Total withdrawal of immunosuppression (TIW) without causing rejection has been reported in some stable liver recipients.1,2,3 Patient characteristics which predict this clinical tolerance have not been determined. Ursodeoxycholic acid (UDCA) has been reported to reduce the risk of early graft rejection following hepatic and cardiac transplantation.4 We conducted a double blind controlled trial of UDCA therapy followed by TIW in 26 liver recipients to (a) determine if UDCA would facilitate TIW, (b) assess the safety of attempting TIW and (c) determine predictors of success of TIW.
Patients and methods
Records were reviewed and all patients who had been free of rejection for a minimum of 2 years, and on single or double drug immunosuppression, with transaminase levels <1.5 times the upper limit of normal, were invited to participate. Twenty six (13 male) patients gave informed consent and entered the study. Baseline liver biopsies were obtained, and a priori data related to pre‐transplant and post‐transplant patient variables were recorded. UDCA (15 mg/kg) or identical placebo capsule was administered, followed by sequential withdrawal of azathioprine (AzA) or prednisone and then graded reduction in ciclosporin (CyA) dose. Endpoints were defined as graft dysfunction (alanine aminotransferase >2× normal) with biopsy confirmation of abnormalities, or 6 months of no immunosuppression and no rejection on repeated biopsy. Rescue therapy for rejection was reinstitution of previous treatment, bolus steroid treatment with tapering or conversion to Tacrolimus based therapy.
Results
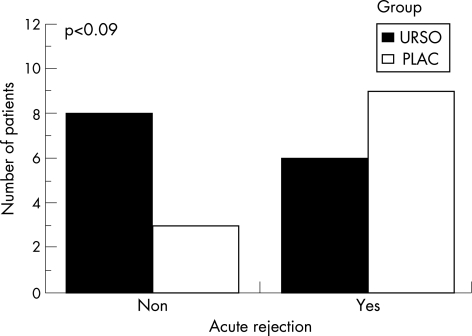
The UDCA and placebo groups had similar baseline characteristics (table 1). Rejection episodes occurred in 6/14 (43%) patients in the UDCA group and in 9/12 (75%) of those on placebo (p = 0.09) (fig 1). Time to rejection, degree of rejection (blind biopsy review) and immunosupression at the time rejection developed were similar in the two groups.
Table 1 Baseline characteristics of liver transplant recipients with ursodeoxycholic acid (UDCA) therapy and controls.
| Characteristic | UDCA group (n = 14) | Placebo group (n = 12) | p Value* |
|---|---|---|---|
| Age (y) | 56.3 (12.6) | 50.7 (15.7) | 0.3 |
| Sex (M:F) | 7:7 | 6:6 | NS |
| OLT indications (%) | |||
| Cryptogenic | 7 | 25 | |
| Cholestatic | 36 | 8 | |
| Alcoholic | 36 | 8 | |
| Autoimmune | 14 | 17 | |
| Miscellaneous | 7 | 42 | |
| No of previous rejections | 0 (1) | 0 (1) | NS |
| Time since OLT (months) | 51.8 (12.8) | 60.2 (29.2) | 0.3 |
| Biochemistry | |||
| ALT (U/l) | 18.8 (8.7) | 29.9 (29.5) | 0.1 (4–26) |
| AST (U/l) | 23.8 (9.5) | 32.8 (20.3) | 0.1 (5–27) |
| ALK PH (U/l) | 93.1 (25.2) | 123 (50.6) | 0.08 (16–98) |
| Bilirubin (μmol/l) | 12.4 (4.5) | 15.2 (6.6) | 0.2 (3.4–17.1) |
| Creatinine (μmol/l) | 144.1 (55.7) | 163.6 (60.3) | 0.3 (71–168) |
| Immunosuppression | |||
| CyA dose (mg twice daily) | 128 (42.6) | 139.58) | 0.6 |
| CyA level (ng/ml) | 130.3 (68) | 172.5 (50) | 0.1 |
| Regimens | NS | ||
| CyA+AzA | n = 8 | n = 6 | |
| CyA+prednisone | n = 2 | n = 3 | |
| CyA alone | n = 4 | n = 3 |
Data are expressed as mean (SD).
ALK PH, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AzA, azathioprine; CyA, ciclosporin; OLT, orthotopic liver transplantation.
*t test or Fisher's exact test as appropriate.
Figure 1 Number of patients with acute rejection in the ursodeoxycholic acid (UDCA) and placebo groups after complete immunosuppression withdrawal.
All responded to rescue therapy; none developed chronic rejection. All rejection episodes developed during CyA tapering, with a mean daily dose of 105 mg with whole blood levels <50 ng/ml in all patients. Only 1/6 patients (17%) with alcoholic liver developed rejection. Nine of the remaining 11 patients developed graft dysfunction without evidence of rejection. Three of four patients (75%) with autoimmune hepatitis (AIH) had recurrence of the disease with immunosuppression withdrawal.
One year after withdrawal only two patients were completely free of immunosupression usage but 4/5 previous users of prednisone were using no steroids, and the mean dose of CyA was <50% of that at entry. Age greater than 60 years, underlying primary alcoholic liver disease (ALD) and no (CyA+AzA) immunosuppression regimen were the only three favourable variables that could predict successful total immunosuppression withdrawal in 82% of cases.
Discussion
UDCA did not appear to decrease the frequency of acute rejection although the number of patients is insufficient to exclude an effect. UDCA may prevent rejection by decreasing HLA class 1 antigen and interleukin 6 expression, both important mediators in the process of transplant rejection.5,6,7 Although the rate and intensity of rejection were similar to that of previous reports,1,2,3 all but one patient responded to rescue therapy and no cases of chronic rejection or mortality were seen. The mechanism of recurrence of AIH early after withdrawal of immunosuppressions is unknown. However, HLA‐DR3 or HLA‐DR4 positive recipients are at risk of recurrence regardless of donor HLA status.8
The reasons why liver grafts develop tolerance in ALD patients after immunosuppression withdrawal in unclear. One explanation is that these patients may have continued to drink alcohol after liver transplantation which maintains some degree of immunosuppression9; however, liver biopsy at 6 months did not show any evidence of alcoholic injury. Whether these patients had more liver endothelial cell chimerism or more diminished dendritic cell numbers than those experiencing rejection is under investigation.
Conclusion
We have shown that late total immunosuppression withdrawal in stable liver transplant recipients is safe but seldom successful and most useful for patients transplanted for ALD and not for patients transplanted for autoimmune liver disease. We suggest that the search for an accurate means of identifying allograft tolerance among immunosuppressed recipients should become a priority in liver transplantation.
Footnotes
Competing interests: None.
References
- 1.Pons J A, Yelamos J, Ramirez P.et al Endothelial cell chimerism does not influence allograft tolerance in liver transplant patients after withdrawal of immunosuppression. Transplantation 2003751045–1047. [DOI] [PubMed] [Google Scholar]
- 2.Ramos H C, Reyes J, Abo‐Elmagd K.et al Weaning of immunosuppression in long term liver transplant recipients. Transplantation 199559212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandborn W J, Hay J E, Porayko M K.et al Cyclosporin withdrawal for nephrotoxicity in liver transplant recipients does not result in sustained improvement in kidney function and causes cellular and ductopenic rejection. Hepatology 199419925–932. [PubMed] [Google Scholar]
- 4.Presson H, Friman S, Schersten T.et al Ursodeoxycholic acid for prevention of acute rejection in liver transplant recipients. Lancet 199033652–53. [DOI] [PubMed] [Google Scholar]
- 5.Lazaridis K N, Gores G J, Lindor K D. Ursodeoxycholic acid mechanisms of action and clinical use in hepatobiliary disorders. J Hepatol 200135134–146. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa M Y, Tsujii T, Matsunura Y.et al Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology 199216358–364. [DOI] [PubMed] [Google Scholar]
- 7.Calmus Y, Guechot J, Podevin P.et al Differential effects of chenodeoxycholic acid and ursodeoxycholic acids on interleukin 1, interleukin and tumor necrosis factor alpha production by monocytes. Hepatology 199216719–723. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez A, Czaja A, Carpenter H.et al Recurrent autoimmune hepatitis after othotopic liver transplantation. Liver Transpl 20017302–310. [DOI] [PubMed] [Google Scholar]
- 9.Pageaux G P, Bismuth M, Perney P.et al Alcohol relapse after liver transplantation for alcoholic liver disease: does it matter? J Hepatol 200338629–634. [DOI] [PubMed] [Google Scholar]