Abstract
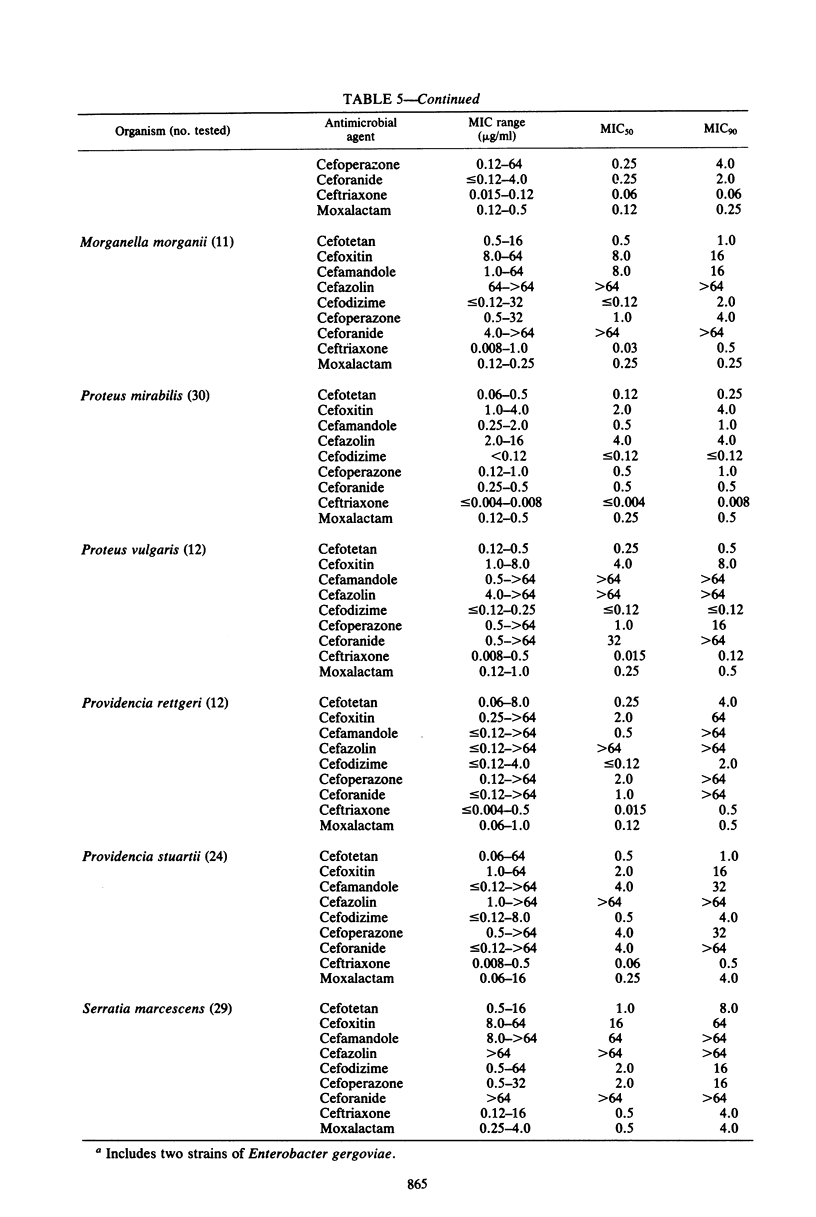
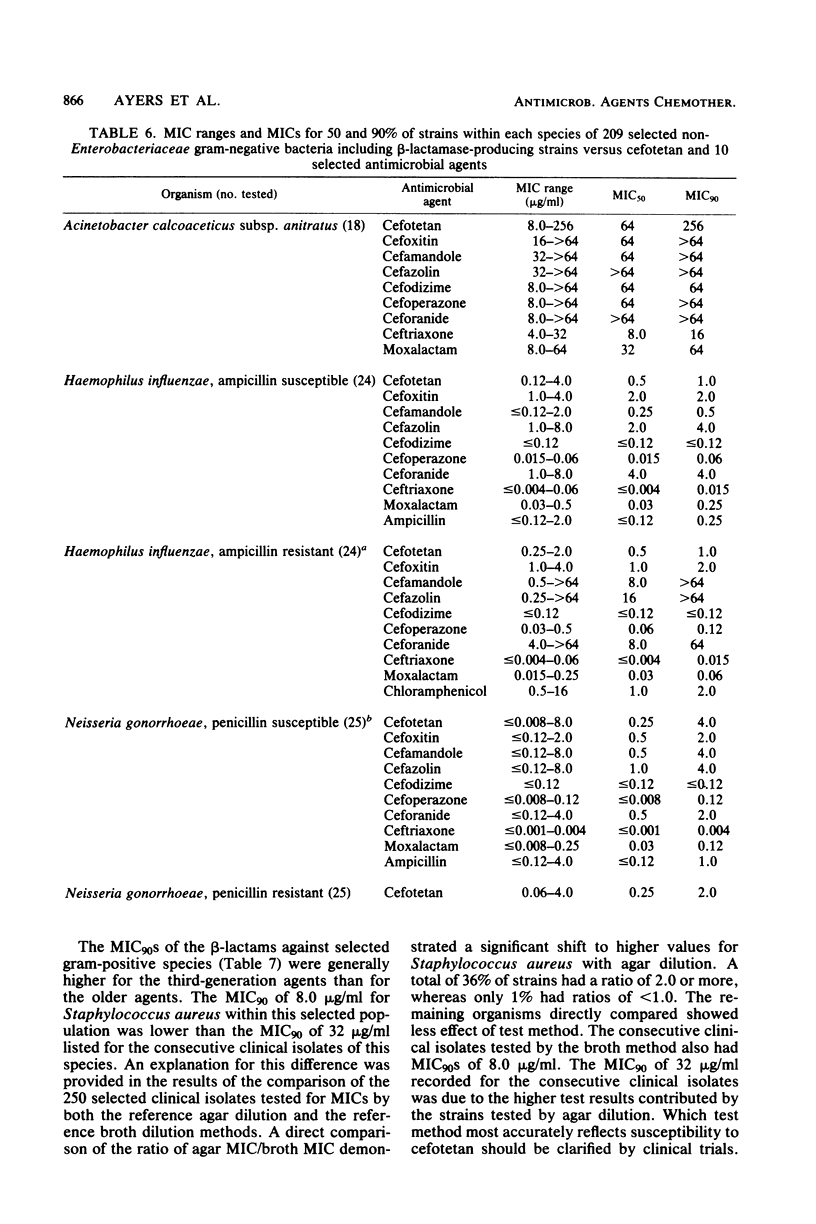
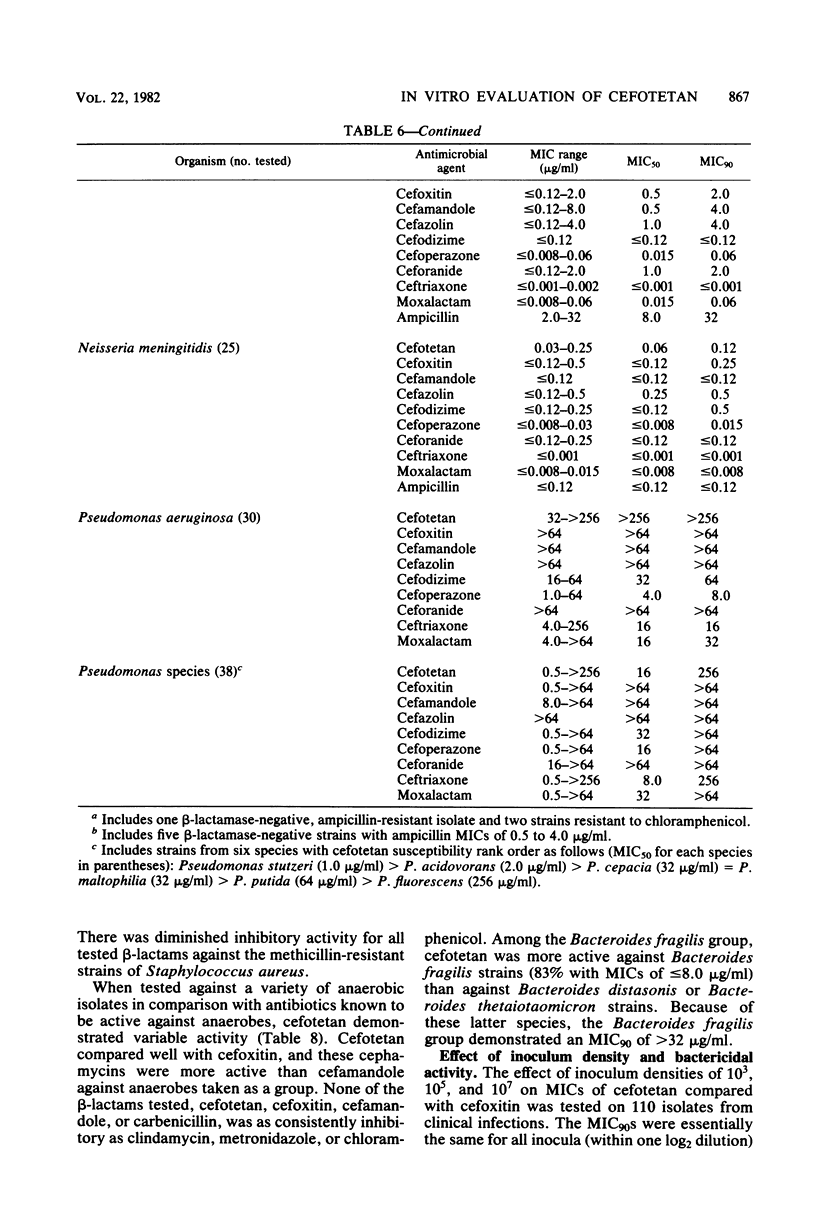
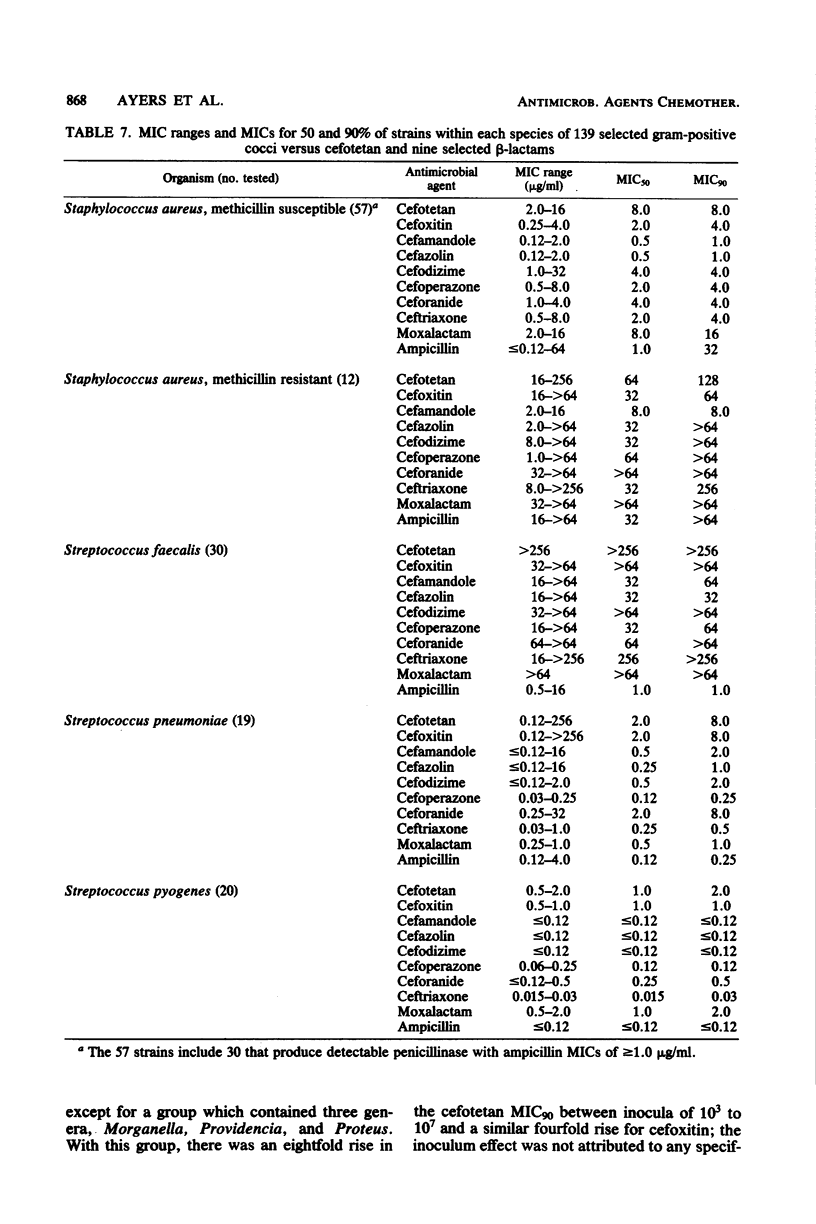
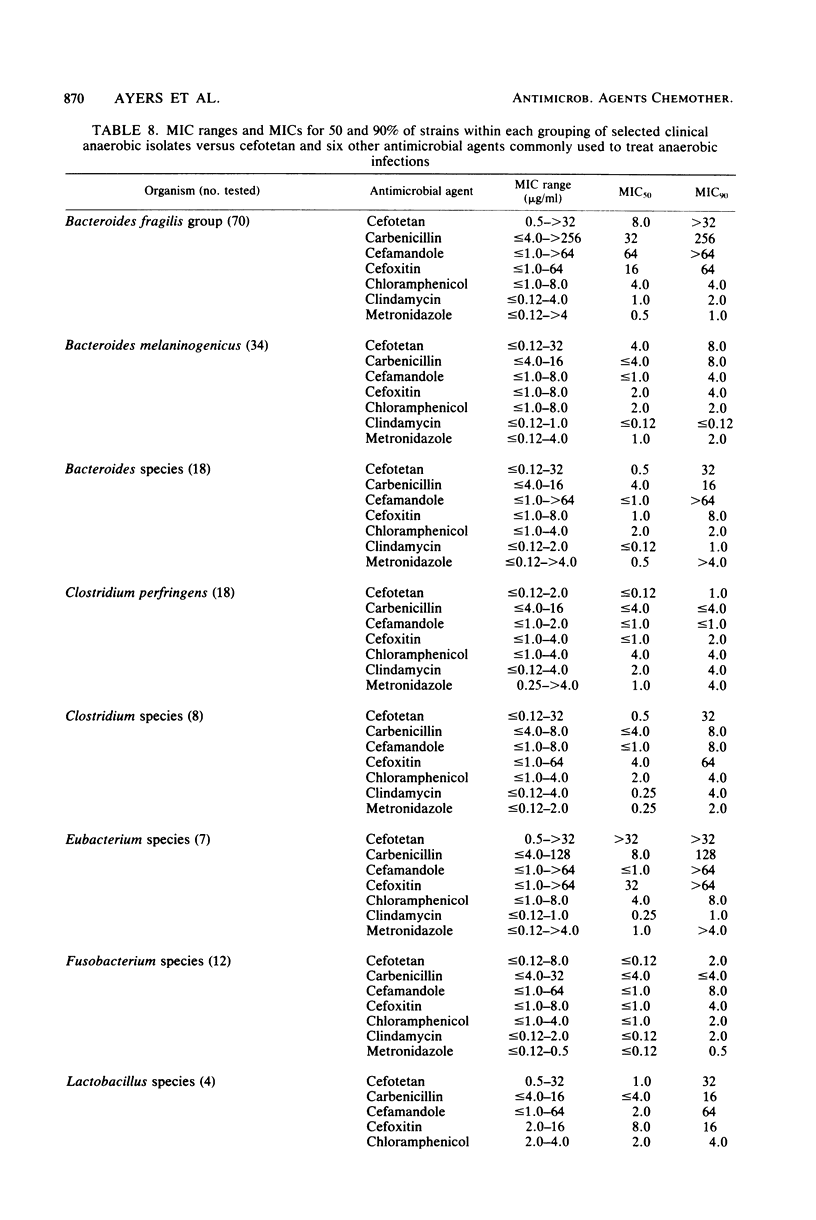
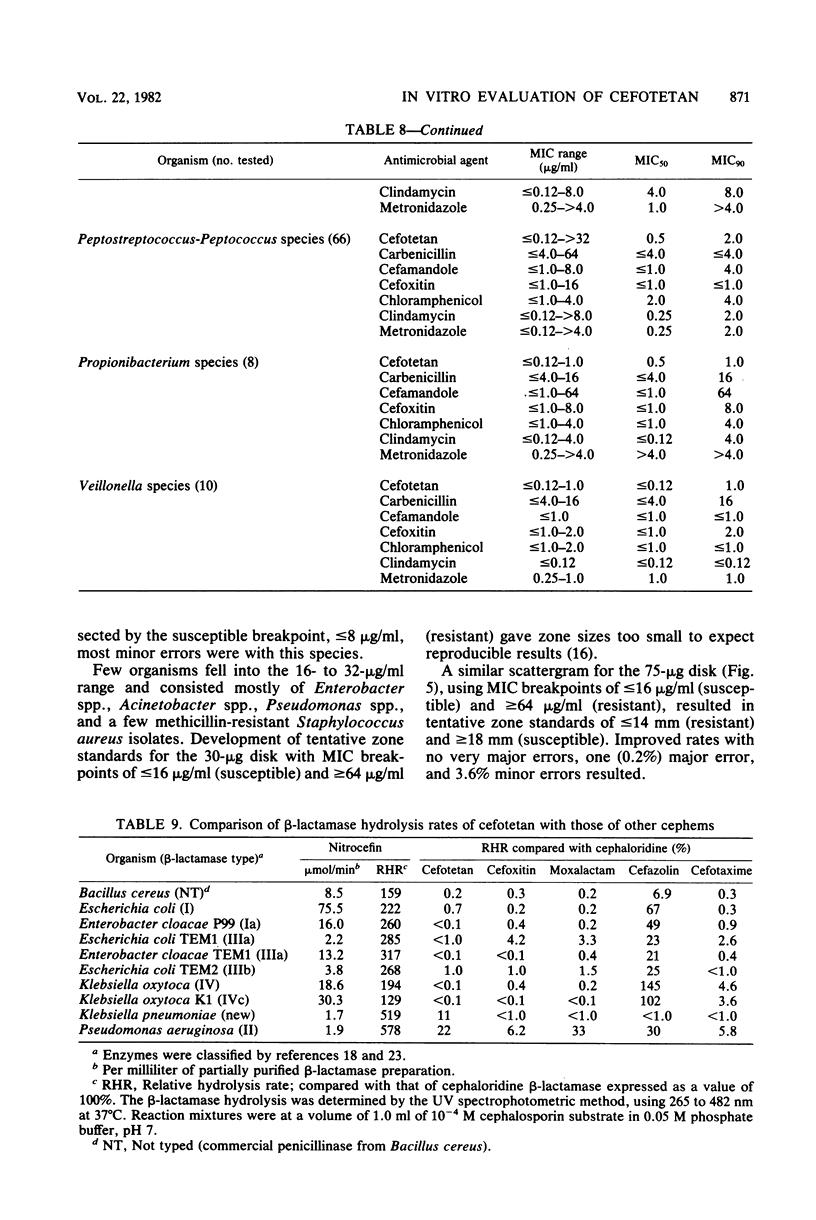
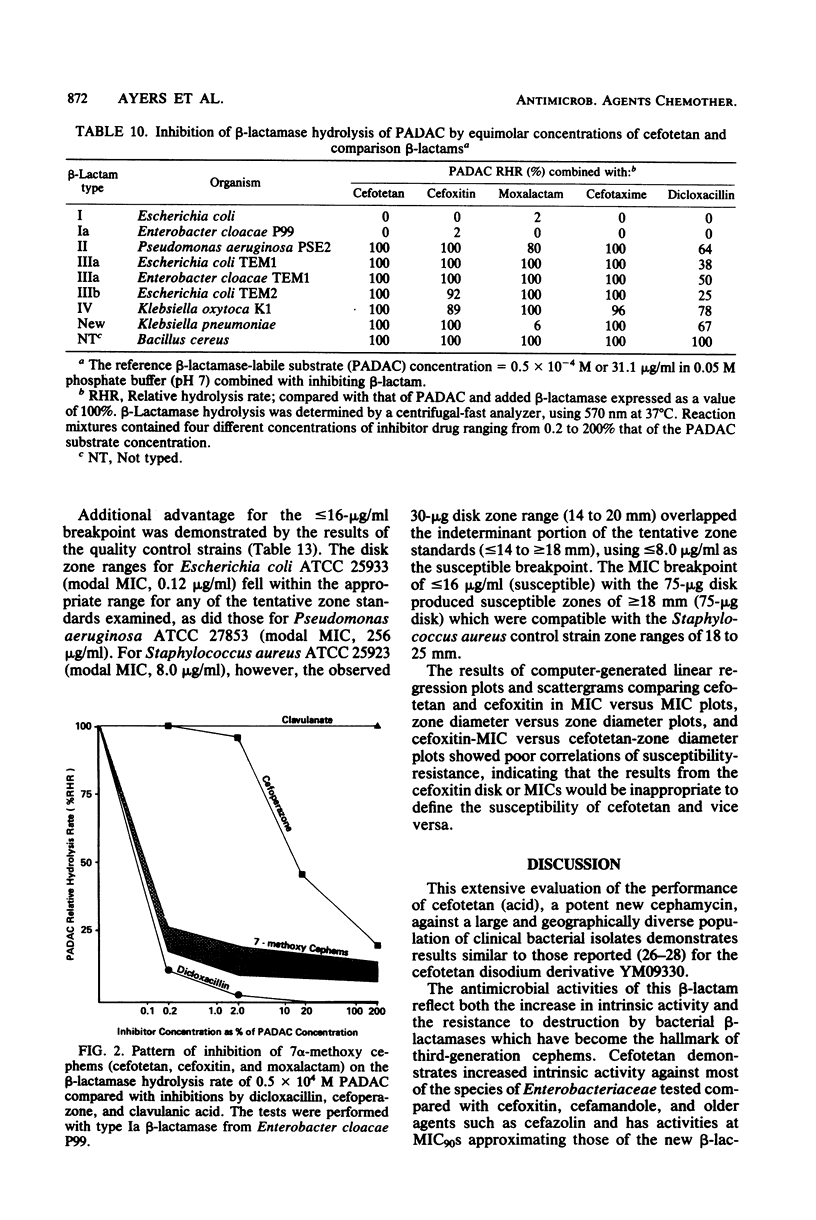
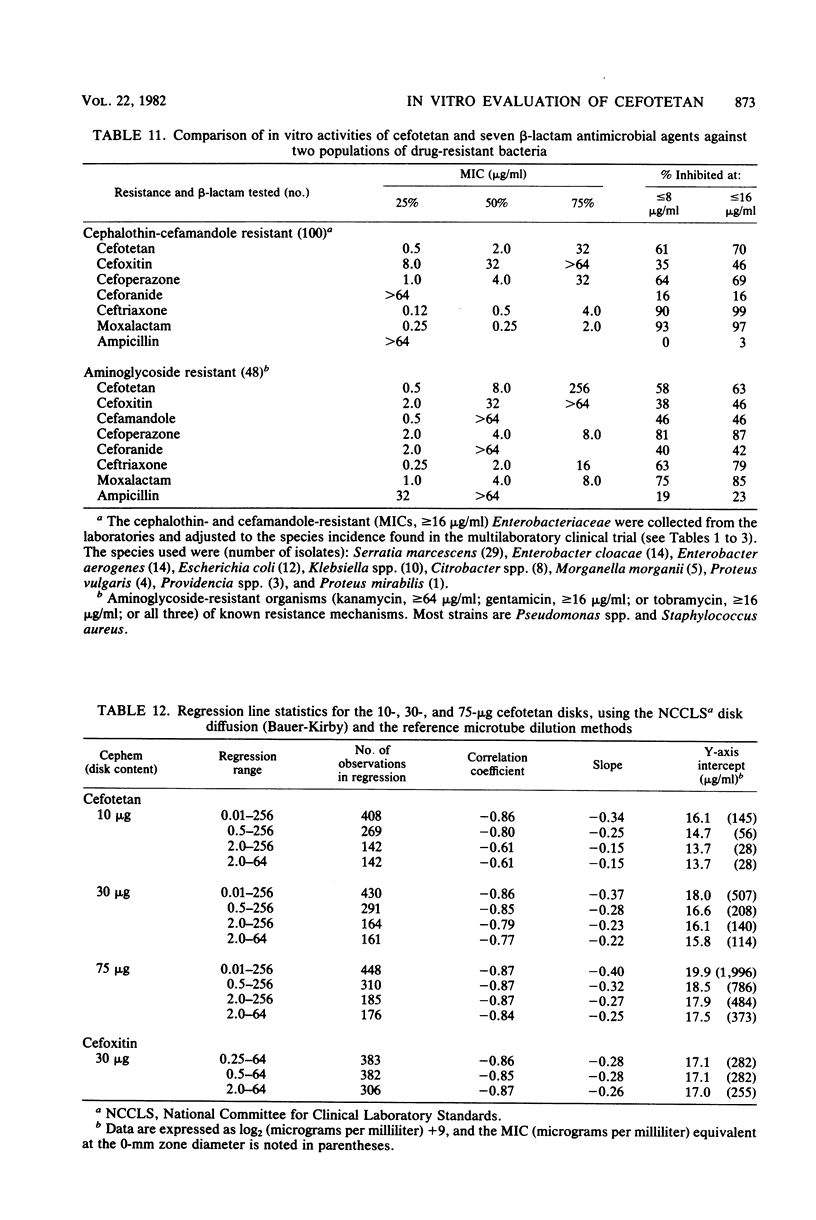
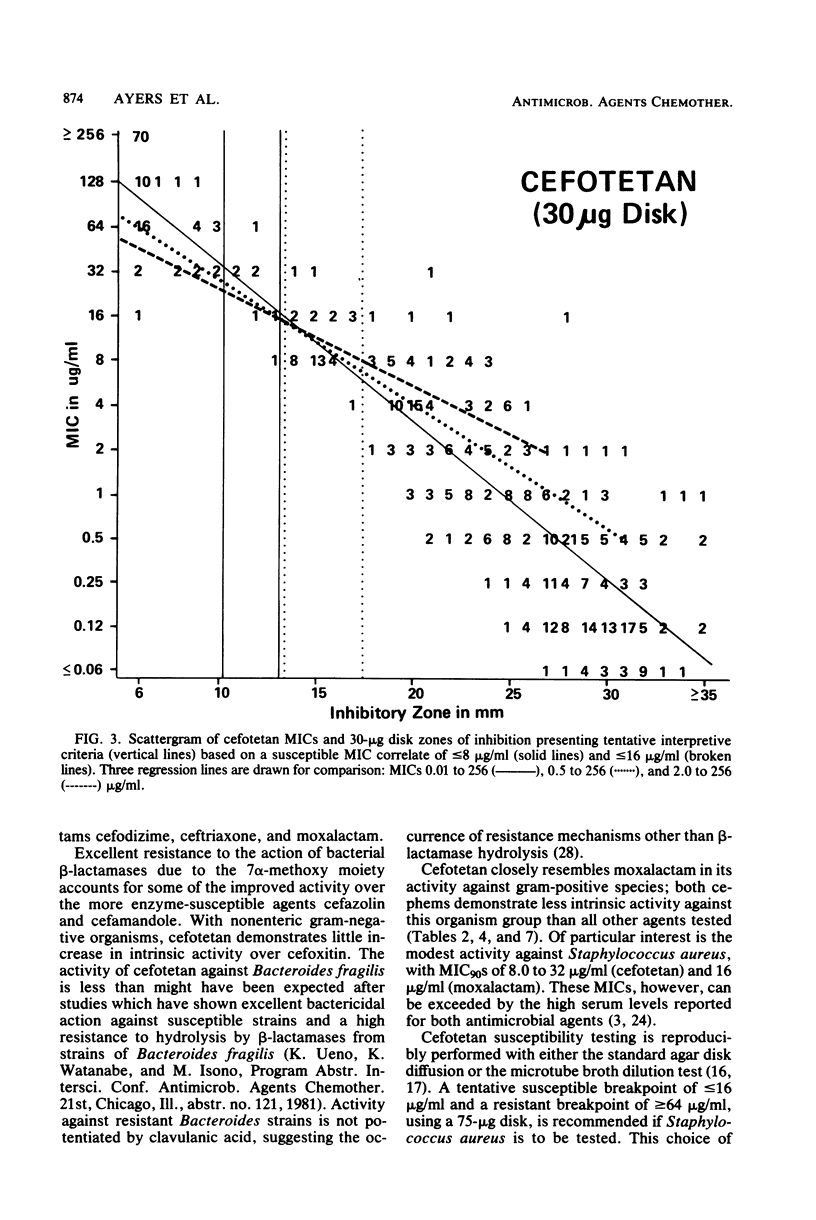
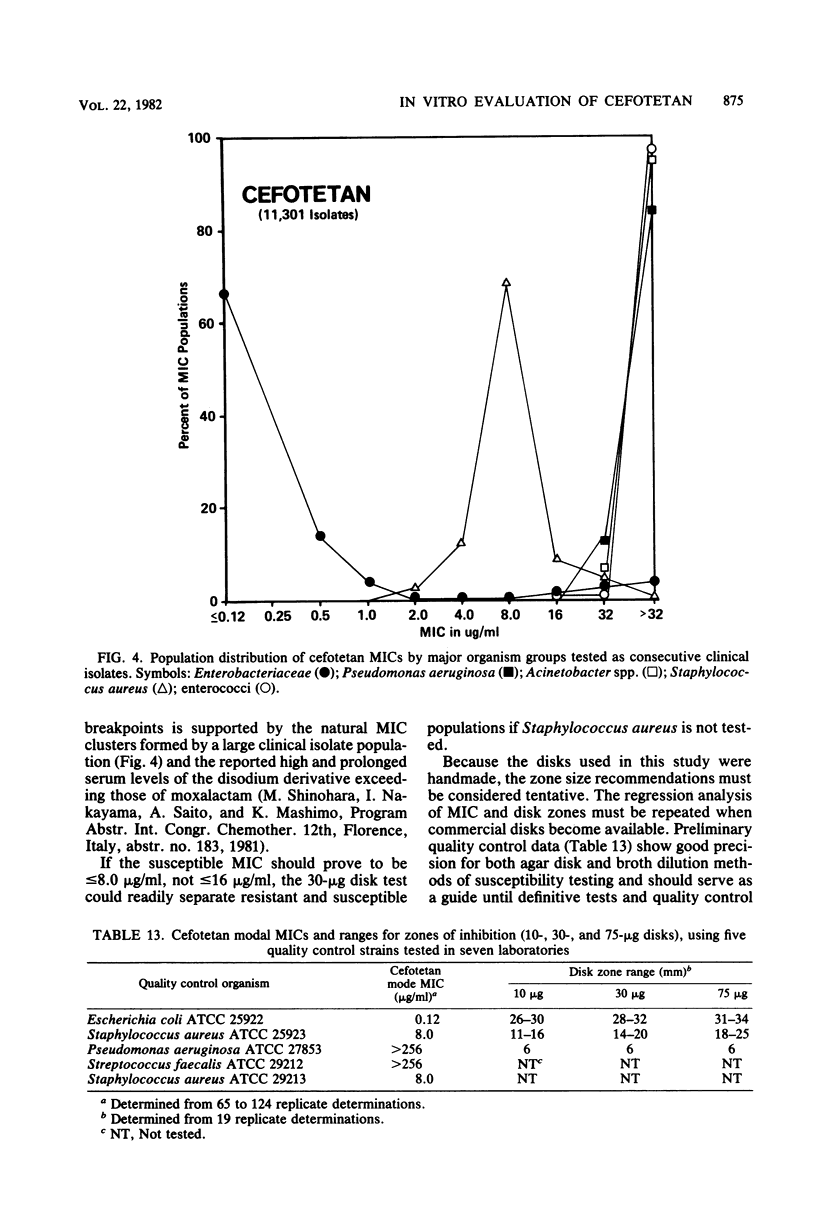
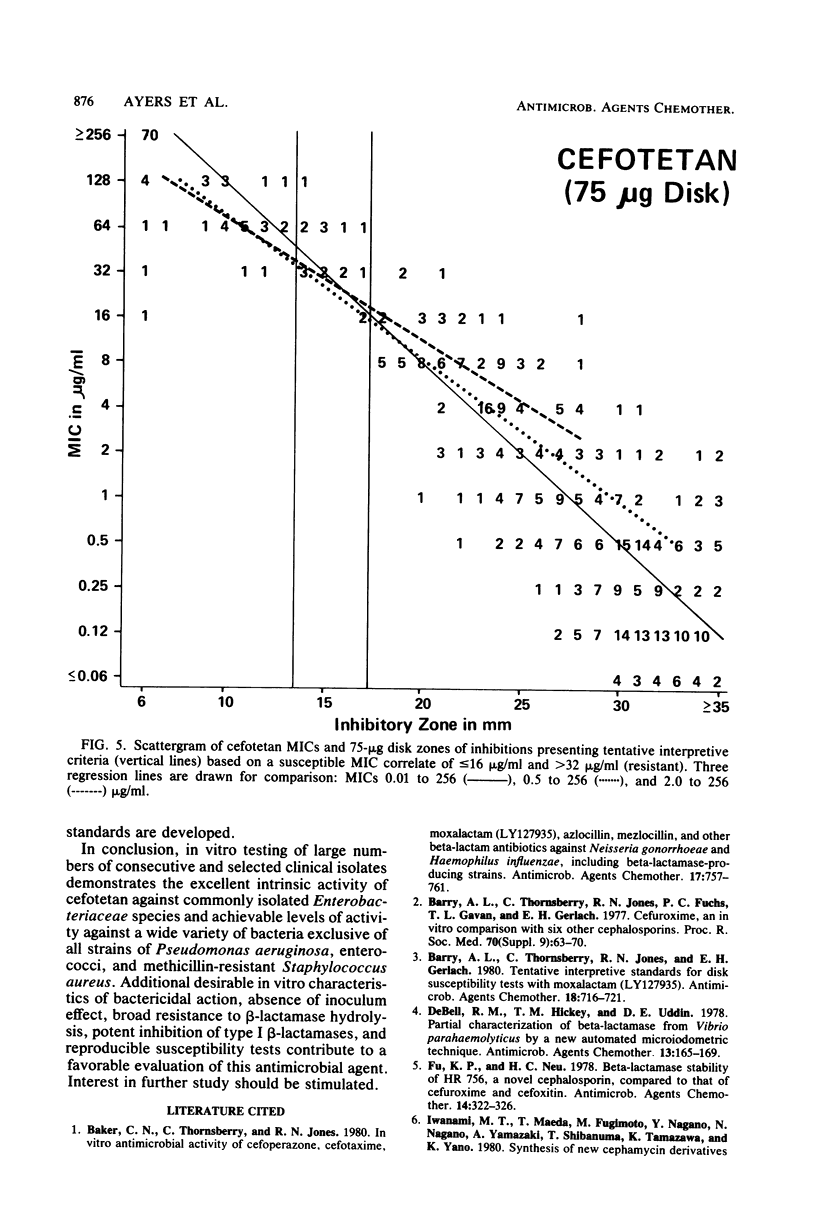
Cefotetan is a new, potent, 7 alpha-methoxy cephalosporin (cephamycin). The in vitro activity of cefotetan tested in a multiphasic, collaborative study against 12,260 consecutive clinical isolates and 448 selected isolates showed 93% of Enterobacteriaceae, 90% of methicillin-susceptible Staphylococcus aureus (broth dilution), 83% of Bacteroides fragilis, and 72% of non-enterococcal streptococci to be inhibited by less than or equal to 8 micrograms/ml. Beta-Lactamase-producing and -nonproducing Haemophilus influenzae strains were inhibited by less than or equal to 1.0 micrograms/ml. Cefotetan's inhibitory spectrum paralleled those of the newest generation of cephems and exceeded those of cefoxitin and cefamandole. No useful activity was present against Streptococcus faecalis or Pseudomonas aeruginosa. Cefotetan was bactericidal without significant inoculum effect and was highly resistant to hydrolysis by Richmond-Sykes types I, III, and IV beta-lactamases. Hydrolysis of the chromogenic cephalosporin PADAC (pyridine-2-azo-p-dimethylaniline cephalosporin) by type I beta-lactamases was markedly inhibited by concentrations of cefotetan similar to those of the potent inhibitor dicloxacillin. Analysis of agar disk diffusion for several disk potencies and broth dilution susceptibility tests by regression and error rate-bounding methods produced preliminary tentative zone standards (30-micrograms disk, using minimal inhibitory concentration breakpoints of less than or equal to 8 micrograms/ml susceptible and greater than 32 micrograms/ml resistant, or 75-micrograms disk, using minimal inhibitory concentration breakpoints of less than or equal to 16 micrograms/ml susceptible and greater than or equal to 64 micrograms/ml resistant) of greater than or equal to 18 mm susceptible, less than or equal to 14 mm resistant, and 15 to 17 mm indeterminate. Staphylococcus aureus testing with the 30-micrograms disk is not recommended.
Full text
PDF
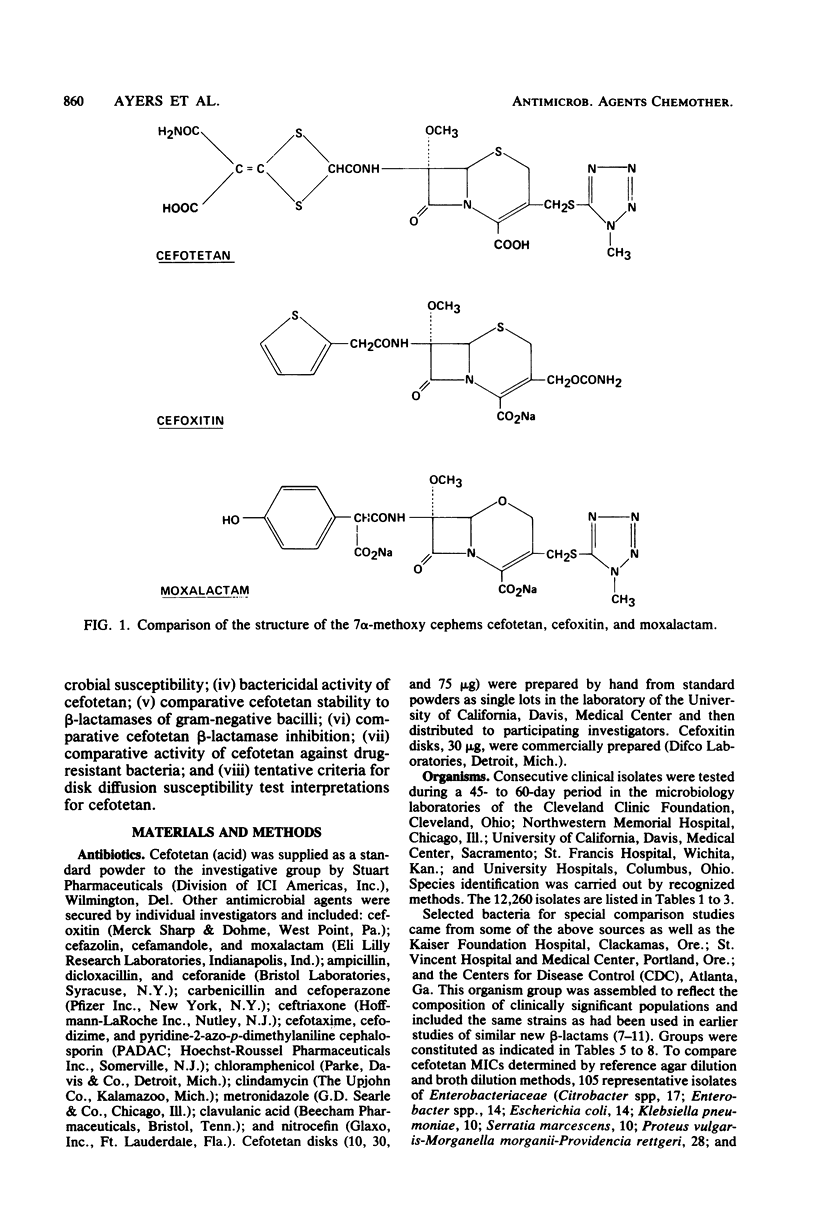
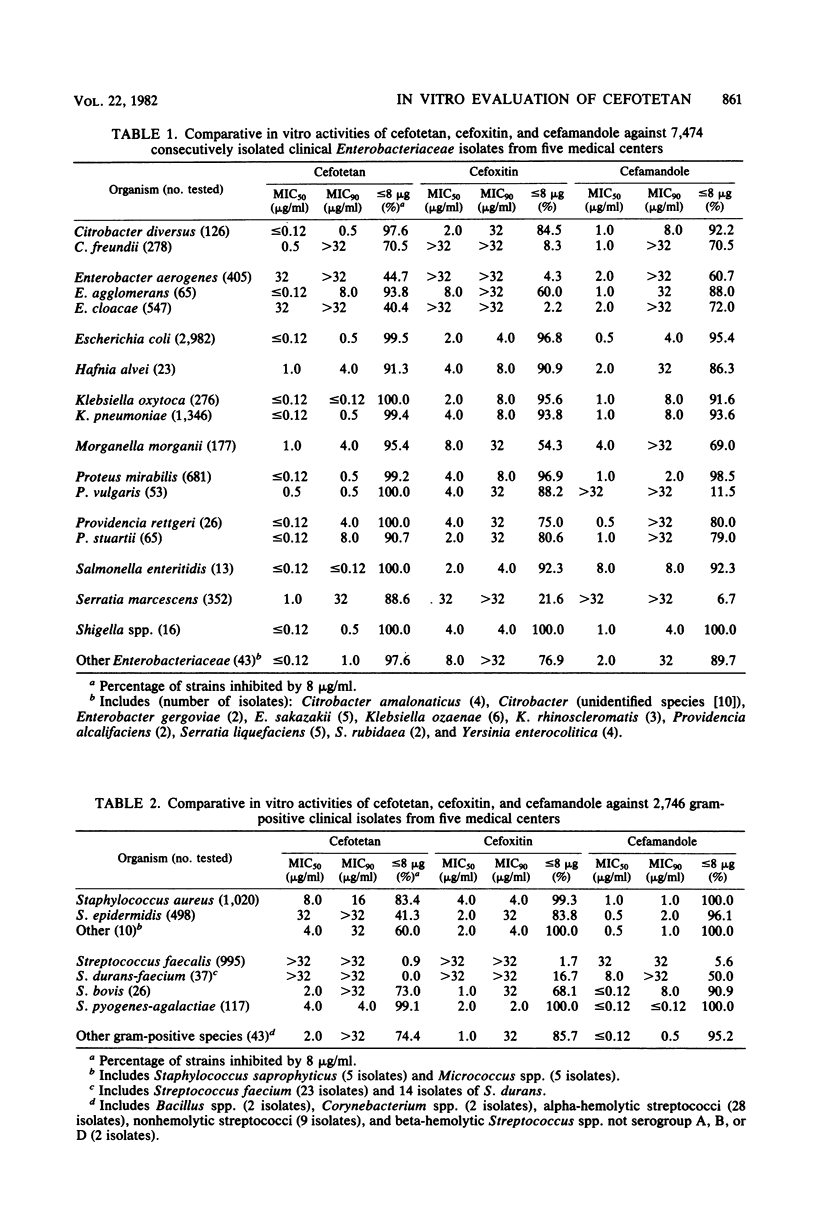
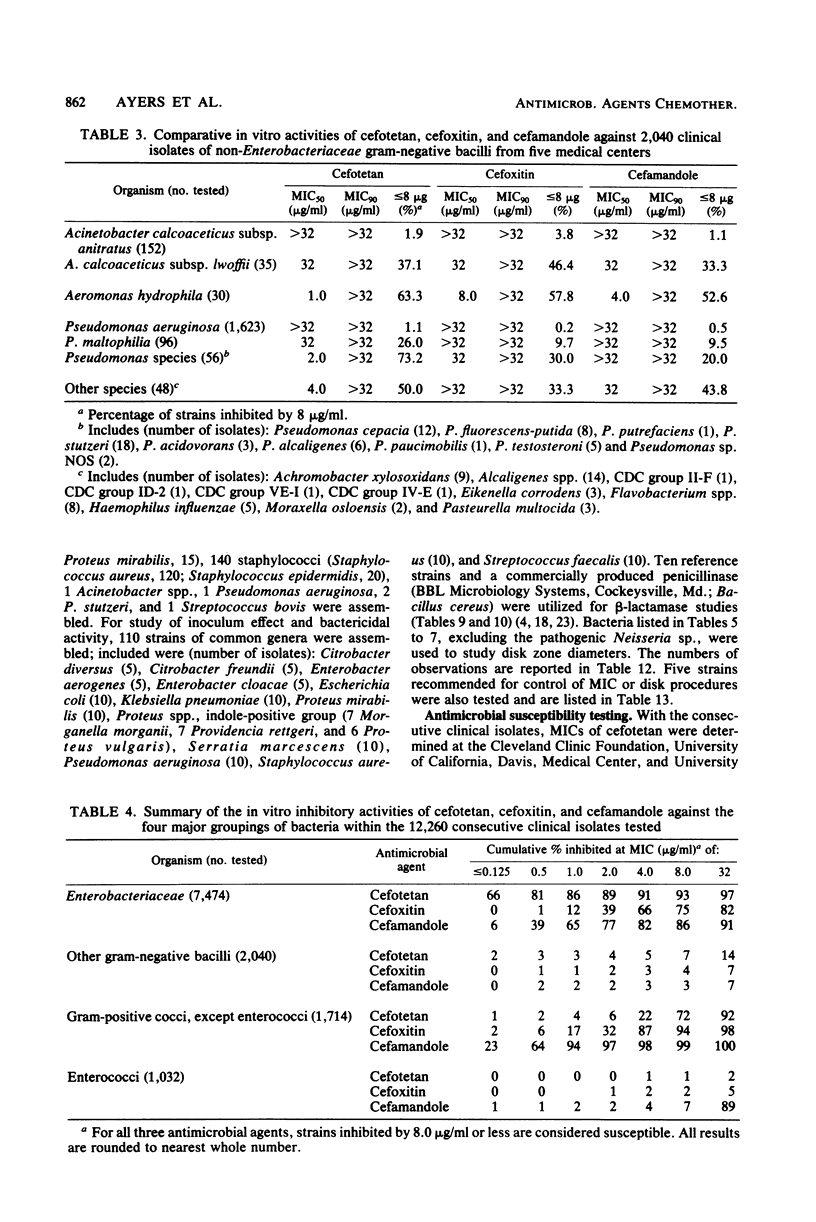

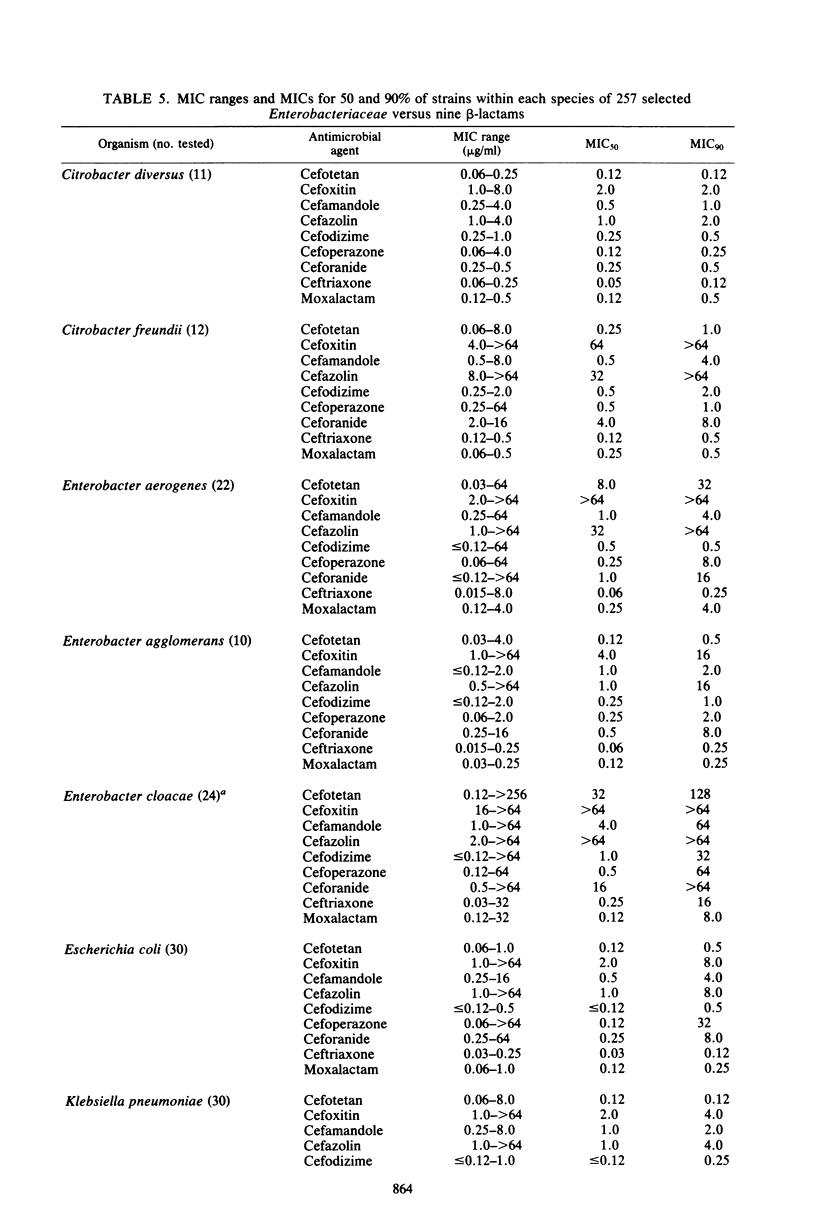


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Thornsberry C., Jones R. N. In vitro antimicrobial activity of cefoperazone, cefotaxime, moxalactam (LY127935), azlocillin, mezlocillin, and other beta-lactam antibiotics against Neisseria gonorrhoeae and Haemophilus influenzae, including beta-lactamase-producing strains. Antimicrob Agents Chemother. 1980 Apr;17(4):757–761. doi: 10.1128/aac.17.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N., Fuchs P. C., Gavan T. L., Gerlach E. H. Cefuroxime, an in vitro Comparison with Six Other Cephalosporins. Proc R Soc Med. 1977;70(Suppl 9):63–71. doi: 10.1177/00359157770700S912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N., Gerlach E. H. Tentative interpretive standards for disk susceptibility tests with moxalactam (LY127935). Antimicrob Agents Chemother. 1980 Nov;18(5):716–721. doi: 10.1128/aac.18.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBell R. M., Hickey T. M., Uddin D. E. Partial characterization of a beta-lactamase from Vibrio parahaemolyticus by a new automated microiodometric technique. Antimicrob Agents Chemother. 1978 Feb;13(2):165–169. doi: 10.1128/aac.13.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. beta-lactamase stability of HR 756, a novel cephalosporin, compared to that of cefuroxime and cefoxitin. Antimicrob Agents Chemother. 1978 Sep;14(3):322–326. doi: 10.1128/aac.14.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C., Wilson H. W. In vitro antimicrobial activity evaluation of cefodizime (HR221), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1981 Dec;20(6):760–768. doi: 10.1128/aac.20.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Barry A. L., Gavan T. L., Sommers H. M., Gerlach E. H. Cefoperazone (T-1551), a new semisynthetic cephalosporin: comparison with cephalothin and gentamicin. Antimicrob Agents Chemother. 1980 Apr;17(4):743–749. doi: 10.1128/aac.17.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Gavan T. L., Gerlach E. H., Barry A. L., Thornsberry C. Cefuroxime, a new parenteral cephalosporin: collaborative in vitro susceptibility comparison with cephalothin against 5,887 clinical bacterial isolates. Antimicrob Agents Chemother. 1977 Jul;12(1):47–50. doi: 10.1128/aac.12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Wilson H. W., Novick W. J., Jr In vitro evaluation of pyridine-2-azo-p-dimethylaniline cephalosporin, a new diagnostic chromogenic reagent, and comparison with nitrocefin, cephacetrile, and other beta-lactam compounds. J Clin Microbiol. 1982 Apr;15(4):677–683. doi: 10.1128/jcm.15.4.677-683.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Crawford S. A., Alexander G. A. Comparison of moxalactam (LY127935) and cefotaxime against anaerobic bacteria. Antimicrob Agents Chemother. 1980 May;17(5):901–904. doi: 10.1128/aac.17.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya M., Kikuchi Y., Tachibana A., Yano K. Pharmacokinetics of new broad-spectrum cephamycin, YM09330, parenterally administered to various experimental animals. Antimicrob Agents Chemother. 1981 Aug;20(2):176–183. doi: 10.1128/aac.20.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotimi V. O., Turk D. C. Transferable multiple antibiotic resistance in Haemophilus influenzae. J Antimicrob Chemother. 1981 Sep;8(3):187–192. doi: 10.1093/jac/8.3.187. [DOI] [PubMed] [Google Scholar]
- Sutter V. L., Barry A. L., Wilkins T. D., Zabransky R. J. Collaborative evaluation of a proposed reference dilution method of susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1979 Oct;16(4):495–502. doi: 10.1128/aac.16.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., Baker C. N., Jones R. N. In vitro antimicrobial activity of piperacillin and seven other beta-lactam antibiotics against Neisseria gonorrhoeae and Haemophilus influenzae, including beta-lactamase producing strains. J Antimicrob Chemother. 1979 Mar;5(2):137–142. doi: 10.1093/jac/5.2.137. [DOI] [PubMed] [Google Scholar]
- Toda M., Ikeuchi T., Tajima M., Inoue M., Mitsuhashi S. Comparative inhibition of beta-lactamases by cephamycin antibiotics. J Antibiot (Tokyo) 1981 Jan;34(1):114–117. doi: 10.7164/antibiotics.34.114. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Hancox J. In vitro activity of cefotetan, a new cephamycin derivative, compared with that of other beta-lactam compounds. Antimicrob Agents Chemother. 1982 Mar;21(3):486–491. doi: 10.1128/aac.21.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]



