginkel P R G R, Walker T M, Hu D N.et al The identification and differential expression of calcium‐binding proteins associated with ocular melanoma. Biochim Biophys Acta 19981448290–297. [DOI] [PubMed] [Google Scholar]
- 23.Iacobuzio‐Donahue C A, Maitra A, Olsen M.et al Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol 20031621151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grutzmann R, Pilarsky C, Ammerpohl O.et al Gene expression profiling of microdissected pancreatic ductal carcinomas using high‐density DNA microarrays. Neoplasia 20046611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Corte V, Van Impe K, Bruyneel E.et al Increased importin‐beta‐dependent nuclear import of the actin modulating protein CapG promotes cell invasion. J Cell Sci 20041175283–5292. [DOI] [PubMed] [Google Scholar]
- 26.De Corte V, Bruyneel E, Boucherie C.et al Gelsolin‐induced epithelial cell invasion is dependent on Ras‐Rac signaling. EMBO J 2002216781–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Tan D, Asch H L.et al Prognostic significance of gelsolin expression level and variability in non‐small cell lung cancer. Lung Cancer 20044629–42. [DOI] [PubMed] [Google Scholar]
- 28.Onoda K, Yu F X, Yin H L. gCap39 is a nuclear and cytoplasmic protein. Cell Motil Cytoskeleton 199326227–238. [DOI] [PubMed] [Google Scholar]
- 29.Van Impe K, De Corte V, Eichinger L.et al The nucleo‐cytoplasmic actin‐binding protein CapG lacks a nuclear export sequence present in structurally related proteins. J Biol Chem 200327817945–17952. [DOI] [PubMed] [Google Scholar]
- 30.Onoda K, Yin H L. gCap39 is phosphorylated. Stimulation by okadaic acid and preferential association with nuclei. J Biol Chem 19932684106–4112. [PubMed] [Google Scholar]
- 31.Prasad N B, Biankin A V, Fukushima N.et al Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res 2005651619–1626. [DOI] [PubMed] [Google Scholar]