Abstract
Walleye dermal sarcoma (WDS) is a common disease of walleye fish in the United States and Canada. These proliferative lesions are present autumn through winter and regress in the spring. Walleye dermal sarcoma virus (WDSV), a retrovirus distantly related to other members of the family Retroviridae, has been etiologically linked to the development of WDS. We have reported that the D-cyclin homologue [retroviral (rv) cyclin] encoded by WDSV rescues yeast conditionally deficient for cyclin synthesis from growth arrest and that WDSV-cyclin mRNA is present in developing tumors. These data strongly suggest that the rv-cyclin plays a central role in the development of WDS. To test the ability of the WDSV rv-cyclin to induce cell proliferation, we have generated transgenic mice expressing the rv-cyclin in squamous epithelia from the bovine keratin-5 promoter. The transgenic animals were smaller than littermates, had reduced numbers of hair follicles, and transgenic females did not lactate properly. Following injury the transgenic animals developed severe squamous epithelial hyperplasia and dysplasia with ultrastructural characteristics of neoplastic squamous epithelium. Immunocytochemistry studies demonstrated that the hyperplastic epithelium stained positive for cytokeratin and were abnormally differentiated. Furthermore, the rv-cyclin protein was detected in the thickened basal cell layers of the proliferating lesions. These data are the first to indicate that the highly divergent WDSV rv-cyclin is a very potent stimulator of eukaryotic cell proliferation and to demonstrate the potential of a cyclin homologue encoded by a retrovirus to induce hyperplastic skin lesions.
Knowledge concerning mechanisms of cell cycle control and cancer has been greatly enhanced through the study of oncogenic animal viruses. Walleye dermal sarcoma (WDS) is a cutaneous tumor that was first reported on feral walleye fish (Stizostedion vitreum) from Oneida Lake in New York by Walker (1) who later reported the presence of type C retroviral particles, walleye dermal sarcoma virus (WDSV) in lesions (2). The sarcoma is neither a rare nor a geographically limited disease, as up to 27% of the walleye population of Oneida Lake have tumors in some years and the disease has been reported on walleyes throughout the higher latitudes of North America (3, 4). One of the remarkable characteristics of WDS is its seasonal induction and regression. Tumors are observed from late fall through early spring when they regress; lesions are absent in the summer months. The molecular events leading to the seasonal induction and regression of WDS are not known but likely include complex interactions of host factors, e.g., hormonally regulated changes in viral gene expression and variations in the immune response of fish at different water temperatures (5). This tenet is supported by (i) the presence of retroviral type C particles in regressing tumors but not in developing tumors; (ii) observations that the gene expression pattern of WDSV changes both quantitatively and qualitatively during the course of the disease, with low levels of multiply spliced RNA transcripts produced in developing tumors and high levels of spliced and unspliced viral transcripts produced in regressing tumors (6–8); and (iii) the experimental transmission of lesions to walleye and sauger (Stizostedion canadense) fingerlings using cell-free extracts from regressing tumors but not developing tumors (9–14). Experimental transmission studies typically have produced the benign skin tumors observed on feral walleye, but importantly, they have also resulted in the generation of invasive tumors (13, 15).
We have recently reported that the orf-A open reading frames of WDSV, and the related viruses, walleye epidermal hyperplasia viruses types 1 and 2 (WEHV1 and WEHV2) encode unique rv-cyclin homologues that are distantly related to walleye cellular cyclins and all other cellular and viral cyclins (16). Although only distantly related to known cyclins, these cyclins are most similar to cellular cyclin D1s (16, 17). This important finding suggested a possible mechanism for WDSV induction of WDS tumorigenesis analogous to cyclin D1-induced tumorigenesis in human parathyroid adenomas and lymphomas (18, 19). This hypothesis is consistent with the spliced mRNA species present in developing WDS encoding the rv-cyclin and the ability of the WDSV rv-cyclin to support cell cycle progression in cyclin-deficient yeast (Saccharomyces cerevisiae) (16). Taken together, these observations suggest that the rv-cyclins play a central role in inducing cell proliferation, leading to WDS and walleye epidermal hyperplasia.
To investigate the ability of these novel rv-cyclins to induce cellular proliferation, we have generated transgenic mice that express the WDSV rv-cyclin. Analogous to earlier experiments with human cyclin D1 expression, the WDSV rv-cyclin was expressed in squamous epithelia in transgenic mice from the bovine keratin-5 promoter (ker-5p) (20, 21). In contrast with the results of experiments using human cyclin D1, the WDSV rv-cyclin transgenic animals were reduced in body size compared with littermate control animals, presented a severe alopecia, and females failed to lactate properly. Additionally, transgenic mice developed moderate to severe squamous epithelial hyperplasia and dysplasia apparently associated with skin injury. Ultrastructurally, the skin lesions had characteristics of neoplastic squamous epithelium, including the ability to transmigrate through basal lamina. Immunocytochemistry studies demonstrated that the hyperplastic epithelium stained positive for cytokeratin, but were abnormally differentiated as indicated by aberrant expression of involucrin. Importantly, the rv-cyclin protein was detected in the proliferating lesions in thickened basal layers, indicating that the WDSV rv-cyclin protein expression directly correlated with the cell proliferation. These are the first data demonstrating the potential of a cyclin homologue encoded by a retrovirus to induce hyperplastic skin lesions, suggesting a novel system to investigate mechanisms of retroviral-mediated carcinogenesis.
Materials and Methods
Derivation of WDSV rv-Cyclin Transgenic Mice.
The plasmid pDL1, containing the complete WDSV genome, was constructed by sequentially cloning the two SmaI fragments from a λ clone into pBluescript SK− (Strategene) (22, 23). The WDSV rv-cyclin was PCR amplified to include a consensus Kozak sequence for efficient translation from the full-length plasmid clone, pDL1, and cloned into pBS K5 197 (provided by Claudio Conti, M.D. Anderson Cancer Center, Smithville, TX), using NotI and NheI to make pk5svcyc. After confirming the sequence of the rv-cyclin (Sequenase; Pharmacia Biotechnology), the fragment of pk5svcyc containing the bovine ker-5p, the β-globin intron, the WDSV rv-cyclin, and the 3′ polyadenylation region was cut from vector sequences with KpnI and gel purified using a GFX PCR DNA and gel band purification kit (Amersham Pharmacia) and adjusted 2 μg/ml for pronuclear injection (24). Transgenic animals were generated by pronuclear injection of (C57BL/6 × SJL)F2 zygotes. Mouse genomic DNA was extracted from tail clips and hybridized with the WDSV rv-cyclin PCR fragment by a dot-blot assay to identify founder animals (24). Founder animals were bred to C57BL/6 or SJL mice and transgenic animals and control nontransgenic littermates were identified by dot-blot assay. Animal studies were approved by an institutional review board and conducted according to U.S. Public Health Service guidelines for the care and use of laboratory animals.
Gross, Microscopic, and Ultrastructural Pathology Examination.
Mice were euthanized by injection with a pentobarbitol solution before necropsy. Tissues were excised and fixed in 10% buffered neutral Formalin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). In addition to sections from all major organ systems, standardized (approximately 1.0 cm2) skin sections were examined and compared from the tail, dorsal back, ear, and shoulder regions. Selected skin sections were processed for transmission electron microscopic examination by fixing tissues in 3.0% glutaraldehyde followed by postfixation in 1.3% osmium. Fixed samples were embedded in Eponate 12 resin (Ted Pella, Redding, CA) and stained with uranyl acetate and lead citrate.
Immunohistochemistry.
Skin sections (5 μm) were prepared from unstained paraffin-embedded tissue sections. Skin sections were examined for the expression of cytokeratin, involucrin, and rv-cyclin. Slides were deparaffinized and rehydrated, endogenous peroxidases were quenched (0.3% H2O2, 20 min), and antigen recovery was treated with 0.1% trypsin (10 min, 37°C) and blocked with 1.5% normal goat serum before primary antibodies were applied. Three polyclonal anti-rabbit primary antibodies: cytokeratin (1:200; Zymed), involucrin (1:20,000; Berkeley Antibody, Richmond, CA), and rv-cyclin (raised against recombinant orf-A protein; Volker Vogt and Sharon Fodor, Cornell University, 1:40,000) were used in conjunction with a biotinylated secondary antibody and an avidin–biotin peroxidase reagent (Vector Laboratories). Metal-enhanced diaminobenzidine (Pierce) was used to visualize the final antibody reaction product. The rv-cyclin antibody was treated with acetone powders of mouse skin to absorb and precipitate nonspecific binding proteins (25). Control slides were reacted as described above, but with primary and secondary antibodies each separately omitted.
Results
Generation of rv-Cyclin Transgenic Mice.
Transgenic mice were created by pronuclear injection of a construct designed to express rv-cyclin in the basal layers of stratified squamous epithelium from the 5′ regulatory fragment of the bovine keratin K5 gene (Fig. 1). We initially identified 12 founder mice harboring the keratin-5 rv-cyclin transgene (data not shown). Founder animals 10 and 110 were severely runted and died before they could be bred. The remaining founders were bred with SJL mice to produce transgenic offspring. All of the transgenic animals were estimated by the intensity of hybridization signals from Southern blots to contain 10–20 copies of the transgene (data not shown). Analysis of transgene junction fragments indicated only a single site of integration in each of the founders; subsequent breeding was also consistent with a single transgene integration site. Six of the founders produced transgenic offspring that were maintained and examined for the development of lesions (Table 1).
Figure 1.
Diagram of rv-cyclin construct (pk5svcyc) used for generation of transgenic mice. WDSV rv-cyclin was PCR amplified from the full-length plasmid clone pDL1, cut with NotI and NheI, and cloned into pBS K5 197, kindly provided by Claudio Conti (21): β-globin, β-globin + β-globin intron 2; rv-cyclin, WDSV rv-cyclin; poly(A), poly(A) additional sequences.
Table 1.
Transgenic lines and lesions
| Line | Founder | Total no. | Phenotypic lesions* |
|---|---|---|---|
| 29 | Male | 19† | H ++++, R +++, A +++ |
| 33 | Male | 5 | H ++, R ++, A ++ |
| 105 | Female | 4 | H ++, R ++, A ++ |
| 120 | Male | 4 | H ++, R ++, A +++ |
| 150 | Male | 1 | H ++++, R ++, A ++ |
| 180 | Female | 3 | H +, R +, A +++ |
Lesions were ranked as follows: hyperplasia (H): +, mild; ++, moderate with dermal extension; +++, moderate to severe with dysplasia; ++++, extensive with dysplasia, and ulceration. Runting (R): +, body wt. 85–90% of siblings; ++, body wt. 75–84% of siblings; +++, body wt. 50–75% of siblings. Alopecia (A): ++, hair loss 20–45%; +++, hair loss 45–75%. Sporadic lesions included toenail hyperkeratosis, dilated sex glands, and lymphoid hyperplasia.
F1 and F2 generations.
Gross Lesions of Transgenic Mice.
Founder mouse 29 had stunted growth and developed severe, diffuse dermal hyperplasia, including a bulbous tail growth at the point of clipping for DNA extraction. Each of the transgenic pups derived from founder 29 was characterized by a similar phenotype (Fig. 2 and Table 1). The transgenic F1 animals derived from founder 29 (n = 9) exhibited dermal hyperplasia, including similar tail lesions (likely due to tail clipping for DNA samples and bite or scratch wounds), and failed to nurse their pups due to poor lactation (females) or had improperly descended testicles (males). Additionally, all mice derived from line 29 were runted. On average (n = 7), the transgenic F1 females were ≈60% of the body weight of their nontransgenic female siblings at 20 days of age but had 90% of the body weight of their female siblings by 45 days of age (data not shown). In contrast, the average weight of the transgenic males (n = 2) was ≈50% of the average weight of their male nontransgenic siblings (n = 4) at 20 days of age and only about 65% of the body weight of their male siblings at 45 days. The phenotype persisted and segregated with the presence of the rv-cyclin transgene through the F2 generation from line 29 (Fig. 2). Founder 33 appeared to be normal and sired 35 pups, 3 of which were transgenic for rv-cyclin. Each of the transgenic pups exhibited severe proliferative and ulcerative skin lesions, prominent on the tail and ears, as well as narrowed facial features, stunted growth, and diffuse alopecia. Based, in part, on the limited production of transgenic offspring, the normal appearance of founder 33 was likely due to the mosaicism commonly observed in founder stocks of transgenic mice. The remainder of the founders (lines 105, 120, 150, and 180) were also likely to be highly mosaic. They presented with mild alopecia, but their transgenic offspring were characterized by the severe phenotype typical of lines 29 and 33 (Table 1). Some mice of these lines had keratin extensions from the toenails producing a curled appearance to their digits. Several animals had impacted secondary sex glands that produced prominently dilated cyst-like structures that prevented normal hind limb extension. Animals with ulcerated skin lesions often had moderate to severe lymph node and splenic hyperplasia.
Figure 2.
(A) Runting and alopecia exhibited by mouse 96 (F2 progeny from line 29) transgenic for rv-cylin compared with normal sibling mouse 216 (line 29). (B) Mouse 249 (F2 progeny from line 29) illustrating runting, generalized alopecia, and extensive epithelial thickening along tail. Inset (B): Epithelial proliferation of the tail of mouse 249. Note proliferative skin lesion with ulceration at site of tail cropping.
Microscopic Lesions in Transgenic Mice.
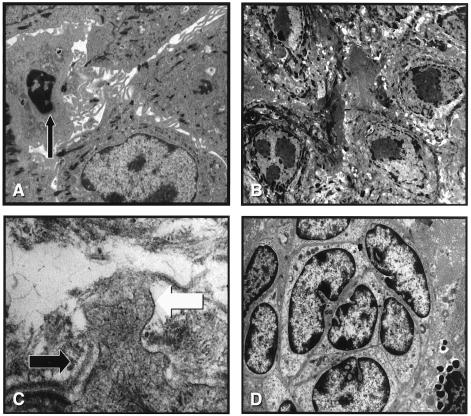
Tissue samples from the rv-cyclin transgenic mice were compared with normal control sibling mice. Transgenic mice had various degrees of squamous epithelial proliferation in standardized skin samples taken from the tail, dorsal rump, shoulder region, and ear. The lesions from the tail and ear sections characteristically contained severe epidermal hyperplasia with moderate to severe hyperkeratosis (Fig. 3). The epithelium within these regions while appearing to undergo differentiation to keratinocytes contained large cells, separated from adjoining cells (interpreted as dysregulated growth). These cells had a clear cytoplasm often with dark shrunken nuclei, suggestive of cellular apoptosis. In addition, numerous whorls of large pleomorphic epidermal cells (dysplasia: abnormal differentiation) were found throughout the most severely affected skin sections. The most severely affected regions of tail and ear skin sections also contained papillary outgrowths of keratinized epidermis (Fig. 3 A and B). The dermis was marked by mixed inflammatory infiltrates in areas of ulcerated skin sections. Projections of basal epithelia, often having atypical cell morphology and containing mitotic figures, were observed invading the dermis in numerous sections (Fig. 3 C and D). Systemic metastases from the lesions were not observed in any of the transgenic mice. Control skin sections from nontransgenic sibling mice had normal morphology. Secondary sex glands from some transgenic mice were prominently dilated and contained whorls of proteinaceous debris. Lymph nodes from mice with ulcerative skin lesions were hyperplastic and contained numerous germinal centers.
Figure 3.
Microscopic lesions (hyperplasia and dysplasia) in rv-cylin transgenic mice. (A) Cross section of pinna of ear from mouse 274 (line 150) transgenic for rv-cylin illustrating marked epithelial proliferation (hyperplasia and dysplasia). Inset (A): Normal pinna of ear from mouse 216 (line 29) (×40, H&E stain). (B) Skin section from tail of mouse 274 (line 150) with severe dysplastic epithelium with altered epithelial cell layer organization and hyperkeratosis (×200, H&E stain). (C) Skin section from tail of mouse 274 (line 150) with thickened epithelium, hyperkeratosis, and epithelial layer invasion into dermis (arrow). Inset (C): Normal skin section from tail of mouse 216 (line 29) (×100, H&E stain). (D) Higher magnification of dermal invasion by epithelium from tail of mouse 274 (line 150) illustrated in C (arrow) (×200, H&E stain).
Electron photomicrographs of skin lesions indicated a pleomorphic epithelium with prominent tonofilaments, interdigitating plasma membrane extensions, and large and irregular nucleolar morphology (Fig. 4 A and B). In some sections, cells contained small compact nuclei with dark condensed nuclear chromatin (interpreted as apoptotic cell death).
Figure 4.
Ultrastructure of skin lesions from rv-cylin transgenic mice. (A and B) Skin sections from tail lesions from mouse 70 (line 29) illustrating pleomorphic epithelium with prominent tonofilaments, interdigitating plasma membrane extensions (A), and large and irregular nucleolar morphology (B). Cells with features of apoptotic cell death (small compact nuclei with dark condensed nuclear chromatin indicated by arrow in A). (C) Basement membranes (black arrow) disrupted by cytoplasmic extensions of epithelial cells (white arrow) from tail lesions from mouse 70 (line 29). (D) Nests of epithelial cells in a whorl pattern with convoluted nuclei from tail skin lesions from mouse 274 (line 150).
Periodically, basement membranes were disrupted by cytoplasmic extensions of epithelial cells (Fig. 4C). Nests of epithelial cells in a whorl pattern with convoluted nuclei were also observed (Fig. 4D).
Immunohistochemical Staining of Skin Lesions.
Skin sections were immunohistochemically stained to identify features of proliferative epithelium and to document the expression of rv-cyclin in lesion sites. rv-Cyclin was expressed exclusively in basal epithelial cells in transgenic mice (Fig. 5A). The staining pattern for rv-cyclin correlated with the degree of squamous epithelium hyperplasia and was restricted to basal epithelial cells. Control skin sections from normal siblings were negative for rv-cyclin antibody staining (data not shown). As expected, epidermal cells from both transgenic and normal siblings stained diffusely for cytokeratin (Fig. 5B). Involucrin staining was performed to determine the degree of squamous epithelial differentiation in both normal and transgenic mice skin sections. As expected, sections containing differentiated squamous epithelial cells from both groups of animals stained positively; however, the degree of involucrin staining was more intense and extensive in deeper epithelial layers in rv-cyclin transgenic mice skin compared with controls, suggesting alteration of normal patterns of epithelial differentiation in lesions (Fig. 5 C and D).
Figure 5.
Immunohistochemical staining of hyperplastic and dysplastic epithelium from rv-cylin transgenic mice. (A) rv-Cyclin expression (arrow) in basal epithelial cells in tail skin lesion from mouse 274 (line 150) (×40). (Inset) Tail skin of normal sibling. (B) Epidermal cells from both transgenic and normal siblings (inset) illustrating diffuse cytokeratin staining (arrows in B and arrowhead in inset). (C and D) Involucrin staining with H&E counterstain illustrating atypical squamous epithelial differentiation in hyperplastic (C, ×200) and dysplastic (D, ×100) skin lesion from mouse 274 (line 150). Skin sections from normal siblings exhibited normal involucrin staining in superficial layers of epidermis (C, inset, ×200).
Discussion
The study of DNA and RNA oncogenic animal viruses has led to the identification of a wide variety of oncogenes whose characterization has been critical to efforts to define mechanisms of carcinogenesis (26–30). We generated transgenic mice that express the first cyclin homologue to be discovered in a retrovirus to test its functional significance. The transgenic animals expressing the WDSV rv-cyclin were smaller than nontransgenic littermates, had mild to severe alopecia, and developed severe hyperplastic and dysplastic skin lesions at sites of injury. Several mice developed impacted accessory sex glands and females failed to lactate properly, both lesions likely due to abnormal duct epithelial proliferation. Importantly, rv-cyclin protein was detected in the thickened germinal cell layers of lesions, implicating the viral protein directly in the cell proliferation observed in WDS.
WDS are present late fall through early spring, when lesions regress; lesions are absent during the summer months. Lethal and metastatic tumors have not been documented for WDS, suggesting that regression is complete. Experimental transmission of WDS has generally produced tumors typical of developing tumors on feral walleye, but after lengthy incubation periods some locally invasive tumors penetrating bones have been observed (13, 15). We have molecularly cloned and sequenced the retroviruses likely to be the etiological agents of WDS (WDSV) and WEHV1 and WEHV2 (6, 17, 31, 32). These viruses are only distantly related to the other retroviruses and are the only retroviruses directly linked to the development of skin tumors. Recently, WDSV, WEHV1, and WEHV2 were classified as a distinct genus among the Retroviridae, the Epsilonretroviruses (33).
The orf-A open reading frames of WDSV, WEHV1, and WEHV2 encode unique retroviral cyclin D homologues (rv-cyclins) that are distantly related to all cellular and viral cyclins (16). The WDSV rv-cyclin and human cyclin D1 share 19% and 29% overall amino acid identity and similarity within their cyclin boxes, but importantly, the most highly conserved regions are those that constitute the cyclin-dependent kinase (cdk)-binding domain sequences within the cellular cyclin D1 (16). The WDSV rv-cyclin supports cell cycle progression in cyclin-deficient yeast and low levels of small subgenomic transcripts from orf-A are found in developing WDS, suggesting a functional role for the rv-cyclin in cell proliferation (16). Interestingly, our data indicate that the expression of the rv-cyclin in squamous epithelial cells of transgenic mice produced a pervasive phenotype not observed in transgenic mice expressing human cyclin D1 (21). Transgenic mice expressing human cyclin D1 had a normal coat and were of normal size, but did have severe thymic hyperplasia, and a mild skin hyperplasia. In addition, human cyclin D1 transgenic mice apparently did not respond to injury with massive skin hyperproliferation as did our rv-cyclin transgenic mice. Although our rv-cyclin transgenic mice exhibited normal thymic and mammary gland involution with age, we cannot exclude the possibility of subtle lesions in these organ systems in neonates or lactating females.
Although it can be argued that the phenotype of the transgenic mice expressing human cyclin D1 may differ from the transgenic mice generated here, two of the founder mice expressing human cyclin D1 from the ker-5p promoter had the same genetic background of our transgenic mice (21). These data suggest that the effects of rv-cyclin and human cyclin D1 expression were not due to the genetic backgrounds of the mouse strains. Additionally, the transgene copy number harbored by individual lines of transgenic mice in both studies likely overlapped, arguing that the phenotypic differences of these animals were not due to transgene copy number. Furthermore, the cyclin genes were both positioned downstream of the ker-5p and each cyclin is in a favorable context for efficient translation, suggesting that expression of cyclin D1 and the rv-cyclin was comparable in these studies (21). However, the lack of PEST sequences in the rv-cyclin may make it a longer lived protein than cyclin D1, potentially leading to it being present at higher levels in cells than cyclin D1.
Alternatively, the rv-cyclin has properties divergent from cellular cyclins. This is certainly possible based on its divergent sequence and the properties of cyclins encoded by γ herpesviruses (34–38). The sequences of the Kaposi's sarcoma-associated herpesvirus and herpesvirus saimiri cyclin homologues, like the rv-cyclins are very divergent from but most closely related to the cellular type D cyclins. The Kaposi sarcoma-associated herpesvirus cyclin rescues human Saos-2 osteosarcoma cells from Rb-mediated cell cycle arrest, demonstrating that it, like the WDSV rv-cyclin, has the potential to mediate G1-to-S transition (36). The Kaposi sarcoma-associated herpesvirus cyclin–Cdk6 complex phosphorylates the Rb protein and histone H1 (39), is resistant to a number of proteins that negatively regulate cyclin–cdk complexes (p16Ink4a, p21Cip1, and p27Kip1) (40), and stimulates degradation of the p27Kip Cdk inhibitor by phosphorylation (41, 42). Alternatively, cyclins and cyclin-like proteins are known to serve as components of protein complexes associated with cellular gene expression, e.g., the yeast RNA polymerase II holoenzyme and the basal transcription factor TFIIH of higher eukaryotes (43, 44). Additionally, cyclin T is a cellular component of the TAR-binding complex of HIV (45). Biochemical analysis of the rv-cyclin to identify interacting cellular and viral proteins may offer a new model to assess how retroviruses influence cell cycle regulation.
Viruses associated with chronic infections or cancer must develop strategies to ensure their transmission and survival (26–30). We suggest that the WDSV rv-cyclin promotes WDSV replication by interacting with cellular proteins to stimulate cell proliferation. WDSV rv-cyclin and the rv-cyclins of WEHV1 and WEHV2 are transcribed at low levels in developing lesions (8, 17). Peak viral gene expression and viral production likely contributes to the regression of WDS and walleye epidermal hyperplasia and the release of virions into the environment during the walleye spring spawning run for horizontal transmission of these diseases (7, 17, 46). The role of the rv-cyclin in WDS regression is not known, but very high levels of cyclin expression may induce apoptosis typical of regressing WDS (S. Bloom and D.L.H., unpublished data). These observations suggest that WDSV induction of and subsequent regression of WDS is an efficient strategy to ensure viral spread.
Thirteen neoplasms of fish are tentatively attributed to the presence of retroviruses (46). These systems may yield new oncogenes or new oncogene homologues that have evolved under different selective pressures from their cellular counterparts analogous to the WDSV rv-cyclin. The characterization of these as tumor models provides the opportunity to investigate novel mechanisms of cell division, cell cycle regulation, and viral evolution. In addition, some of the piscine tumors, like WDS and WEH spontaneously regress, thereby providing important models for the study of natural tumor death.
Acknowledgments
We thank C. Conti for the kind gift of the pBS K5 197 plasmid, T. Vojt for assistance with the photomicrographs, E. Handley for electron microscopy, V. Vogt and S. Fodor for antiserum to rv-cyclin, and D. Wight, E. Holle, and D. Buckley of the Edison Biotechnology Institute for assistance in transgenic animal production. We also thank P. Green, L. LaPierre, and K. Boris-Lawrie for their insightful comments regarding this manuscript. This study was supported by Grants AI-01474 and CA-16058 from the National Institutes of Health.
Abbreviations
- WDS
walleye dermal sarcoma
- WDSV
walleye dermal sarcoma virus
- ker-5p
keratin-5 promoter
- H&E
hematoxylin and eosin
- WEHV1 and 2
walleye dermal hyperplasia viruses types 1 and 2
- rv-cylin
retroviral cyclin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110024497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110024497
References
- 1.Walker R. Natl Cancer Inst Monogr. 1969;31:195–207. [PubMed] [Google Scholar]
- 2.Walker R. Anat Rec. 1947;99:559–560. [PubMed] [Google Scholar]
- 3.Yamamoto T, Kelley R K, Nielsen O. Fish Pathol. 1985;20:361–372. [Google Scholar]
- 4.Bowser P R, Wolfe M J, Forney J L, Wooster G A. J Wildlife Dis. 1988;24:292–298. doi: 10.7589/0090-3558-24.2.292. [DOI] [PubMed] [Google Scholar]
- 5.Anders K, Yoshimizu M. Dis Aquat Org. 1994;19:215–232. [Google Scholar]
- 6.Holzschu D L, Martineau D, Fodor S K, Vogt V M, Bowser P R, Casey J W. J Virol. 1995;69:5320–5331. doi: 10.1128/jvi.69.9.5320-5331.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulet F M, Bowser P R, Casey J W. In: Retroviruses of Fish, Reptiles, and Molluscs. Levy J A, editor. Vol. 3. New York: Plenum Press; 1994. pp. 1–38. [Google Scholar]
- 8.Quackenbush S L, Holzschu D L, Bowser P R, Casey J W. Virology. 1997;237:107–112. doi: 10.1006/viro.1997.8755. [DOI] [PubMed] [Google Scholar]
- 9.Bowser P R, Martineau D, Wooster G A. J Aquat Anim Health. 1990;2:157–161. [Google Scholar]
- 10.Bowser P R, Wooster G A. J Aquat Anim Health. 1993;6:178–179. [Google Scholar]
- 11.Bowser R N, Wooster G A, Quackenbush S L, Casey R N, Casey J W. J Aquat Anim Health. 1996;8:78–81. [Google Scholar]
- 12.Bowser P R, Wooster G A, Earnest-Koons K. J Aquat Anim Health. 1997;9:274–279. [Google Scholar]
- 13.Holzschu D L, Wooster G A, Bowser P R. Dis Aquat Org. 1998;32:9–14. doi: 10.3354/dao032009. [DOI] [PubMed] [Google Scholar]
- 14.Martineau D, Bowser P R, Wooster G A, Armstrong G A. Veter Pathol. 1990;27:230–234. doi: 10.1177/030098589002700403. [DOI] [PubMed] [Google Scholar]
- 15.Earnest-Koons K, Wooster G A, Bowser P A. Dis Aquat Org. 1996;24:227–232. [Google Scholar]
- 16.LaPierre L A, Casey J W, Holzschu D L. J Virol. 1998;72:8765–8771. doi: 10.1128/jvi.72.11.8765-8771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaPierre L A, Holzschu D H, Bowser P R, Casey J W. J Virol. 1999;73:9393–9403. doi: 10.1128/jvi.73.11.9393-9403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold A. J Invest Med. 1995;43:543–549. [PubMed] [Google Scholar]
- 19.Motokura T, Bloom T, Kim H G, Juppner H, Ruderman J V, Kronenberg H M, Arnold A. Nature (London) 1991;350:512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez A, Bravo A, Jorcano J C, Vidal M. Differentiation. 1994;58:53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- 21.Robles A I, Larcher F, Whalin R B, Murillas R, Richie E, Gimenez Conti I B, Jorcano J L, Conti C J. Proc Natl Acad Sci USA. 1996;93:7634–7638. doi: 10.1073/pnas.93.15.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martineau D, Renshaw R, Williams J R, Casey J W, Bowser P R. Dis Aquat Org. 1991;10:153–158. [Google Scholar]
- 23.Martineau D, Bowser P R, Renshaw R R, Casey J W. J Virol. 1992;66:596–599. doi: 10.1128/jvi.66.1.596-599.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogan B, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 25.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY.: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 26.Vogt P K. In: Retroviruses. Coffin J M, Hughes S M, Varmus H E, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1997. pp. 1–26. [PubMed] [Google Scholar]
- 27.Hunter T. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 28.Nevins J R, Vogt P K. In: Fields Virology. Fields B N, Knipe D M, Howley P M, Channock J L, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Philadelphia, PA: Lippincott-Raven; 1996. pp. 301–304. [Google Scholar]
- 29.Scherr C T. Science. 1996;274:1674–1677. [Google Scholar]
- 30.Rasheed S. In: Retroviruses and Oncogenes. Levy J A, editor. Vol. 4. New York: Plenum Press; 1995. pp. 293–408. [Google Scholar]
- 31.Holzschu D L, Quackenbush S L, Bowser P R, Casey J W. Leukemia Suppl. 1997;3:172–175. [PubMed] [Google Scholar]
- 32.LaPierre L A, Holzschu D L, Wooster G A, Bowser P R, Casey J W. J Virol. 1998;72:3484–3490. doi: 10.1128/jvi.72.4.3484-3490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy F A. In: Veterinary Virology. Murphy F A, Gibbs E P J, Horzinek M C, Studdert M J, editors. New York: Academic Press; 1999. pp. 23–42. [Google Scholar]
- 34.Albrecht J-C, Nicholas J, Biller D, Cameron K R, Biesinge B, Newman C, Wittmann S, Craxton M A, Coleman H, Fleckenstein B, Honess R W. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, M. K D. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang Y, Moore P S, Talbot S J, Boshoff C H, Zarkowska T, Godden H, Paterson H, Weiss R A, Mittnacht S. Nature (London) 1996;382:410–411. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 37.Jung J U, Stager M, Desrosiers R C. Mol Cell Biol. 1994;14:7235–7244. doi: 10.1128/mcb.14.11.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M, Lee H, Yoon D W, Albrecht J C, Fleckenstein B, Neipel F, Jung J U. J Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godden-Kent D, Talbot S J, Boshoff C, Chang Y, Moore P, Weiss R A, Mittnacht S. J Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanton C, Mann D J, Fleckenstein B, Neipel F, Peters G, Jones N. Nature (London) 1997;390:184–187. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 41.Ellis M, Chew Y P, Fallis L, Freddersdorf S, Boshoff C, Weiss R A, Lu X, Mittnacht S. EMBO J. 1999;18:644–653. doi: 10.1093/emboj/18.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann D J, Child E S, Swanton S, Laman H, Jones N. EMBO J. 1999;18:654–663. doi: 10.1093/emboj/18.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao S M, J, Z, Jeffery D A, Koleske A J, Thompson C M, M, C D, Viijoen M, Vuuren H J J V, Young R A. Nature (London) 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 44.Makela T P, Parvin J D, Kim J, Huber L J, Sharp P A, Weinberg R A. Proc Natl Acad Sci USA. 1995;92:5174–5178. doi: 10.1073/pnas.92.11.5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei P, Garber M E, Fang S-M, Fischer W H, Jones K A. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 46.Getchell R G, Casey J W, Bowser P R. J Aquat Anim Health. 1998;10:191–201. [Google Scholar]