Abstract
Background
Abdominal surgery results in a molecular and cellular inflammatory response in the intestine, leading to postoperative ileus. It was hypothesised that resident macrophages within the intestinal muscularis have an important role in this local inflammation.
Aims
To investigate whether chemical or genetic depletion of resident muscularis macrophages would lead to a reduction in the local inflammation and smooth‐muscle dysfunction.
Methods
Two rodent models were used to deplete and inactivate macrophages: (1) a rat model in which resident macrophages were depleted by chlodronate liposomes; (2) a model of mice with osteopetrosis mice, completely lacking the resident muscularis macrophages, used as an additional genetic approach. Animals with normal or altered intestinal macrophages underwent surgical intestinal manipulation. The inflammatory response was investigated by quantitative reverse transcriptase‐polymerase chain reaction for mRNA of MIP‐1α, interleukin (IL)1β, IL6, intracellular adhesion molecule 1 (ICAM‐1) and monocyte chemotractant protein 1 (MCP)‐1 in the isolated small bowel muscularis. In addition, muscularis whole mounts were used for histochemical and immunohistochemical analysis to quantify leucocyte infiltration and detect cytokine expression. Subsequently, in vitro muscle contractility and in vivo gastrointestinal transit were measured.
Results
Both models resulted in markedly decreased expression of MIP‐1α, IL1β, IL6, ICAM‐1 and MCP‐1 after manipulation compared with controls. In addition to this decrease in inflammatory mediators, recruitment of leucocytes into the muscularis was also diminished. Macrophage‐altered animals had near normal in vitro jejunal circular muscle function and gastrointestinal transit despite surgical manipulation.
Conclusions
Resident intestinal muscularis macrophages are initially involved in inflammatory responses resulting in postoperative ileus. Depletion and inactivation of the muscularis macrophage network prevents postoperative ileus.
Postoperative ileus remains a major clinical problem after surgical trauma of the abdominal cavity and other types of surgery. The prolonged dysfunction of bowel motility lasts for days, with accumulation of secretions and gas, abdominal distension, nausea, vomiting, need for gastric tubes, discomfort, pain and respiratory abnormalities including aspiration of gastrointestinal fluids. The cost of this postoperative complication has been estimated at US$1 billion/year in the US.1
Various pathophysiological mechanisms have been investigated and the pathophysiology seems to be multifactorial.2,3,4 One of the underlying factors is local inflammation of the bowel wall as a consequence of the intestinal manipulation. Our group has focused on the activation of local molecular and cellular immune mechanisms. In brief, we have shown that intestinal manipulation activates an inflammatory cascade that results in the extravasation of leucocytes into the intestinal muscularis, which has a duration of several days.5,6 Subsequent to this local muscularis inflammatory response, gastrointestinal motility is diminished, resulting in ileus.7,8
Macrophages in the intestinal tract are studied mainly at the mucosal level. Their role in the gut‐associated lymphatic tissue has been studied in numerous articles.9 Macrophages are known to secrete inflammatory mediators and kill microbial pathogens on activation.10,11 The existence of macrophages in the intestinal smooth muscle has been mentioned first by Taxi in 1965.12 Their anatomical structure and presence was further studied by Mikkelsen et al.13
Recent findings in rodent models and investigations on the human muscularis externa showed that the activation of these resident macrophages, that normally lay inactive as a hidden line of defence in the intestinal muscle layer, may have an important role in the inflammatory reaction leading to ileus.7,14,15,16 The aim of this study was to investigate whether chemical or genetic depletion of resident muscularis macrophages would lead to a reduction in the local inflammation and smooth‐muscle dysfunction. For this purpose, a rat model with intravenous application of liposome‐encapsulated dichloromethylene diphosphonate (clodronate (Cl2MDP)) was used to induce apoptosis of macrophages.17 By this application, a selective depletion of macrophages could be attained in vivo in various models.17,18,19 Additionally, inactivation of macrophages was achieved by injection of gadolinium chloride (GdCl3), a selective inhibitor of stretch‐activated ion channels.20 In a second model, a mouse with naturally occurring osteopetrosis served as a tissue‐specific macrophage‐deficient model. Mikkelsen et al showed previously that these mice, carrying a mutation in the colony stimulating factor‐1 gene,21 completely lack intestinal muscularis macrophages and have a diminished number of mucosa macrophages.22 The animals in both models underwent a standardised surgical manipulation of the entire small bowel, which has been shown to cause postoperative ileus comparable to clinical practice.6 The local muscularis inflammatory response was quantified by analysis of macrophage activation (MIP‐1α), inflammatory mediators interleukin (IL)1β and IL6, chemokines (MCP‐1), adhesion molecules (ICAM‐1) and leucocyte infiltration. The subsequent functional sequelae of smooth‐muscle inhibition and intestinal dysmotility were investigated by in vitro muscle contractility and in vivo gastrointestinal transit studies. Our findings show that intestinal muscularis macrophages are initially involved in the postoperative local inflammatory response. Therefore, abrogation of macrophage function diminished the molecular and cellular inflammatory response, which resulted in a prevention of the postsurgical muscle dysfunction and improved postoperative gastrointestinal motility.
Methods
Animals
Male Sprague–Dawley rats (220–280 g body weight) were obtained from Harlan–Winkelmann (Borchen, Germany). Homozygotic mice with osteopetrosis (op‐/‐) were bred from heterozygotic op+/‐ breeding pairs, obtained from Charles River (Sulzfeld, Germany), and maintained with self‐made jelly food under pathogen‐free conditions. All experiments were performed in accordance with the federal law regarding the protection of animals. The principles of laboratory animal care were followed.
Operative procedures
The small bowel of the animals was subjected to a standardised, surgical manipulation as described previously.23 In brief, after anaesthesia a mid‐line abdominal incision was made into the peritoneal cavity. The entire small bowel was eventrated and lightly manipulated using two moist cotton applicators. After manipulation, the laparotomy was closed by two layers of continuous sutures. All animals recovered rapidly from bowel manipulation procedure.
Experimental groups
In pilot experiments, various depletion protocols were evaluated to achieve the most effective depletion and inactivation of resident muscularis macrophages. Rats (n = 2–3 per group) were injected intravenously with Cl2MDP liposomes at different time points and with different frequencies with or without GdCl3, manipulated at day 0 and killed at day 1. In the final protocol, rats were given 1 ml Cl2MDP liposomes (50 mg chlodronate/kg) per 100 g body weight at a size of 400 nm on days −4 and −2 (before the operative procedure) and alternate administration of 0.5 ml GdCl3 (10 mg/kg) per 100 g body weight on days −3 and −1. This pharmacologically treated group is designated DI rats. Normal rats received chlodronate‐free liposomes as vehicle administration. All experiments were performed with operated and non‐operated (controls) animals (n = 5–8 each group). For this study, animals were killed between 0.5 and 24 h after manipulation and the isolated jejunal muscularis externa used for histochemistry, immunohistochemistry, mRNA extraction and in vitro muscle contractility. In vivo gastrointestinal transit studies were performed with the complete small and large intestines, including the stomach.
Preparation of liposomes
Cl2MDP liposomes were prepared according to the protocol of van Rooijen and Sanders.19 In brief, 86 mg L‐α‐phosphatidylcholin and 8 mg cholesterol were dissolved in chloroform, vacuum evaporated and dissolved in a clodronate solution (2.5 g clodronate/10 ml aqua dest.). After waterbath sonication non‐encapsulated clodronate was removed from the suspension by centrifugation (10 000 for 15 min). The Cl2MDP liposomes which formed a band were washed twice with phosphate‐buffered solution (PBS) and resuspended in 4 ml PBS for injection. The Cl2MDP‐liposome suspension contained about 5 mg clodronate/ml.
For further experiments, liposomes were extruded with a Mini‐Extruder through a polycarbonate membrane of pore size 400 or 100 nm (Avanti Polar Lipids, Alabaster, Alabama, USA). The liposome radius was measured by dynamic light scattering using ALV‐NIBS/HPPS (high‐sensitivity version) and the ALV‐NIBS/HPPS V.0.3.0 software (ALV‐GmbH, Langen, Germany).
Histochemistry and immunohistochemistry
Histochemical examination was performed on whole mounts of the distal jejunum, as described before in detail (n = 5 each group).24 In brief, jejunal segments were opened, immersed in chilled Krebs–Ringer buffer (KRB), and fixed in 100% ethanol for 10 min. After washing with KRB mucosa and submucosa were stripped off under microscopic observation and mucosa‐free muscularis whole mounts were cut into 0.5×1 cm pieces and used for staining procedures. To detect myeloperoxidase (MPO)‐positive cells, freshly prepared whole mounts were stained with Hanker–Yates reagent as described before.24 MPO‐positive cells were counted under a microscope (SM‐LU, Leica, Bensheim) in five randomly chosen areas in each specimen at a magnification of ×160. Muscularis whole mounts were also used for immunohistochemical analysis of ED1 (monocyte‐specific antibody, 5 µg/ml; Serotec, Düsseldorf, Germany), ED2 (resident macrophage‐specific antibody, 5 µg/ml, Serotec), IL1β (IL1β rabbit anti‐rat, 30 µg/ml, Endogen, Woburn, Massachusetts, USA), IL6 (IL6 goat anti‐rat, 30 µg/ml, R&D Systems, Minneapolis, Minnesota, USA) and F4/80 (MCA497CA, rat anti‐mouse, 10 µg/ml, Serotec).
Each whole mount was incubated overnight in the primary antibody, washed three times in PBS and then incubated in the appropriate secondary antibody (goat anti‐mouse, Dako, Hamburg, Germany; 20 µg/ml, goat anti‐mouse Alexa 568, 10 µg/ml; donkey anti‐goa –Alexa 555 IgG, 30 µg/ml; goat anti‐rabbit Alexa 555 IgG, 30 µg/ml, Molecular Probes, Leiden, The Netherlands) at 4°C overnight. Whole mounts were coverslipped and inspected by fluorescent microscopy after staining (TE‐2000, Nikon, Duesseldorf, Germany). Leucocytes were counted in five randomly chosen areas in each specimen at a magnification of ×200.
Real‐time reverse transcriptase‐polymerase chain reaction
Total RNA was extracted from the isolated muscularis of rats and mice23 at specific time points (0.5–24 h after intestinal manipulation; n = 3–5 each group). Total RNA extraction was performed using the RNeasy Mini extraction kit (Qiagen, Hilden, Germany) followed by DNAse‐I treatment (Ambion, Austin, Texas, USA). cDNA was synthesised using the Omniscript RT kit (Qiagen). Expression of mRNA was quantified in triplicates by a real‐time reverse transcriptase‐polymerase chain reaction (PCR) with SYBR Green or Tagman probes (for sequences, see table 1). The PCR reaction mixture was prepared using the SYBR Green PCR Mastermix (Applied Biosystems, Darmstadt, Germany), and amplification of cDNA was carried out for 40 cycles (95°C×15 s, 60°C×1 min) on an AbiPrism 7900 HT cycler. Relative data quantitation was performed by the ΔΔCT method, described in the User Bulletin 2 of the Abi Prism manufacturer.
Table 1 Nucleotide sequences of mouse and rat oligonucleotide primers and Tagman assays .
| Gene | Sense primer | Antisense primer |
|---|---|---|
| 18S‐rRNA (r) | 5′‐gtggagcgatttgtctggtt‐3′ | 5′‐cgctgagccagttcagtgta‐3′ |
| β‐actin (m) | 5′‐agagggaaatcgtgcgtgac‐3′ | 5′‐caatagtgatgacctggccgt‐3′ |
| IL1β (r) | 5′‐tctcacagcagcatctcgac‐3′ | 5′‐catcatcccacgagtcacag‐3′ |
| IL6 (r) | 5′‐ccactgccttccctacttca‐3′ | 5′‐acagtgcatcatcgctgttc‐3′ |
| IL6 (m) | 5′‐aagtcggaggcttaattacacatgt‐3′ | 5′‐ccattgcacaactcttttctcattc‐3′ |
| ICAM 1 (r) | 5′‐aggtatccatccatcccaca‐3′ | 5′‐gccacagttctcaaagcacag‐3′ |
| ICAM‐1 (m) | 5′‐agggctggcattgttctcta‐3′ | 5′‐cttcagaggcaggaaacagg‐3′ |
| MCP 1 (r) | 5′‐ttctggacccattccttattg‐3′ | 5′‐gctgaagaccttagggcaga‐3′ |
| MCP 1 (m) | 5′‐cccaatgagtaggctggaga‐3′ | 5′‐gctgaagaccttagggcaga‐3′ |
| MIP 1α (m) | 5′‐accatgacactctgcaacca‐3′ | 5′‐cccaggtctctttggagtca‐3′ |
| MIP 1α (r) | #Rn00564660_m1 | Applied Biosystems, Germany |
ICAM, intracellular adhesion molecule; IL, interleukin; m, mouse; MCP, monocyte chemotractant protein 1; r, rat.
Functional studies
Jejunal smooth‐muscle activity was measured as described previously.6 Briefly, after preparation, mucosa‐free circular muscularis strips were equilibrated in KRB‐perfused organ chambers at 37°C for 1 h. One end of each strip was tied to a fixed post and the other attached to an isometric force transducer (WPI, Sarasota, Florida, USA). Dose–response curves of muscle contraction were generated by exposing the muscle strips to increasing concentrations of the muscarinic agonist bethanechol (0.1–300 µmol/l) for 10 min, followed by a wash period (KRB) of 10 min. The contractile response was analysed with the Acknowledge software (Biopac Systems, Santa Barbara, California, USA) and the contractions were calculated as grams per square millimeter per second by conversion of weight and length of the strip to square millimeters of tissue.
Gastrointestinal transit was measured in controls and surgically manipulated animals 24 h postoperatively by evaluating the intestinal location of fluorescein‐labelled dextran (70 000 MW, Molecular Probes, Leiden, The Netherlands; n = 5–8 each). At 22 h after the bowel manipulation, animals were lightly anaesthetised and given dextran (200 µl of 6.25 mg/ml stock solution in rats and 70 µl in mice) via a gastric tube into the stomach. Animals were euthanised 90 min after administration, the entire gastrointestinal tract was divided into 15 segments opened and fluorescein‐labelled dextran was washed out by saline. Probes were centrifuged at 12 000 rpm and fluorescence of supernatants was read at 494 nm/521 nm wavelength in a fluorescence reader (Safire, Tecan, Crailsheim, Germany). The data were expressed as the percentage of activity per segment and plotted in a median histogram. Gastrointestinal transit was calculated as the geometric centre (GC) of distribution of fluorescence marker using the following formula:
C=Σ (% of total fluorescent signal per segment×segment number)/100.
Drugs and solutions
A standard KRB was used with the following constituents (concentrations expressed as mm/l): Na+, 137.4; K+, 5.9; Ca2+, 2.5; Mg2+, 1.2; Cl−, 134; HCO3−, 15.5; H2PO4−, 1.2; and glucose, 11.5. Antibodies were diluted in PBS containing 0.2% bovine serum. Clodronate was a kind gift of Roche Molecular Biochemicals (Mannheim, Germany). All other chemicals used for this study (if not separately mentioned) were purchased from Sigma (Taufkirchen, Germany).
Data analysis
Data were compiled as mean (standard deviation (SD)). Statistical analysis was performed using two‐way analysis of variance with a significance level of p<0.05 (*), p<0.01 (**) or p<0.001 (***), followed by a Bonferoni post‐test.
Results
Muscularis macrophage distribution
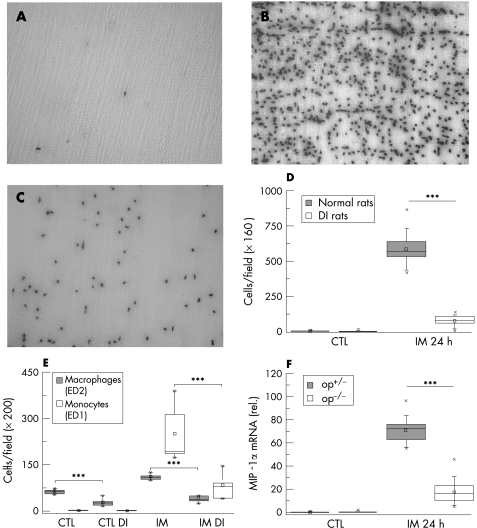
In control whole mounts of normal rats, we found a dense network of ED‐2 positive cells (resident macrophages; mean 65.36 (SD 3.67) cells at ×200; fig 1A). Intravenous pretreatment with Cl2MDP liposomes and GdCl3 caused a significant macrophage depletion of 52% compared with control rats (31.44 (6.18)). Liposomes of smaller size have been shown to circulate longer in blood and are taken up less effectively by the liver and spleen than larger ones.25 The size of the unfiltrated liposomes was 1000–3000 nm. Therefore, we prepared 400 nm and 100 nm liposomes and tested the effectiveness of these smaller liposomes. Application of 400 nm liposomes and GdCl3 resulted in a 85% depletion of resident muscularis macrophages (9.96 (1.7)); treatment with 100 nm liposomes was less effective (74%, 17.3 (0.64)). The remaining muscularis macrophages showed morphological changes, the dendrites were short and margins of the cell were not clearly visible (fig 1B). The treatment did not alter the number of constitutively present MPO‐positive (polymorphonuclear neutrophils (PMNs) and ED1‐positive cells (monocytes) in comparison to controls.
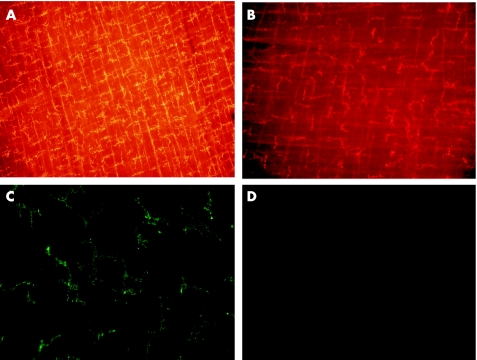
Figure 1 Intestinal macrophages in rat and mouse were detected in muscularis whole mounts by immunofluorescence with monoclonal antibodies recognising ED2 and F4/80, respectively. Whole mount from a control rat (A) showing the dense homogeneous distribution of ED2‐positive resident macrophages. After treatment with liposome‐encapsulated dichloromethylene diphosphonate (clodronate) (Cl2MDP) liposomes and gadolinium chloride (GdCl3), just a few phenotype‐altered residual macrophages are detected (B). In op+/‐ mice, F4/80 staining also results in detection of the typical dendrite‐shaped muscularis macrophages (C), whereas no cells are stained in homozygotic osteopetrosis mice (op‐/‐; D). Micrographs were taken with original magnification of ×100 (A,B) and ×200 (C,D).
Immunohistochemical stainings with markers against mouse macrophages (F4/80 and CD11b) also showed the known regular distribution of muscularis macrophages in normal and heterozygotic op+/‐ mice (fig 1C); these macrophages were completely absent in homozygotic op‐/‐ mice (fig 1D).
Macrophage activation
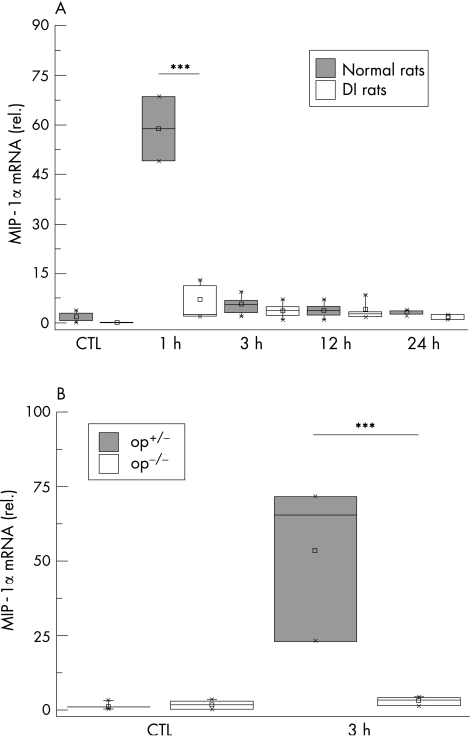
Activation of the local macrophages was postulated to be an early step of the inflammatory reaction leading to dysmotility. Therefore, we measured MIP‐1α mRNA expression in muscularis extracts. MIP‐1α expression in normal rats increased 57‐fold at 1 h after surgical intervention. In DI rats, no significant changes in MIP‐1α expression were detected compared with controls (fig 2A). In the mouse model heterozygotic op+/‐ mice showed a MIP‐1α elevation 3 h after intestinal manipulation (41‐fold), but in op‐/‐ mice MIP‐1α expression was not significantly up regulated (fig 2B).
Figure 2 MIP‐1α expression after surgical trauma. Normal and macrophage‐depleted and inactivated rats (DI rats) (A) and op+/‐ and op‐/‐ mice (B) were subjected to intestinal manipulation. At different time points cDNA was generated from muscularis externa and analysed by quantitative polymerase chain reaction for MIP‐1α expression. The massive increase at 1 h after intestinal manipulation is completely abrogated in DI rats. Corresponding, op‐/‐ mice do not show increased MIP‐1α levels compared with op+/‐ mice 3 h after trauma. All samples are compared with untreated control (CTL) animals. Values are means and asterisks indicate significant differences between the indicated samples (n = 4–5).
Inflammatory reaction in the muscularis
After establishing that macrophage activation was an initial step, the following cascade of inflammatory events was sequentially investigated on transcriptional, translational and cellular levels.
Inflammatory mediators
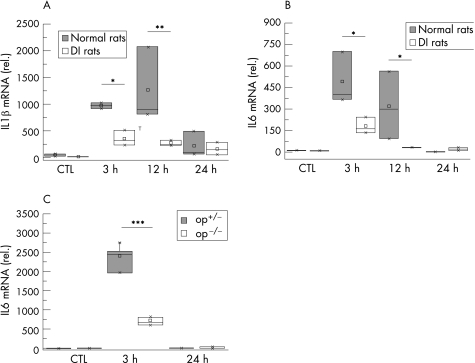
We investigated the expression of prototypic proinflammatory cytokines (IL1β and IL6) in the muscularis. Only a basal cytokine expression was observed in specimens from control animals (fig 3). As illustrated in fig 3, IL1β and IL6 were rapidly activated and peaked within 3 h after manipulation (IL1β: 20.3‐fold; IL6: 33.2‐fold increase over controls). Both cytokines returned to control levels 24 h after manipulation.
Figure 3 Interleukin (IL)1β and IL6 expression after surgical trauma. Normal and macrophage‐depleted and inactivated rats (DI rats) were subjected to intestinal manipulation. After the indicated time, cDNA was generated from muscularis externa and analysed by quantitative polymerase chain reaction for IL1β (A) and IL6 (B) expression. In op+/‐ and op‐/‐ mice IL6 expression levels were determined 3 and 24 h after surgical trauma (C). In both normal rats and op+/‐ mice, maximal cytokine expression is found 3 h after trauma. In DI rats and op‐/‐ mice, this up regulation is significantly diminished. All samples are compared with untreated control (CTL) animals. Values are means and asterisks indicate significant differences between the indicated samples (n = 4–5).
In DI rats, this up‐ regulation in both cytokines was significantly reduced early postoperatively (IL1ß: 63.5%; IL6: 50.6%, compared with manipulated normal rats;fig 3). This reduction persisted at 12 h (IL1ß: 61.6%; IL6: 84.4%).
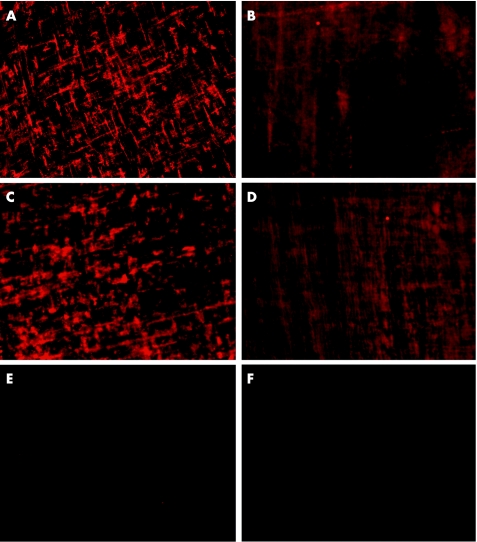
To confirm these results on the translational level, immunohistochemical analysis was performed on muscularis whole mounts for IL1β and IL6 protein localisation. At 24 h after manipulation, a strong fluorescence was observed. Figure 4 shows the typical dendritic morphology, which is known to represent muscularis macrophages. By contrast, in DI rats no specific staining pattern of cells was visible.
Figure 4 Immunohistochemistry of intestinal muscularis whole mounts showing interleukin (IL)1β and IL6 protein expression 24 h after intestinal manipulation in rats. In normal rats, strong IL1β (A) and IL6 (C) signals are found within the dense network of resident macrophages. By contrast, IL1β (C) and IL6 (D) immunoreactivity is nearly absent after depletion and inactivation of muscularis macrophages (macrophage‐depleted and inactivated rats (DI rats)). Micrographs were taken with an original magnification of ×200. Control stainings of anti‐rabbit (E) and anti‐goat (F) secondary antibodies were performed with a false primary immunoglobulin G (IgG) from mouse.
Expression of IL6 mRNA was also measured in the mouse model. Heterozygotic op+/‐ mice showed a marked up regulation (2365‐fold) peaking at 3 h after trauma. In op‐/‐ mice, this upregulation was considerably diminished (673‐fold over control; fig 3C).
Chemokine and adhesion molecules
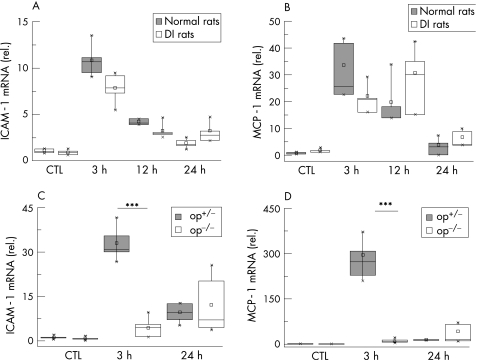
As previously shown, ICAM‐1 is one of the key adhesion molecules and MCP‐1 an important chemokine after intestinal trauma.26,27 Expression of ICAM‐1 and MCP‐1 mRNA was maximum at 3 h after trauma in normal rats (ICAM‐1: 10.6‐fold, MCP‐1: 20.6‐fold increase) with a gradual decrease up to 24 h (fig 5A, B).
Figure 5 Intracellular adhesion molecule (ICAM)‐1 and monocyte chemotractant protein 1 (MCP)‐1 expression after surgical trauma. Normal and macrophage‐depleted and inactivated rats (DI rats) and op+/‐ and op‐/‐ mice were subjected to intestinal manipulation. At different time points, cDNA was generated from muscularis externa and analysed by quantitative polymerase chain reaction for expression of ICAM‐1 and MCP‐1. Expression of ICAM‐1 (A) and MCP‐1 (B) is diminished 3 h after surgical trauma. In op‐/‐ mice, the expression of ICAM‐1 (C) and MCP‐1 (D) is also considerably decreased at 3 h. Note that this effect is much more dramatical in mice compared with the rat model. All samples are compared with untreated control (CTL) animals. Values are means and asterisks indicate significant differences between the indicated samples (n = 4–5).
ICAM‐1 mRNA expression was measured in op+/‐ and op‐/‐ mice at 3 and 24 h. Heterozygotic op+/‐ mice showed a significant increase at 3 h (29.6‐fold), but no significant up regulation was found in op‐/‐ mice. Furthermore, op+/‐ mice showed an MCP‐1 mRNA up regulation of 288‐fold, whereas this up regulation was significantly decreased in op‐/‐ mice (15.4‐fold). Again the 24‐h time point did not show any difference (fig 5C, D).
Cellular infiltration
Intestinal manipulation in normal rats resulted in an infiltration of PMNs (155‐fold increase over unoperated rats) and monocytes (50.8‐fold) and an increase of macrophages (1.7‐fold) in the intestinal muscularis at 24 h (fig 6). In DI rats, manipulation resulted in a significant reduction of leucocyte extravasation compared with manipulated normal rats (PMNs 75.6%, monocytes 57.2% and macrophages 81.9%; fig 6D, E).
Figure 6 Recruitment of leucocytes after surgical trauma. Myeloperoxidase‐staining (MPO) for polymorphonuclear neutrophils (PMNs) within muscularis whole mounts of control (CTL) rats (A), manipulated normal (B) and macrophage‐depleted and inactivated rats (DI rats) (C) was performed 24 h after surgical trauma. Depletion and inactivation of resident muscularis macrophages (DI rats) result in a significantly reduced infiltration 24 h after surgical manipulation (D). Monocytes and macrophages were immunohistochemically stained and counted at ×200 magnification. In DI rats, the infiltrating monocyte population is significantly decreased compared with normal rats 24 h after surgical manipulation (E). In addition, op‐/‐ mice also show a significantly diminished number of PMNs 24 h after manipulation in myeloperoxidase‐staining (F). Rat MPO (A–D) was performed at ×160 magnification. ED1 and ED2 immunohistochemistry in rats (E) and MPO in mice (F) are quantified at ×200 magnification. Values are means and asterisks indicate significant differences between the indicated samples (n = 5). IM, intestinal manipulation.
In contrast with op+/‐ mice (mean 0.1 (SD 0.01) cells/×200 magnification), neutrophil counts in the normal muscularis of unmanipulated op‐/‐ mice did not show any positively stained cells. After manipulation, cellular infiltration in op+/‐ mice showed a significant increase in recruited leucocytes (mean 70.7 (SD 1.46)). This increase was again significantly diminished in op‐/‐ mice (mean 17.2 (SD 3.0); fig 6F).
Muscle function
The degree of muscular dysfunction was analysed in the following experiments by measurement of in vitro muscle contractility and in vivo gastrointestinal transit. Both methods have been proved to be highly reliable in previous investigations using different strains of rodents.5,8,28
In vitro contractility
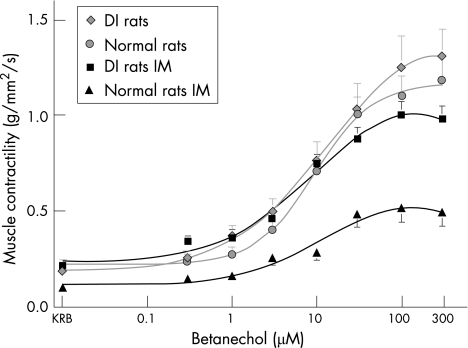
In rats control jejunal muscle strips exhibited spontaneous regular monophasic contractions with a mean (SD) frequency of 33 (0.9) events/min. Figure 7 shows the responses of the circular muscle to increasing doses of bethanechol (0.3–300 µmol/l). Stimulation of control muscle strips with the muscarinic agonist caused a dose‐dependent increase in the generation of large phasic contractions overlying a tonic contractile component, as shown before.6 Figure 7 shows that after intestinal manipulation, spontaneous and bethanechol‐stimulated muscle contractility were significantly decreased (53.7%) compared with controls (100 µM bethanechol: mean (SD) 0.5 (0.07) v 1.1 (0.11) g/mm2/s). In DI rats, this suppression was prevented (100 µM bethanechol: 1.00 (0.07) v 0.5 (0.07) g/mm2/s DI v control rats) and contractile force returned almost to normal levels (fig 7).
Figure 7 Bethanechol dose–response curves of jejunal circular smooth‐muscle contractile activity. Intestinal manipulation (IM, ▴); reduces muscle function compared with controls (•). Depletion and inactivation of macrophages (macrophage‐depleted and inactivated rats (DI rats)) prevent the surgical‐induced suppression and normalised the contractility (▪). Values are displayed as means (SD; n = 5). KRB, Krebs–Ringer buffer.
In vivo gastrointestinal transit
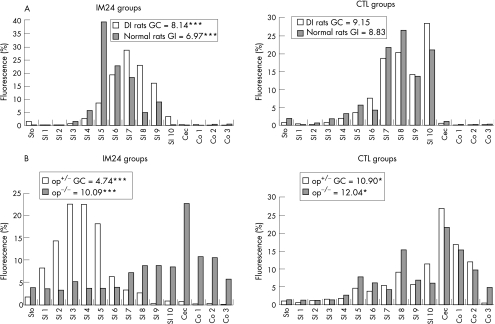
We could show that in DI rats the fluorescence marker migrated to the distal end of the bowel, with a maximum in the last segment of the small bowel. The calculated mean (SD) geometric centres of unmanipulated normal and DI rats were 8.83 (0.54) and 9.15 (0.40), respectively. Normal rats subjected to intestinal manipulation showed a significant decrease (geometric centre 6.97 (0.75)), whereas operated DI rats had a regular transit (geometric centre 8.14 (0.25)) that did not significantly differ from healthy animals (fig 8A).
Figure 8 Transit histogram for the distribution of non‐absorbable fluorescein‐labelled dextran along the gastrointestinal tract after oral administration. In rats (A), intestinal manipulation (IM24) causes a significant delay in intestinal transit (geometric centre (GC) = 6.97). Depletion and inactivation of macrophages (macrophage‐depleted and inactivated rats (DI rats)) avert the delay in transit significantly (GC = 8.14; p<0.001; n = 5–8). In unmanipulated (CTL) groups, no difference in transit could be detected. In heterozygotic op+/‐ mice (B), surgical manipulation results in an even stronger deceleration in intestinal transit (GC = 4.74) compared with unmanipulated control mice (GC = 10.9). In homozygotic op‐/‐ (op) mice transit is not significantly affected (GC = 10.09) by intestinal manipulation (IM) compared with unmanipulated control op‐/‐ mice (GC = 12.04). Values (n = 5–8) are displayed as GCs for the 15 intestinal segments stomach (Sto), small intestine (SI) 1–10, cecum (Cec) and colon (Co).
The effect of intestinal manipulation was verified using the mouse model. Heterozygotic op+/‐ and op‐/‐ mice showed a normal transit with mean (SD) geometric centres of 10.9 (0.46) and 12.0 (0.22), respectively. Intestinal manipulation led to a significant slow‐down of gastrointestinal transit in op+/‐ mice (geometric centre 4.7 (0.35)), whereas op‐/‐ mice were not significantly influenced by the surgical trauma and showed a normal transit with a geometric centre of 11.0 (0.91) (fig 8B).
Discussion
Postoperative ileus is a problem of nearly every abdominal surgery.1 Although this postoperative problem occurs often, little is known about the underlying pathophysiological pathways and mechanisms.1,29,30,31,32 Previously, we have shown a direct link between the postsurgical inflammation of the intestinal smooth muscle and subsequent muscle dysfunction.5,6,26 Further, we were able to show an early activation of resident muscularis macrophages and the release of prototypic proinflammatory mediators from these phagocytes within the smooth muscle after surgery.15,24,33 The inflammatory potential of intestinal macrophages in postoperative ileus is supported in several recent studies,34,35 however, mast cells are also considered to be a regulatory cell population necessary for the inflammatory response.36 Our recent studies led to the hypothesis that intestinal muscularis macrophages have an initial role in the propagation of the inflammatory reaction and that the depletion of these—normally resident—cells might be a therapeutically interesting approach. In this set of experiments, we aimed to study the role of these macrophages using two different animal models. In the rat model, macrophages are pharmacologically depleted and inactivated. The second model used the mutant osteopetrosis mice, which have been shown to completely lack macrophages within the intestinal smooth muscle.22,37 Both models showed convincingly that restricting the functional activity of this macrophage population resulted in an abrogation of the inflammatory cascade normally set into motion by surgical manipulation. The results showed a down regulation of macrophage activation, inflammatory mediators, adhesion molecules and chemokine expression, leading to a reduction in cellular infiltration and a subsequent improvement in muscle dysfunction.
Pharmacological depletion of macrophages results in a diminished number of these phagocytes in various tissue compartments. In previous protocols, Cl2MDP liposomes were used for depletion of macrophages in several organs—for example, liver, spleen and lung.38,39 Using the original protocol of van Rooijen,40 we did not achieve a substantial depletion of muscularis macrophages. Limitation of the maximal liposome size to 400 nm proves to be effective, with a depletion rate of 80–90%, but residual muscularis macrophages are still present. Even though our histological findings suggest that these remaining cells are severely altered, we additionally inactivate these phagocytes by GdCl2. Although this method systemically depletes macrophages, the advantage of this pharmacological approach is that only mature macrophages are able to phagocyte the chlodronate liposomes in an effective (toxic) quantity. Leucocytes and immature, bone marrow‐derived cells are not affected by this treatment. We further thought to confirm these results using a genetic approach, the mouse with osteopetrosis. As shown by Mikkelsen et al, these mice possess a reduced number of macrophages within all organs. In the gut, mucosal macrophages are also significantly reduced, but, the benefit of this model is that macrophages are completely absent in the smooth‐muscle layer.22 The disadvantage is that the mutation in the colony stimulation factor gene results in a monocytopenic phenotype and therefore in a less effective immune system in these animals. However, they are still able to defend bacterial infections.41 In addition, the disadvantage of the monocytopenic phenotype in this model is compensated by our pharmacological rat model, where only mature phagocytes are depleted. Therefore, each of these two models bridges the gap of the other.
As we have hypothesised that activation of the muscularis macrophages is an initial step in the post‐surgical inflammatory cascade, we measured the well‐established macrophage activation marker MIP‐1α in both macrophage‐deficient models. The early expression of MIP‐1α within the first 60–180 min after surgical manipulation indicates that macrophage activation is an initial step of the resulting inflammation cascade. The ensuing and prompt decrease of MIP‐1α to normal levels underlines our hypothesis of an initial inflammation‐triggering stimulus by a resident macrophage population.
Inflammatory reactions depend largely on the expression of proinflammatory mediators. Interleukins—especially IL6—are known prototypic proinflammatory cytokines, which have been shown to be involved in the local inflammatory reaction within the intestinal muscularis after surgical insult.15,33 Again, in this study, the maximum peak of cytokine expression for IL1β and IL6 is found early at 3 h postoperatively. In immunohistochemical staining, both cytokines are clearly visible within muscularis macrophages after manipulation of normal rats. In contrast with these animals DI rat muscularis whole mounts show no macrophage‐specific staining. Our macrophage‐deficient mouse model shows an even lower expression of IL6, strengthening the results found in the rat.
In parallel to the expression of proinflammatory cytokines, we find a concomitant upregulation of adhesion molecules and chemokines. A major link between IL6 and the induction of adhesion molecules as well as chemokines—ICAM‐1 and MCP‐1—has been described in signal transduction pathways of the STAT transcription factor family.42,43 Herein, we report that the inhibition of macrophage function in two different animal models shows a down regulation of ICAM‐1 and MCP‐1 mRNA expression. However, in the rat model this effect does not reach statistical significance. This finding can be explained by the residual 10–20% rat muscularis macrophages after depletion, as we are not able to ensure that all of the remaining macrophages are completely inactivated. The mouse model, which is completely devoid of muscularis macrophages, supports this theory owing to the nearly complete abrogation of ICAM‐1 and MCP‐1 expression. Altogether, these results once again strengthen the importance of the inclusion of the macrophage‐lacking mouse model in this set of experiments.
The direct consequence of the inflammatory process can be monitored by quantification of the cellular infiltration into the damaged tissue. In both models of macrophage inhibition, we show a dramatic reduction in leucocyte recruitment into the intestinal muscularis after surgery. Of note, the decrease in infiltrating neutrophils in inflammatory responses seems not to be associated with a functional change of these cells in op‐/‐ mice. Peritoneal infection with Escherichia coli also results in a significant down regulation in neutrophil recruitment, but shows a normal functional activity of these cells.44 In addition, Jiang et al45 observed functionally normal neutrophil responses in the liver of mice with osteopetrosis after LPS administration.
Clinical relevance of the results mentioned so far can only be judged by measurement of smooth‐muscle function in both models. In vitro measurement of smooth‐muscle contractility in the rat model shows a significant improvement of muscle function in manipulated DI rats, in contrast with manipulated rats with normal macrophage function. To verify these results in an in vivo setting, we also performed gastrointestinal transit studies. Again, the transit is almost normalised in DI rats postoperatively. Again, in the mouse model the reduction in motility was completely abolished in op‐/‐ mice subjected to intestinal manipulation, resulting in prevention and rapid restoration of regular small bowel motor function.
Conclusion
This study describes for the first time a direct link of intestinal muscularis macrophage activation and postoperative ileus. In the first model of systemic macrophage depletion and inactivation in rats, we have achieved a significant reduction of resident macrophages. This pharmacological treatment abrogates smooth‐muscle dysfunction after surgery owing to a significantly diminished molecular and cellular inflammatory response and subsequent leucocyte recruitment. These findings are confirmed and further strengthened by the second mouse model, which shows a complete absence of intestinal muscularis macrophages. Consequently, inhibited macrophage function results in normalised intestinal smooth‐muscle function. Therefore, this study shows that resident intestinal muscularis macrophages are initially involved in the surgically induced local inflammatory response resulting in postoperative ileus. The inhibition of this specific macrophage function could be a promising clinical approach to prevent postoperative gut motor dysfunction.
Abbreviations
Cl2MDP - liposome‐encapsulated dichloromethylene diphosphonate (clodronate)
GdCl3 - gadolinium chloride
ICAM - intracellular adhesion molecule
KRB - Krebs–Ringer buffer
MCP - monocyte chemotractant protein 1
MPO - myeloperoxidase
PBS - phosphate‐buffered saline
OP - osteopetrosis
PCR - polymerase chain reaction
PMN - polymorphonuclear neutrophils
Footnotes
Funding: This work was supported, partly, by a grant from the German Research Council (DFG) to the Clinical Research Group (KFO 115), and through BONFOR grants O‐112.0018 and O‐112.0025.
Competing interests: None.
Clodronate was a generous gift of Roche Diagnostics GmbH, Mannheim, Germany.
References
- 1.Livingston E H, Passaro E P. Postoperative ileus. Digest Dis Sci 199035121–132. [DOI] [PubMed] [Google Scholar]
- 2.Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg 2003138206–214. [DOI] [PubMed] [Google Scholar]
- 3.Baig M K, Wexner S D. Postoperative ileus: a review. Dis Colon Rectum 200447516–526. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H. Postoperative ileus. Gut 200047(Suppl 4)iv85–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalff J C, Buchholz B, Eskandari M K.et al Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rats. Surgery 1999126498–509. [PubMed] [Google Scholar]
- 6.Kalff J C, Schraut W H, Simmons R L.et al Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 1998228652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalff J C, Schraut W H, Billiar T R.et al Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 2000118316–327. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz N T, Kalff J C, Turler A.et al Prostanoid production via COX‐2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 20011211354–1371. [DOI] [PubMed] [Google Scholar]
- 9.Spahn T W, Kucharzik T. Modulating the intestinal immune system: the role of lymphotoxin and GALT organs. Gut 200453456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan C F. Secretory products of macrophages. J Clin Invest 198779319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosser D M. The many faces of macrophage activation. J Leukoc Biol 200373209–212. [DOI] [PubMed] [Google Scholar]
- 12.Taxi J. Contribution à l'étude des connexions des neurones moteurs du systeme nerveux autonome. Ann Natur‐Zool Biol Animale 19657413–674. [Google Scholar]
- 13.Mikkelsen H B, Rumessen J J. Characterization of macrophage‐like cells in the external layers of human small and large intestine. Cell Tissue Res 1992270273–279. [DOI] [PubMed] [Google Scholar]
- 14.Hori M, Kita M, Torihashi S.et al Upregulation of iNOS by COX‐2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol Gastrointest Liver Physiol 2001280G930–G938. [DOI] [PubMed] [Google Scholar]
- 15.Kalff J C, Turler A, Schwarz N T.et al Intra‐abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg 2003237301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Winter B Y, van Nassauw L, De Man J G.et al Role of oxidative stress in the pathogenesis of septic ileus in mice. Neurogastroenterol Motil 200517251–261. [DOI] [PubMed] [Google Scholar]
- 17.van Rooijen N, Sanders A, van den Berg T K. Apoptosis of macrophages induced by liposome‐mediated intracellular delivery of clodronate and propamidine. J Immunol Methods 199619393–99. [DOI] [PubMed] [Google Scholar]
- 18.Naito M, Nagai H, Kawano S.et al Liposome‐encapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro. J Leukoc Biol 199660337–344. [DOI] [PubMed] [Google Scholar]
- 19.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 199417483–93. [DOI] [PubMed] [Google Scholar]
- 20.Adding L C, Bannenberg G L, Gustafsson L E. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardiovasc Drug Rev 20011941–56. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Hayashi S, Kunisada T.et al The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990345442–444. [DOI] [PubMed] [Google Scholar]
- 22.Mikkelsen H B, Thuneberg L. Op/op mice defective in production of functional colony‐stimulating factor‐1 lack macrophages in muscularis externa of the small intestine. Cell Tissue Res 1999295485–493. [DOI] [PubMed] [Google Scholar]
- 23.Kalff J C, Schwarz N T, Walgenback KJ et a l. Molecular and functional studies on expression and activation of COX‐2 in the human intestinal muscularis during abdominal surgery. Neurogastroenterol Motil 19981078 [Google Scholar]
- 24.Kalff J C, Schwarz N T, Walgenbach K J.et al Leukocytes of the intestinal muscularis externa: their phenotype and isolation. J Leukoc Biol 199863683–691. [DOI] [PubMed] [Google Scholar]
- 25.Hu Q, van Rooijen N, Liu D. Effect of macrophage elimination using liposome‐encapsulated dichloromethylene diphosphate on tissue distribution of liposomes. J Liposome Res 19966681–698. [Google Scholar]
- 26.Kalff J C, Carlos T M, Schraut W H.et al Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology 1999117378–387. [DOI] [PubMed] [Google Scholar]
- 27.Turler A, Schwarz N T, Turler E.et al MCP‐1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol 2002282G145–G155. [DOI] [PubMed] [Google Scholar]
- 28.Behrendt F F, Tolba R H, Overhaus M.et al Indocyanine green fluorescence measurement of intestinal transit and gut perfusion after intestinal manipulation. Eur Surg Res 200436210–218. [DOI] [PubMed] [Google Scholar]
- 29.Condon R E, Frantzides C T, Cowles V E.et al Resolution of postoperative ileus in humans. Ann Surg 1986203574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois A, Weise V K, Kopin I J. Postoperative ileus in the rat: physiopathology, etiology and treatment. Ann Surg 1973178781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothnie N G, Harper R A K, Catchpole B N. Early postoperative gastrointestinal activity. Lancet 1963264–67. [DOI] [PubMed] [Google Scholar]
- 32.Smith J, Kelly K A, Weinshilboum R M. Pathophysiology of postoperative ileus. Arch Surg 1977112203–209. [DOI] [PubMed] [Google Scholar]
- 33.Wehner S, Schwarz N T, Hundsdoerfer R.et al Induction of IL‐6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery 2005137436–446. [DOI] [PubMed] [Google Scholar]
- 34.de Jonge W J, van der Zanden E P, The F O.et al Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2‐STAT3 signaling pathway. Nat Immunol 20056844–851. [DOI] [PubMed] [Google Scholar]
- 35.Metz C N, Tracey K J. It takes nerve to dampen inflammation. Nat Immunol 20056756–757. [DOI] [PubMed] [Google Scholar]
- 36.de Jonge W J, The F O, van der Coelen D.et al Mast cell degranulation during abdominal surgery initiates postoperative ileus in mice. Gastroenterology 2004127535–545. [DOI] [PubMed] [Google Scholar]
- 37.Galeazzi F, Lovato P, Blennerhassett P A.et al Neural change in Trichinella‐infected mice is MHC II independent and involves M‐CSF‐derived macrophages. Am J Physiol Gastrointest Liver Physiol 2001281G151–G158. [DOI] [PubMed] [Google Scholar]
- 38.van Rooijen N, Kors N, vd Ende M.et al Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome‐encapsulated dichloromethylene diphosphonate. Cell Tissue Res 1990260215–222. [DOI] [PubMed] [Google Scholar]
- 39.Thepen T, van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 1989170499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Rooijen N. The liposomes‐mediated macrophages “suicide” technique. J Immunol Methods 19891241–6. [DOI] [PubMed] [Google Scholar]
- 41.Schonlau F, Schlesiger C, Ehrchen J.et al Monocyte and macrophage functions in M‐CSF‐deficient op/op mice during experimental leishmaniasis. J Leukoc Biol 200373564–573. [DOI] [PubMed] [Google Scholar]
- 42.Caldenhoven E, van Dijk T B, Solari R.et al Stat3beta, a splice variant of transcription factor Stat3, is a dominant regulator of transcription. J Biol Chem 199627113221–13227. [DOI] [PubMed] [Google Scholar]
- 43.Romano M, Sironi M, Toniatti C. Role of IL‐6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 19976315–325. [DOI] [PubMed] [Google Scholar]
- 44.Wiktor‐Jedrzejczak W, Dzwigala B, Szperl M.et al Colony‐stimulating factor 1‐dependent resident macrophages play a regulatory role in fighting Escherichia coli fecal peritonitis. Infect Immun 1996641577–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang S, Naito M, Kaizu C.et al Lipopolysaccharide‐induced cytokine and receptor expression and neutrophil infiltration in the liver of osteopetrosis (op/op) mutant mice. Liver 200020465–474. [DOI] [PubMed] [Google Scholar]