Abstract
Background and aims
Patients with inflammatory bowel disease often present with abnormal gut motility away from the inflammatory site. We studied remote motility disturbances and their pathophysiology in a rat model of colitis.
Methods
Colitis was induced 72 h prior to experiments using trinitrobenzene sulphate (TNBS) instillation. Inflammation was verified using histology and myeloperoxidase (MPO) measurements. To assess gut motility, we determined gastric emptying, distal front and geometric centre (GC) of intestinal transit 30 min after intragastric administration of a semiliquid Evans blue solution. The effects of hexamethonium (20 mg/kg), capsaicin (125 mg/kg) and pelvic nerve section on colitis induced motility changes were evaluated. c‐Fos expression was studied in the pelvic nerve dorsal root ganglion (DRG) S1.
Results
Colitis reduced gastric emptying from 38.4 (3.6)% in controls to 22.7 (4.4)% in TNBS treated rats in the absence of local gastric inflammation. Colitis had no effect on the distal front or on the geometric centre of small intestinal transit. Hexamethonium reduced gastric emptying in controls to 26.3 (4.1)% but restored it to 35.8 (4.4)% in TNBS treated rats. Capsaicin significantly impaired gastric emptying in controls from 33.1 (5.2)% to 9.5 (3.3)% while this effect was less pronounced in TNBS treated rats (from 19.2 (2.3)% to 11.5 (3.8)%; NS). In TNBS treated rats, pelvic nerve section completely restored gastric emptying from 19.8 (5.3)% to 52.5 (6.3)% without any effect on gastric emptying in control rats. TNBS colitis induced de novo c‐Fos expression in the DRG S1.
Conclusions
Experimental colitis in rats delays gastric emptying via a neuronal pathway involving pelvic afferent nerve hyperactivity.
Inflammatory bowel disease (IBD) comprises two distinct pathophysiological and clinical entities: Crohn's disease and ulcerative colitis.1 Both are accompanied by chronic inflammation of the bowel wall resulting from an inappropriate activation of the immune system. Therapeutic efforts are mainly anti‐inflammatory and, as a consequence, the bulk of current experimental and clinical research on IBD focuses on the role of immunological events and the development of novel immune modulating drugs.2
However, the effects of IBD on gastrointestinal sensorimotor events are equally clinically relevant.3 IBD associated abdominal pain and diarrhoea are common symptoms related to alterations in visceral sensitivity and motility, respectively, and may lead to weight loss and malnutrition. These neurogastroenterological symptoms are obvious during both inflammatory episodes and remission. Indeed, the prevalence of irritable bowel‐like symptoms in inactive ulcerative colitis patients is 2–3 times higher than that in a healthy population,4,5 imposing a significant load on the patient's quality of life.6 The occurrence of neurogastroenterological symptoms in IBD is supported by objective measurements of gut motility and sensitivity. Patients with mild to moderate active ulcerative colitis show an increase in fasting colonic motility yet a decrease in the gastrocolonic response to food ingestion.7 Modified motor behaviour is not just confined to the region of inflammation as patients with Crohn's disease without gastroduodenal involvement are known to suffer from a reduced rate of gastric emptying.8,9 A similar “long distance” effect has been reported for visceral sensitivity in patients with ulcerative colitis: sensitivity thresholds to intrarectal balloon distension were significantly attenuated during inflammation in this patient group10 but were equally reduced when a balloon was distended in the oesophagus.11
There is extensive experimental literature supporting the concept that during gastrointestinal inflammation, a complex interaction occurs between immune cells, epithelial and mesenchymal cells, and the neurones of both the intrinsic and extrinsic innervation of the gut.12 Importantly, these events are not restricted to the inflamed region.13,14
The aim of our study was to document the effects of experimentally induced colitis in rats on in vivo parameters of gastrointestinal motor function, focusing on regions remote from the inflammatory site, and to investigate the pathophysiology of these remote motility disturbances.
Materials and methods
Animal model
Distal colitis was induced in male Wistar rats (200–225 g) according to published methods.15 Rats were fasted for 24 h. After pentobarbital anaesthesia (45 mg/kg) an enema of 0.5 ml of saline (controls) or trinitrobenzene sulphate (7.5 mg TNBS dissolved in 50% ethanol) was administered into the colorectum. All further experiments were performed 72 h after induction of colitis. All procedures were approved by the Committee for Medical Ethics and the Use of Experimental Animals at the University of Antwerp.
Macroscopic and microscopic evaluation of colitis
After each experiment, the colorectum was removed and macroscopic mucosal damage was assessed using a standardised scoring system, ranging from 0 to 10.16 Rats were included if the score was >5, indicating major sites of damage. Tissue samples were harvested for histological assessment of the inflammatory infiltrate. The colonic segment was fixed in 4% formaldehyde and embedded in paraffin for haematoxylin–eosin staining.
Myeloperoxidase activity assay
Tissue myeloperoxidase (MPO) activity, directly related to the myeloid cell infiltrate in the inflamed tissue,17 was assayed according to published methods14 in full thickness tissue samples taken from the distal colon, jejunum and stomach.
In vivo measurements of gastrointestinal motility
A protocol was adapted from De Winter et al.18,19 Rats were fasted for 48 h with free access to tap water containing 5% glucose. On the day of the experiment, 1 ml of Evans blue, a semiliquid non‐nutrient dye (50 mg/ml dissolved in 0.5% methylcellulose), was instilled intragastrically. Thirty minutes later, rats were anaesthetised and sacrificed. The stomach and small intestine were carefully removed. Intestinal transit was measured from the pylorus to the most distal point of migration and expressed as a percentage of the total length of the small intestine. The small intestine was then divided into 10 segments of equal length. These 10 segments and the stomach were placed in 25 ml of 0.1 N NaOH, minced and put in an ultrasonic bath for 1 h (Bransonic 2000; Branson Cleaning Equipment Co., Shelton, Connecticut, USA). The resulting suspension was left at room temperature for 1 h. The supernatant (5 ml) was then centrifuged at 1356 g for 20 min at 4°C. Samples were further diluted (1:5 for intestinal specimens and 1:50 for the stomach), and absorbance (A) was read at wavelength 565 nm. Gastric emptying was calculated as
%gastric emptying = [A(small intestine)/A(stomach+small intestine)]×100.
The geometric centre (GC) of intestinal transit20 was calculated both including (GCS+I) and omitting (GCI) the stomach segment, as the latter method provides a better idea of small intestinal peristaltic function independent of gastric emptying efficacy. These values were calculated as
geometric centre = Σ[%A(segment)×(number of segment)]/100.
Experimental protocols
Firstly, the effect of TNBS induced colitis on gastric emptying, intestinal transit and geometric centre was assessed.
Secondly, the nicotinic receptor blocker hexamethonium (20 mg/kg intraperitoneally)21 or its vehicle (saline) was administered to TNBS treated rats and controls, 1 h before Evans blue gavage.
Thirdly, 14 days before the experiments, rats were pretreated with the sensory neurotoxin capsaicin (total dose 125 mg/kg subcutaneously) or its vehicle (10% ethanol, 10% Tween 80, 80% saline) according to published methods.22 The effectiveness of capsaicin pretreatment was assessed clinically by ocular chemosensitivity to a drop of capsaicin (40 μmol/l) and by immunocytochemical staining of calcitonin gene related peptide (CGRP) containing extrinsic afferent nerve fibres in the gastric wall.23
Fourthly, the effects of pelvic nerve sectioning were examined. This procedure eliminates the sacral (parasympathetic) portion of the extrinsic innervation of the distal colon. Rats were anaesthetised with pentobarbital (45 mg/kg intraperitoneally). A laparotomy was performed and the pelvic nerves were isolated bilaterally and transected. In sham operated rats, the pelvic nerves were also isolated but not transected. All operated rats were allowed to recover for 4 days before induction of colitis. As the pelvic nerve also provides the parasympathetic innervation for the bladder, its section resulted in urine retention. To prevent globus formation and subsequent urinary infections, the bladders of transected rats were manually drained twice a day (Credé manoeuvre).
Immunocytochemistry
For CGRP staining, rats were anaesthetised and sacrificed by exsanguination. The stomach was removed and rinsed in Krebs solution. It was then filled with and immersed for 2 h at room temperature in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.0). After fixation, strips were processed for cryosectioning according to published methods.24
All incubations were performed at room temperature. Antibodies were diluted in phosphate buffered saline (PBS) containing 10% normal goat serum (Dako Denmark A/S, Glostrup, Denmark), 0.01% bovine serum albumin, 0.05% thimerosal and 0.01% sodium azide (PBS). Sections were immersed for 30 min in PBS containing 1% Triton X‐100 followed by incubation for 16 h with a guineapig polyclonal anti‐CGRP antibody (diluted 1:500; B‐GP 470‐1, Euro‐Diagnostica BV, Arnhem, the Netherlands). After rinsing in PBS, they were incubated for 1 h with Cy3 conjugated donkey anti‐guineapig IgG (diluted 1:500; Jackson, West Grove, Pennsylvania, USA). For negative controls, the primary antiserum was omitted.
For determination of Fos expression, rats were anaesthetised using pentobarbital (45 mg/kg intraperitoneally). An intracolonic balloon (4–5 cm length) was left non‐distended (sham distension) or was inflated to 60 mm Hg for 30 s at 90 s intervals (distension). After 45 min, rats were perfused in vivo with Krebs–Ringer solution followed by phosphate buffer. The dorsal root ganglion (DRG) S1 was dissected out, rinsed in PBS and processed for paraffin embedding. Fos was detected in 4 µm thick sections using the EnVision method (Dako Denmark A/S) as described previously,25 using a rabbit polyclonal anti‐Fos antibody (PC38; Merck Biosciences–Calbiochem, Darmstadt, Germany), diluted 1:10 000 in PBS containing 0.01% sodium azide and 0.1% bovine serum albumin. For quantification of Fos immunoreactivity, the number of positive nuclei was counted on 5 slides per ganglion and expressed as a percentage of the total number of neuronal nuclei identified.
Solutions and drugs
Evans blue, hexadecyltrimethylammonium bromide, hexamethonium, o‐dianisidine dihydrochloride and Tween 80 were purchased from Sigma‐Aldrich Inc. (St Louis, Missouri, USA); hydrogen peroxide and diethyl ether were purchased from Merck (Darmstadt, Germany); capsaicin and TNBS were obtained from Fluka (Neu Ulm, Germany); and pentobarbital (Nembutal) was purchased from Ceva (Brussels, Belgium).
Presentation of results and statistical analysis
Parametric values are shown as mean (SEM); n indicates the number of rats used. For statistical analysis, two way analysis of variance (ANOVA) was performed. Post hoc testing was performed using Student–Newman–Keuls analysis where appropriate. For non‐parametric data, results are presented as median with 25th and 75th percentiles. Non‐parametric analysis was performed using the Mann–Whitney U test. A p value <0.05 was considered statistically significant. Data were analysed using SPSS 11.5 software (SPSS Inc., Chicago, USA) and GraphPad Prism 4.00 (GraphPad Software, California, USA).
Results
Effects of TNBS induced colitis on clinical and macroscopic appearance
Within 72 h after TNBS treatment, rats developed loose stools and lost 10% of their body weight. Macroscopically, colitis was characterised by bowel wall thickening, adhesions and ulcerations. Accumulations of pericolonic mesenteric fat, reminiscent of creeping fat (a key feature of Crohn's disease), were frequently observed. The mucosal surface showed signs of hyperaemic inflammation, focal or linear ulcerations and necrosis. As these severe inflammatory changes covered 2–4 cm of the distal colon mucosa, this resulted in a median macroscopic damage score of 8 (7–9) in TNBS treated rats compared with 0 (0–0) in controls (p<0.05).
Effects of TNBS induced colitis on histology of the gastrointestinal tract
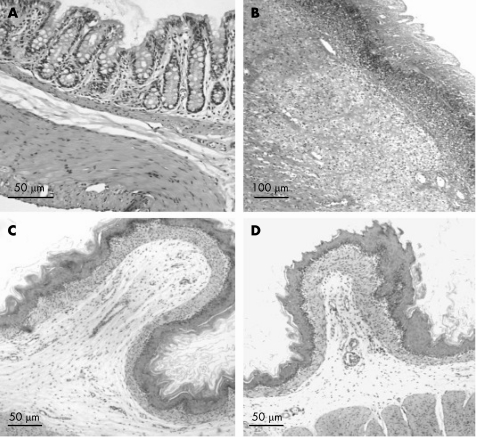
Microscopic evaluation of control rats showed normal features of colon histology (fig 1A). Pronounced inflammatory changes developed 72 h after TNBS induction (fig 1B). Histological evaluation of the gastric fundus and the jejunum in control and TNBS treated rats displayed normal fundic (fig 1C, 1D) and jejunal histology (results not shown).
Figure 1 In control rats, normal histological colon morphology is evident (A, ×20). Seventy two hours after induction of trinitrobenzene sulphate (TNBS) colitis, severe transmural infiltration of the colon wall developed, with submucosal oedema, lymphocytic and neutrophilic infiltration, coagulative necrosis and perivascular fibrosis (B, ×10). Histological study of the gastric fundus showed a normal microscopic anatomy with no apparent differences between control rats (C, ×20) and rats with TNBS colitis (D, ×20). All sections were stained with haematoxylin‐eosin.
Effects of TNBS induced colitis on MPO activity in the gastrointestinal tract
In control rats, the mean colonic MPO activity was 1.8 (0.9) U/g. This value increased to 41.5 (5.8) U/g 72 h after induction of TNBS colitis (p<0.001, n = 6). The jejunal MPO content was 9.4 (3.3) U/g in controls and 4.8 (1.6) U/g in TNBS treated rats (NS, n = 6). MPO activity in the gastric fundus decreased from 1.2 (0.3) U/g in controls to 0.2 (0.1) U/g in TNBS rats (p<0.05, n = 6). However, due to the overall low gastric MPO values, this difference should be interpreted with caution.
Effects of TNBS induced colitis on gastrointestinal motility
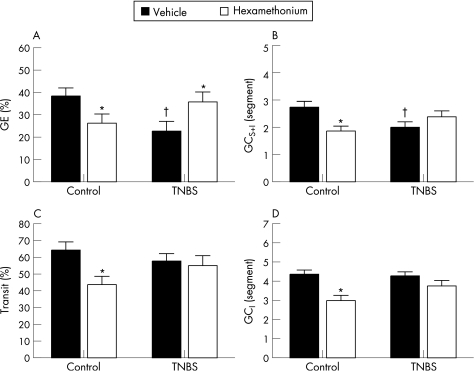
TNBS colitis significantly delayed gastric emptying in TNBS treated rats compared with control rats (p<0.05) (fig 2A). Rats with TNBS colitis also showed a significant reduction in GCS+I (p<0.05) (fig 2B). Experimental colitis had no effect on the distal front of intestinal transit (fig 2C) or on GCI (fig 2D).
Figure 2 Effect of hexamethonium on (A) gastric emptying (GE), (B) the geometric centre of transit including the stomach (GCS+I) (C), the distal front of intestinal transit and (D) the geometric centre of transit omitting the stomach (GCI). Results are expressed as percentage gastric emptying (%GE), percentage intestinal transit (% transit) and segment number (GCS+I and GCI), and are mean (SEM) for n = 12. *p<0.05, significantly different from respective vehicle treatment; †p<0.05, significantly different from vehicle treated controls, two way ANOVA with Student–Newman–Keuls post hoc analysis.
Effects of hexamethonium on TNBS colitis induced gastroparesis
In controls, hexamethonium pretreatment significantly delayed gastric emptying (p<0.05) (fig. 2A). In the TNBS colitis group, however, hexamethonium significantly enhanced gastric emptying (p<0.05) (fig 2A). This effect was reflected in the GCS+I which was decreased by hexamethonium (p<0.05) but increased in TNBS treated rats (NS fig 2B). Hexamethonium significantly reduced the front of intestinal transit (fig 2C) and the GCI (fig 2D) in control rats (p<0.05), an effect which was no longer observed in TNBS treated rats. Hexamethonium pretreatment had no effect on the macroscopic score of inflammation (results not shown).
Effects of capsaicin on TNBS colitis induced gastroparesis
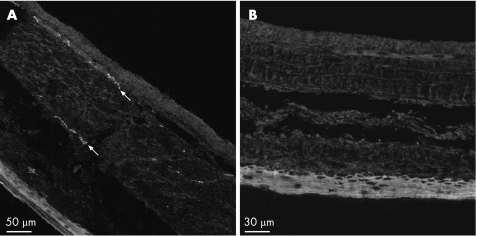
Capsaicin treated rats showed no response to an intraocular drop of capsaicin, indicating loss of function of capsaicin sensitive afferent neurones, in contrast with the writhing behaviour in vehicle treated rats. Similarly, CGRP immunoreactivity was clearly present in nerve fibres and myenteric ganglia throughout the gastric wall in vehicle treated rats (fig 3A) but was absent in the gastric wall obtained from capsaicin treated rats (fig 3B).
Figure 3 Effect of capsaicin pretreatment on calcitonin gene related peptide (CGRP) content of the gastric wall (non‐glandular region), as determined by immunocytochemistry. Note the presence of CGRP‐like immunoreactivity in a nerve fibre and a myenteric ganglion in vehicle treated rats (A, arrows, longitudinal axis) which is no longer visible in capsaicin treated rats (B, circular axis).
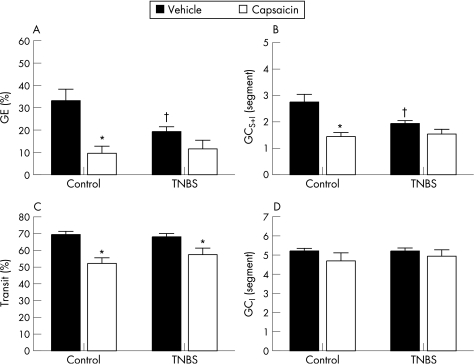
Capsaicin pretreatment had a profound inhibitory effect in control rats for all parameters of gastrointestinal motility, except for the GCI (fig 4A–D). Capsaicin decreased gastric emptying by 71% in control rats (p<0.01) (fig. 4A). This inhibitory effect was less pronounced and not significant in TNBS treated rats: in this group, capsaicin reduced gastric emptying by 40% (NS). This was reflected in the GCS+I which was decreased in capsaicin treated controls compared with vehicle treated controls (p<0.01) (fig 4B). This reduction was also present but again not significant in rats with TNBS colitis. Capsaicin significantly impaired intestinal transit in both controls and TNBS treated rats without significant interaction between the factors (fig 4C). Capsaicin pretreatment had no effect on GCI in control or TNBS treated rats (fig 4D).
Figure 4 Effect of capsaicin pretreatment on (A) gastric emptying (GE), (B) the geometric centre of transit including the stomach (GCS+I), (C) the distal front of intestinal transit and (D) the geometric centre of transit omitting the stomach (GCI). Results are expressed as percentage gastric emptying (%GE), percentage intestinal transit (% transit) and segment number (GCS+I and GCI), and are mean (SEM) for n = 12. *p<0.05, significantly different from respective vehicle treatment; †p<0.05, significantly different from vehicle treated controls, two way ANOVA with Student–Newman–Keuls post hoc analysis.
Capsaicin pretreatment may modulate the inflammatory response itself.14 However, vehicle and capsaicin pretreated rats with TNBS induced colitis showed no differences in macroscopic score (9 (8–10) and 9 (7.5–10), respectively) or MPO content (54.9 (5.9) U/g and 54.4 (7.9) U/g, respectively) (NS).
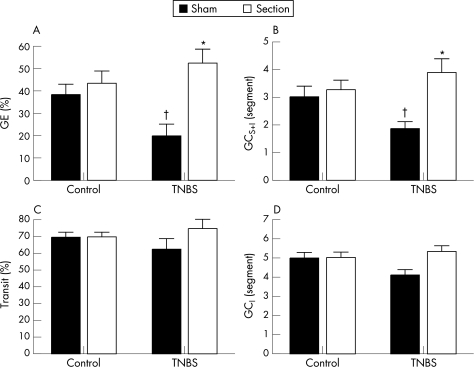
Effects of pelvic nerve section on TNBS colitis induced gastroparesis
In controls, pelvic nerve section had no significant effect on gastric emptying compared with sham operated rats (NS) (fig 5A). In contrast, this procedure significantly accelerated gastric emptying in TNBS treated rats (p<0.01). A similar pattern was evident in the geometric centre of gastrointestinal transit where pelvic nerve section increased GCS+I in TNBS treated rats (p<0.05) while it had no effect on GCS+I in control rats (fig 5B). Neither TNBS colitis nor pelvic nerve section had any effect on the front of intestinal transit (fig 5C). GCI was similarly unaffected (fig 5D). No significant differences in macroscopic score (7 (6–8) and 8 (7.5–9)) or MPO content (30.3 (9.5) U/g and 36.4 (4.8) U/g, respectively) were evident in sham operated compared with sectioned TNBS treated rats (p>0.05).
Figure 5 The effect of pelvic nerve section on (A) gastric emptying (GE), (B) the geometric centre of transit including the stomach (GCS+I), (C) the distal front of intestinal transit and (D) the geometric centre of transit omitting the stomach (GCI). Results are expressed as percentage gastric emptying (%GE), percentage intestinal transit (% transit) and segment number (GCS+I and GCI), and are mean (SEM) for n = 12. *p<0.05, significantly different from respective sham operated rats; †p<0.05, significantly different from sham operated controls, two way ANOVA with Student–Newman–Keuls post hoc analysis.
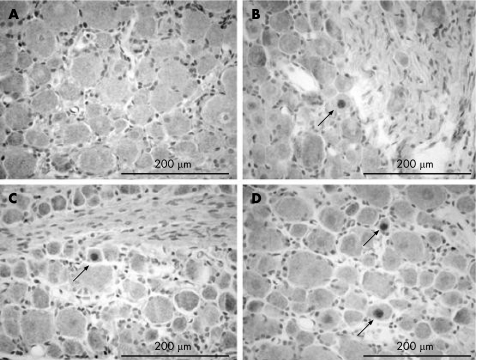
Effects of TNBS induced colitis on c‐Fos expression in DRG S1
In control rats with sham distension, no Fos immunoreactivity was identified in the nuclei of DRG S1 (fig 6A). Fos expressing cells only became visible if prior distension was applied (fig 6C) and represented 0.33 (0.01)% of the total DRG S1 neuronal cell body count (p<0.05 compared with sham distension). In rats with TNBS induced colitis, however, positive cells were already present after sham distension (0.29 (0.03)%) (fig 6B). This number increased proportionally to 0.58 (0.01)% if prior distension was applied (p<0.05 compared with sham) (fig 6D).
Figure 6 Fos immunoreactivity in nuclei of the dorsal root ganglion S1 in (A) control rats and in (B) trinitrobenzene sulphate (TNBS) treated rats without a prior distension period; and in (C) Control rats and (D) TNBS treated rats who underwent colorectal distension prior to tissue presevation. Arrows indicate nuclei staining positive for Fos.
Discussion
In this study, we have reported on the presence and pathogenesis of disturbances in gastric motility in rats with experimental distal colitis. This phenomenon has also been described in patients with IBD8,9 where it may contribute to the frequent occurrence of functional dyspepsia‐like symptoms.3
Disorders of gastric emptying in rats with experimental colitis were previously suggested by McHugh et al26 who weighed gastric content after administration of a large quantity of a high fat diet. They suggested that rats with TNBS induced colitis suffer from reduced gastric emptying capacity, leading to reduced food intake. However, the rats in that study were deprived of food for only 24 h before the experiment, which complicates objective measurements of gastric emptying as we observed that at this time point the stomach was empty in controls but not in rats with TNBS induced ileitis14 or colitis. We used a non‐nutrient semiliquid meal containing the dye Evans blue to measure gastrointestinal motility. Using these techniques, our study showed conclusively that rats with TNBS colitis suffered from impaired gastric emptying and a reduced geometric centre of gastrointestinal transit when the stomach was included. We found no evidence of inflammatory changes at the level of the stomach. Nevertheless, we cannot completely exclude a systemic component acting at sites beyond the scope of our study, such as the central nervous system.27 The manifest therapeutic effects of both hexamethonium and pelvic nerve section, however, suggest that the mechanism responsible for the colitis induced gastroparesis is predominantly neural and peripheral in origin.
In this study, impaired gastric motility in rats with TNBS induced colitis was reversed by pretreatment with the nicotinic acetylcholine receptor blocker hexamethonium. Because neuroneuronal synapses of the autonomic and enteric nervous system are cholinergic (and nicotinic) in nature, it is not surprising that hexamethonium reduced all parameters of gastrointestinal motility in healthy controls, as previously described in vivo20 and in vitro.28 The effects of hexamethonium were different in TNBS treated animals compared with controls: hexamethonium restored gastric emptying and GCS+I to normal levels while it no longer inhibited the front and GCI of intestinal transit. This suggests that even in a situation where colitis has no distinct in vivo effects on intestinal transit, the synaptic circuitry of small intestinal motor function is significantly altered.
Aside from this, the therapeutic effect of hexamethonium on colitis induced gastroparesis clearly shows that synaptic neuronal transmission is responsible for its pathophysiology although it does not disclose the trajectory of the pathway which may be either intrinsic (enteric nervous system) or extrinsic (extramural autonomic reflex).
Therefore, we subsequently investigated the effect of capsaicin pretreatment on colitis induced gastroparesis. Capsaicin selectively deactivates extrinsic sensory neurones29 and may modulate the severity of the inflammatory response.14 Capsaicin pretreatment did not influence the macroscopic score of colitis or colonic MPO content while it dramatically decreased gastric emptying in controls. This finding stresses the important role of the extrinsic nervous system of the gut in a normal physiological context, with a particular role for capsaicin sensitive small diameter afferent neurones.30 Because of the major effects in controls, interpretation of the influence of capsaicin pretreatment is problematic. However, we found that the inhibitory effects of pretreatment were much less pronounced in rats with TNBS induced colitis which may be due to a floor effect as the gastric emptying rate in vehicle treated colitis rats was already very low.
To objectify the role of extrinsic afferent pathways, and considering the lack of an alternative efficacious pharmacological tool to defunctionalise this neuronal population, we surgically denervated the distal colon. The distal colon is innervated by two anatomically and functionally distinct nerve populations.31 The hypogastric and lesser splanchnic nerve project to the inferior mesenteric ganglion and the thoracolumbar spinal cord (DRG T13–L2). The pelvic nerve projects to the lumbosacral spinal cord (DRG L6–S1). We chose to section the latter as it is involved physiologically in wide dynamic range mechanosensation32 and in gut reflex behaviour.33 Moreover, this neural pathway is susceptible to sensitising stimuli such as inflammatory mediators.34 We showed that this pelvic nervous system is involved in colitis induced changes in gastric motility as its surgical elimination restored gastric emptying rates to normal levels in TNBS treated rats. In addition, our finding that Fos expression in DRG S1 was absent in controls but present in rats with TNBS colitis clearly supports the notion of a sensitising effect of colitis on pelvic afferent nerve activity. In contrast with the reported detrimental effects of vagal nerve lesion on colitis,35 pelvic nerve section did not affect the degree of inflammation. This could be due to the high degree of colitis under study, or to the effects of the splanchnic nervous system which was left intact. Nevertheless, the role of the pelvic nerve in inflammatory modulation needs to be studied in more detail and related to the effects of vagal and splanchnic modulation.
In contrast with the marked effect on gastric emptying, experimental colitis had no effect on small intestinal transit in our experiments, influencing neither the front nor the geometric centre of intestinal transit. Modulation of small intestinal physiology at sites remote from an acute inflammatory insult has been described previously by others, with conflicting results. Jacobson et al found that ileal adrenergic nerve function was significantly impaired in a rat model of colitis.36 They attributed this to the systemic spread of interleukin (IL)‐1β originating from the colon. Blandizzi et al reported that experimental colitis attenuated small intestinal nerve function by enhanced expression of prejunctional inhibitory α2a‐adrenoceptors,37 associated with in vivo reduction of the front of intestinal transit. This is in contrast with the results of Aubé et al who found an increase in the frequency of ileal migrating motor complexes in rats with TNBS induced colitis, suggesting enhanced intestinal propulsion.13 In our experiments, colitis had no consistent effect on small intestinal transit. Inhibition of the geometric centre of gastrointestinal transit, including the stomach (GCS+I), in the colitis group was mainly a result of the attenuated gastric emptying component embedded in this parameter. Indeed, if we omitted the stomach from the calculation of the geometric centre of intestinal transit, no differences remained between control and colitis rats.
From our data, we can hypothesise that inflammation of the colon results in sensitisation of extrinsic afferent nerve fibres leading not only to hyperalgesia38,39 but also to hyperactive neuronal reflex pathways affecting gastric motor function. Similar mechanisms have been described for intrinsic gut reflexes as small intestinal inflammation was found to sensitise the local peristaltic reflex apparatus resulting in hypermotility and diarrhoea.40 The phenomenon of colitis induced gastroparesis can thus be seen as the sensitised state of the extrinsic gut reflex, termed cologastric inhibition.41 This represents a potential new strategy in evaluating the in vivo effects of sensitising gut stimuli.
In conclusion, we have shown that rats with TNBS induced colitis suffer from impaired gastric emptying in the absence of local gastric inflammation that is mediated by ganglionic neurotransmission. The neuroanatomical pathway guiding this information from the colon to the stomach involves the afferent portion of the pelvic nerve. These results suggest the existence of inflammation induced “hyperreflexia” next to the well known inflammatory hyperalgesia, and thus provide a novel tool to study the in vivo consequences of colonic afferent nerve sensitisation in the rat. This could contribute to a better understanding of the pathophysiology of motility and sensitivity disturbances in patients with IBD3 which are known to have a significant impact on the quality of life during both inflammatory episodes and periods of remission.6
Abbreviations
ANOVA - analysis of variance
CGRP - calcitonin gene related peptide
DRG - dorsal root ganglion
GCS+I - geometric centre of stomach and intestine
GCI - geometric centre of intestine
IBD - inflammatory bowel disease
MPO - myeloperoxidase
PBS - phosphate buffered saline
TNBS - trinitrobenzene sulphate
Footnotes
Funding: Heiko De Schepper is an aspirant of the Fund for Scientific Research, Flanders (FWO). This work was supported financially by the interuniversity Poles of Attraction (IUPA) program P5/20 and by the FWO (grant No G.0200.05).
Conflict of interest: None.
References
- 1.Podolsky D K. Inflammatory bowel disease. N Engl J Med 2002347417–429. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer S B. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 200612(Suppl 1)S3–S9. [DOI] [PubMed] [Google Scholar]
- 3.Pezzone M A, Wald A. Functional bowel disorders in inflammatory bowel disease. Gastroenterol Clin North Am 200231347–357. [DOI] [PubMed] [Google Scholar]
- 4.Isgar B, Harman M, Kaye M D.et al Symptoms of irritable bowel syndrome in ulcerative‐colitis in remission. Gut 198324190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simren M, Axelsson J, Gillberg R.et al Quality of life in inflammatory bowel disease in remission: The impact of IBS‐like symptoms and associated psychological factors. Am J Gastroenterol 200297389–396. [DOI] [PubMed] [Google Scholar]
- 6.Farrokhyar F, Marshall J K, Easterbrook B.et al Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis 20061238–46. [DOI] [PubMed] [Google Scholar]
- 7.Coulie B, Camilleri M, Bharucha A E.et al Colonic motility in chronic ulcerative proctosigmoiditis and the effects of nicotine on colonic motility in patients and healthy subjects. Aliment Pharmacol Ther 200115653–663. [DOI] [PubMed] [Google Scholar]
- 8.Annese V, Bassotti G, Napolitano G.et al Gastric emptying of solids in patients with nonobstructive Crohns disease is sometimes delayed. J Clin Gastroenterol 199521279–282. [DOI] [PubMed] [Google Scholar]
- 9.Grill B B, Lange R, Markowitz R.et al Delayed gastric emptying in children with Crohn's disease. J Clin Gastroenterol 19857216–226. [DOI] [PubMed] [Google Scholar]
- 10.Rao S C, Read N W, Davison P A.et al Anorectal sensitivity and responses to rectal distension in patients with ulcerative‐colitis. Gastroenterology 1987931270–1275. [DOI] [PubMed] [Google Scholar]
- 11.Galeazzi F, Luca M G, Lanaro D.et al Esophageal hyperalgesia in patients with ulcerative colitis: Role of experimental stress. Am J Gastroenterol 2001962590–2595. [DOI] [PubMed] [Google Scholar]
- 12.Collins S M. The immunomodulation of enteric neuromuscular function: Implications for motility and inflammatory disorders. Gastroenterology 19961111683–1699. [DOI] [PubMed] [Google Scholar]
- 13.Aubé A C, Cherbut C, Barbier M.et al Altered myoelectrical activity in noninflamed ileum of rats with colitis induced by trinitrobenzene sulphonic acid. Neurogastroenterol Motil 19991155–62. [DOI] [PubMed] [Google Scholar]
- 14.Moreels T G, De Man J G, De Winter B Y.et al Effect of 2,4,6‐trinitrobenzenesulphonic acid (TNBS)‐induced ileitis on the motor function of non‐inflamed rat gastric fundus. Neurogastroenterol Motil 20013339–352. [DOI] [PubMed] [Google Scholar]
- 15.Morris G P, Beck P L, Herridge M S.et al Hapten‐induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 198996795–803. [PubMed] [Google Scholar]
- 16.Moreels T G, Nieuwendijk R J, De Man J G.et al Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 20045399–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith J W, Castro G A. Relation of peroxidase‐activity in gut mucosa to inflammation. Am J Physiol 1978234R72–R79. [DOI] [PubMed] [Google Scholar]
- 18.De Winter B Y, Boeckxstaens G E, De Man J G.et al Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br J Pharmacol 1997120464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Depoortere I, De Winter B, Thijs T.et al Comparison of the gastroprokinetic effects of ghrelin, GHRP‐6 and motilin in rats in vivo and in vitro. Eur J Pharmacol 2005515160–168. [DOI] [PubMed] [Google Scholar]
- 20.Miller S M, Galligan J J, Burks T F. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods 19816211–217. [DOI] [PubMed] [Google Scholar]
- 21.Fukumoto S, Tatewaki M, Yamada T.et al Short‐chain fatty acids stimulate colonic transit via intraluminal 5‐HT release in rats. Am J Physiol Regul Integr Comp Physiol 2003284R1269–R1276. [DOI] [PubMed] [Google Scholar]
- 22.Holzer P, Livingston E H, Guth P H. Sensory neurons signal for an increase in rat gastric‐mucosal blood‐flow in the face of pending acid injury. Gastroenterology 1991101416–423. [DOI] [PubMed] [Google Scholar]
- 23.Holzer P, Lippe I T, Amann R. Participation of capsaicin‐sensitive afferent neurons in gastric motor inhibition caused by laparotomy and intraperitoneal acid. Neuroscience 199248715–722. [DOI] [PubMed] [Google Scholar]
- 24.De Jonge F, Van Nassauw L, Adriaensen D.et al Effect of intestinal inflammation on capsaicin‐sensitive afferents in the ileum of Schistosoma mansoni‐infected mice. Histochem Cell Biol 2003119477–484. [DOI] [PubMed] [Google Scholar]
- 25.Sabattini E, Bisgaard K, Ascani S.et al The EnVision™ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol 199851506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHugh K, Castonguay T W, Collins S M.et al Characterization of suppression of food intake following acute colon inflammation in the rat. Am J Physiol 1993265R1001–R1005. [DOI] [PubMed] [Google Scholar]
- 27.Suto G, Kiraly A, Tache Y. Interleukin 1β inhibits gastric emptying in rats: mediation through prostaglandin and corticotropin‐releasing factor. Gastroenterology 19941061568–1575. [DOI] [PubMed] [Google Scholar]
- 28.Bogeski G, Shafton A D, Kitchener P D.et al A quantitative approach to recording peristaltic activity from segments of rat small intestine in vivo. Neurogastroenterol Motil 20057262–272. [DOI] [PubMed] [Google Scholar]
- 29.Szallasi A, Blumberg P M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 199951159–212. [PubMed] [Google Scholar]
- 30.Berthoud H R, Blackshaw L A, Brookes S J.et al Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 200416(Suppl 1)28–33. [DOI] [PubMed] [Google Scholar]
- 31.Brierley S M, Jones R C, iii, Gebhart G F.et al Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 2004127166–178. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta J N, Gebhart G F. Characterisation of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 1994712046–2060. [DOI] [PubMed] [Google Scholar]
- 33.Blackshaw L A, Gebhart G F. The pharmacology of gastrointestinal nociceptive pathways. Curr Opin Pharmacol 20022642–649. [DOI] [PubMed] [Google Scholar]
- 34.Sengupta J N, Snider A, Su X.et al Effects of kappa opioids in the inflamed rat colon. Pain 199979175–185. [DOI] [PubMed] [Google Scholar]
- 35.Mazelin L, Theodorou V, More J.et al Protective role of vagal afferents in experimentally‐induced colitis in rats. J Auton Nerv Syst 19987338–45. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson K, McHugh K, Collins S M. The mechanism of altered neural function in a rat model of acute colitis. Gastroenterology 1997112156–162. [DOI] [PubMed] [Google Scholar]
- 37.Blandizzi C, Fornai M, Colucci R.et al Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by α2‐adrenoceptors in the presence of experimental colitis. Br J Pharmacol 2003139309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebhart G F. Visceral pain‐peripheral sensitisation. Gut 20004754–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gschossmann J M, Adam B, Liebregts T.et al Effect of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Eur J Gastroenterol Hepatol 2002141067–1072. [DOI] [PubMed] [Google Scholar]
- 40.Torrents D, Vergara P. In vivo changes in the intestinal reflexes and the response to CCK in the inflamed small intestine of the rat. Am J Physiol Gastrointest Liver Physiol 2000279G543–G551. [DOI] [PubMed] [Google Scholar]
- 41.Gual O, Bozkurt A, Deniz M.et al Effect of sex steroids on colonic distension‐induced delay of gastric emptying in rats. J Gastroenterol Hepatol 200419975–981. [DOI] [PubMed] [Google Scholar]