Abstract
Background
Zinc carnosine (ZnC) is a health food product claimed to possess health‐promoting and gastrointestinal supportive activity. Scientific evidence underlying these claims is, however, limited.
Aim
To examine the effect of ZnC on various models of gut injury and repair, and in a clinical trial.
Methods
In vitro studies used pro‐migratory (wounded monolayer) and proliferation ([3H]‐thymidine incorporation) assays of human colonic (HT29), rat intestinal epithelial (RIE) and canine kidney (MDCK) epithelial cells. In vivo studies used a rat model of gastric damage (indomethacin/restraint) and a mouse model of small‐intestinal (indomethacin) damage. Healthy volunteers (n = 10) undertook a randomised crossover trial comparing changes in gut permeability (lactulose:rhamnose ratios) before and after 5 days of indomethacin treatment (50 mg three times a day) with ZnC (37.5 mg twice daily) or placebo coadministration.
Results
ZnC stimulated migration and proliferation of cells in a dose‐dependent manner (maximum effects in both assays at 100 µmol/l using HT29 cells), causing an approximate threefold increase in migration and proliferation (both p<0.01). Oral ZnC decreased gastric (75% reduction at 5 mg/ml) and small‐intestinal injury (50% reduction in villus shortening at 40 mg/ml; both p<0.01). In volunteers, indomethacin caused a threefold increase in gut permeability in the control arm; lactulose:rhamnose ratios were (mean (standard error of mean)) 0.35 (0.035) before indomethacin treatment and 0.88 (0.11) after 5 days of indomethacin treatment (p<0.01), whereas no significant increase in permeability was seen when ZnC was coadministered.
Conclusion
ZnC, at concentrations likely to be found in the gut lumen, stabilises gut mucosa. Further studies are warranted.
Currently, there is much interest in the value of natural medicinal products, functional foods and “nutriceuticals” to prevent or treat illness. Unfortunately, current evidence of the scientific validity of many of these traditional and commercial compounds is severely limited.
One such product is zinc carnosine (ZnC), which is an artificially produced derivative of carnosine, where zinc and carnosine are linked in a one‐to‐one ratio to provide a polymeric structure. This product is currently marketed by several companies as a zinc dietary supplement with “added value for gastric health”. Combining zinc with carnosine could theoretically provide added benefits over simple zinc supplementation as carnosine is a dipeptide (comprising β‐alanine and l‐histidine) that is naturally present in long‐living cells such as muscle and nerves, where, among other actions, it probably has a role as an antioxidant.1
To examine further its potential biological actions in a scientific setting, we have performed a series of studies to analyse ZnC in regard to its effects on various mechanisms of gut integrity and repair using well‐validated in vitro and in vivo models, and in a clinical trial.
Materials and methods
All chemicals were purchased from Sigma (Poole, Dorset, UK) unless otherwise stated. ZnC was provided by Lonza Nutrition (USA).
Ethics
All animal experiments were approved by local animal ethics committees and covered by the appropriate licences under the Home Office Animals Procedures Acts, 1986. The clinical trial was approved by a local ethics committee and conformed to national requirements.
Study series A: Effect of ZnC on in vitro models of repair
Background to methods
One of the earliest repair responses after injury to tissue is the migration of surviving cells over any denuded area to re‐establish epithelial integrity. As it is extremely difficult to study this effect in a human or animal, cell culture models are commonly used as surrogate markers of this pro‐migratory response.
Cell migration as a model of wound repair
Cell migration assays were performed using our previously published methods.2 Two cell lines were assessed: the human colonic carcinoma cell line HT29 and the canine epithelial kidney cell line MDCK.
Cells were grown to confluence in six‐well plates in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal calf serum at 37°C in 5% CO2 and were then serum starved for 24 h. The monolayers were then wounded by scraping a disposable pipette tip across the dishes, washed with fresh serum‐free medium and cultured in serum‐free medium in the presence of 1–1000 μM ZnC, equimolar zinc sulphate or equivalent bovine serum albumin (BSA) concentrations (to analyse non‐specific protein effects). Additional monolayers treated with ZnC also had the proliferation inhibitor mitomycin C added at 5 μg/ml to examine whether wound closure was dependent on cell proliferation. The rate of movement of the anterior edges of the wounded monolayers was determined by taking serial photomicrographs at various times after wounding.2 An inverted microscope (Nikon TS100) and a Nikon Coolpix 800 digital camera with 125‐fold magnification were used to obtain photomicrographs. Identical regions were examined at each time point by pre‐marking the base of the plates to facilitate alignment. In all, 20 measurements/field were taken by placing a transparent grid over the photograph and measuring the distance moved from the original wound line. All results are expressed as mean (standard error of mean (SEM)) of three separate experiments.
Cell proliferation
Cell proliferation assays were performed using our previously published methods.3 For the proliferation assays, HT29, MDCK and the rat intestinal epithelial (RIE) cell line were used.
Cells were grown in DMEM containing glutamine and 10% fetal calf serum. Effects of ZnC, zinc sulphate and BSA (to analyse non‐specific protein effects) were subsequently tested under serum‐starved conditions. To assess the rate of cells entering DNA synthesis, [3H]‐thymidine (2 µCi/well) was included 24 h after addition of the test factors, and cells were left for a further 24 h. For each condition, the effects of the solutions were measured in six separate wells. Cell viability, determined by the ability to exclude 0.2% trypan blue, was >90% in all cell lines.
Study series B: Effect of ZnC on in vivo models of repair
Although cell culture studies provide valuable information regarding potential bioactivity, additional information may be gained by extending studies to the in vivo situation. The ability of ZnC to reduce gastric and small‐intestinal damage in rodents was therefore assessed using well‐validated models.2,4
Effect of ZnC on the rat model of gastric damage
Methods used were based on those described previously.2 Male Sprague Dawley rats (225–275 g) were housed in standard cages and fed standard laboratory chow (Special Diet Services, Essex, UK) and tap water ad libitum.
Rats were randomised to receive, by gastric gavage, one of the following factors: 1 ml of saline (negative control), ZnC (1 or 5 mg/ml), or epidermal growth factor (EGF) (25 μg/ml, positive control). All gavage solutions also contained 2% hydroxy‐propyl‐methylcellulose to delay gastric emptying. Thirty minutes after the gavage, all rats received indomethacin (20 mg/kg subcutaneously) and were placed in Bollman‐type restraint cages.
Three hours after indomethacin administration, animals were killed by stunning and cervical dislocation. The stomach was removed and the intragastric pH was determined using a micro pH electrode; the stomach was then inflated with 4 ml of 10% neutral buffered formalin. The next day, the stomachs were opened and placed in fresh formalin before assessment. The stomachs were randomly coded, and all analyses of gastric damage were blindly assessed. Macroscopic injury was assessed using a dissecting microscope (×10) with the aid of a reference square grid. The stomachs were then embedded in wax and the extent of damage assessed microscopically, as previously described.2 Using this system, each stomach was given a score from 0 to 4, where 0 = no damage, 1 = one small erosion (<0.5 mm), 2 = two small or one large erosion (>0.5 mm), 3 = two or more large erosions and 4 = any area of ulceration extending to the muscularis mucosa.
Effect of ZnC on small‐intestinal growth and injury in mice
Methods used were based on those described previously.4 Mice were fed standard laboratory chow (Special Diet Services) and allowed access to water ad libitum. Four groups of animals (n = 6 per group) were used; two groups had ZnC (40 mg/ml) supplemented to their drinking water for 7 days and the other two groups received normal tap water throughout. All animals received a single subcutaneous injection of either indomethacin (85 mg/kg) or carrier alone 12 h before killing. In previous studies we have shown that, at 12 h, the amount of indomethacin induced small intestinal injury is the greatest,4 which is why this time point was chosen. In addition, 1 h before killing, all animals received a single injection of bromodeoxyuridine (BrdU; 50 mg/kg, intraperitoneal) to allow assessment of intestinal proliferation.
After killing, the various sections of the intestine were dissected free. To maintain consistency between animals, the length of the small intestine was expressed as 100% and two serial 1 cm samples were taken from the small intestine at 30% of small‐intestine length (defined as jejunum). The proximal 1 cm segment was collected in Carnoy's solution, left at room temperature for 4 h and stored in 70% alcohol until further assessment of morphology and morphometry. The second 1 cm segment was fixed in neutral buffered formalin for subsequent analysis of proliferation.
Assessment of morphology
Using methods described previously,4 microdissected samples were assessed for villus height and width by tracing the outline of the villi using a precalibrated drawing tube. The tracings were then used to measure the height and width of the villi. In all, 20 individual villi were assessed in each animal, and the mean value from these 20 measurements was used in the subsequent analysis of variance (ANOVA).
Assessment of proliferation
Tissue sections were cut and stained for BrdU and counterstained with haematoxylin. Slides were examined until well‐orientated crypts were found. The number of labelled mitotic cells/crypt was recorded for each animal. In all, 20 crypts/animal were scored at each site and the mean number of labelled cells used in the subsequent ANOVA.
Study series C: Effect of ZnC on indomethacin‐induced changes in gut permeability in humans
Background to method
Assessment of intestinal permeability by quantitating unmediated absorption of at least two sugars of different sizes provides a sensitive index of intestinal damage.5 In a previous study using this method of assessing permeability,6 we included lactulose as the disaccharide probe, and rhamnose and mannitol as two alternative monosaccharide probes in a hypo‐osmolar formulation. Both rhamnose and mannitol have been widely used and provide similar information regarding changes in “paracellular pathways”. However, subsequent analyses showed that the “mannitol peak” in urine samples is sometimes obscured by overlap from other urinary constituents and we therefore presented only data on lactulose and rhamnose.6 To maintain consistency between studies (including osmolality), we used the same mixture as previously, but again, only present the lactulose:rhamnose ratios. This is considered appropriate as this specific combination has been recommended for assessing enteropathy induced by non‐steroidal anti‐inflammatory drugs (NSAIDs).7
Protocol
After an overnight fast, participants emptied their bladders and then drank a standardised sugar solution containing 5 g lactulose, 2 g mannitol and 1 g rhamnose in a total of 450 ml water (calculated osmolality 69 mOs). Participants were allowed unlimited intake of fluid after the first hour of the test to ensure adequate urine output. The urine was collected and pooled over the next 5 h and total volume recorded. Aliquots were centrifuged briefly to remove gross debris and the supernatant was frozen at −20°C until later analysis.
Analyses
Analyses of sugar content in the urine were based on the methods used by our group previously.6 The various sugars were separated using high‐pressure liquid chromatography (HPLC) and quantitated using a pulsed amphometric detector. Using this technique, sugars are oxidised on the gold electrode at the working potential (P = 0.05 V), the current produced being a measure of the amount of sugar present in the sample.8
The system comprised a Hewlett Packard 1100 series HPLC system (Hewlett Packard UK, Bracknell, Berkshire, UK) using a Dionex CarboPac PA10 (Dionex UK, Camberley, Surrey, UK) anion exchange analytical column (4.5×250 mm) with an equivalent guard column. As the metabolite phase, 50 mM NaOH (low in carbonate, BDH Merck (VWR International, Lutterworth, Leicestershire, UK)) was used (1 ml/min, isocratic conditions). Sugars were detected using a Hewlett Packard 1049A Electrochemical Detector with a gold working electrode and a solid reference electrode. The potentials were set as follows: P = 0.05 V, P1 = 0.6 V, P2 = −0.8 V; and T1 = 120 ms, T2 = 120 ms, T3 = 400 ms. Data were analysed using the Hewlett Packard Chemstation software.
Study design
Preliminary studies
To determine the reproducibility of results, a single individual performed permeability studies for four separate days (while not taking any test treatment or NSAIDs). These samples were assayed to determine intrapatient variation and gave a coefficient of variation of about 15%. In addition, a single sample was measured four times to determine intra‐assay variation and gave a coefficient of variation of 8%. These coefficients of variation were similar to those reported by us previously.6
To examine whether ZnC influenced permeability under basal conditions, four participants underwent an initial permeability assessment and then ingested ZnC for 7 days, with a further assessment on the final day.
Main study
Ten volunteers (24–40 years, 5 men and 5 women) who were not taking NSAIDs and who had no conditions likely to affect intestinal permeability—for example, coeliac disease or previous intestinal surgery—were entered into the study. Participants abstained from alcohol consumption and ingestion of any NSAID, including aspirin, for 1 week before starting the study and throughout the remainder of the test period.
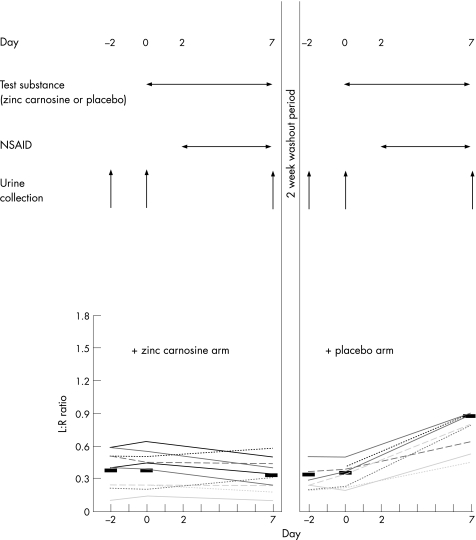
Each participant undertook a total of six permeability assessments (Fig 1). Each arm of the study comprised three collections on days ‐2, 0 and 7. For both arms, samples collected on days ‐2 and 0 were “baseline” analyses when the volunteer was not taking any test substance. After urine collection on day 0, volunteers took the test substance comprising ZnC (37.5 mg) or placebo capsules twice a day for a week. ZnC and placebo capsules were indistinguishable in terms of appearance and taste.
Figure 1 Influence of zinc carnosine (ZnC) on changes in small bowel permeability caused by indomethacin. Healthy volunteers (n = 10) participated in a double‐blind randomised controlled crossover protocol. Each arm comprised two baseline urine collections, followed by a third at the end of that study period. In each arm, volunteers took ZnC (37.5 mg twice daily, orally) or placebo for 7 days, with indomethacin for the final 5 days. Upper panel of the figure shows protocol and lower graph shows individual results expressed as lactulose:rhamnose (L:R) ratios. Black bars show mean values for each stage. When taking placebo, volunteers had a threefold increase in L:R ratios in response to indomethacin administration (p<0.01), whereas no increase was seen if ZnC was also being taken. NSAID, non‐steroidal anti‐inflammatory drug.
After the week's course of test substance (day 7), the third urine collection for that arm of the study was completed. After a 2‐week washout period, each participant repeated the protocol with the other test capsule. The active and placebo capsule arms of the study were administered in random order. In addition, in both arms of the study, for the last 5 days of the study (days 2–7), participants also received an NSAID (indomethacin 50 mg three times a day; fig 1).
Statistics
For the in vitro and in vivo studies, data were analysed by one‐way or two‐way ANOVA as appropriate, using test condition as a factor. For the clinical study, ANOVA was performed using patient, time and arm of study as factors. For all studies, when a significant effect was found (p<0.05) in the ANOVA, individual comparisons were made using t tests based on the residual and degrees of freedom obtained from the ANOVA, a method equivalent to repeated‐measures analyses.
Results
Study series A: Effect of ZnC on in vitro models of repair
Cell migration as a model of wound repair
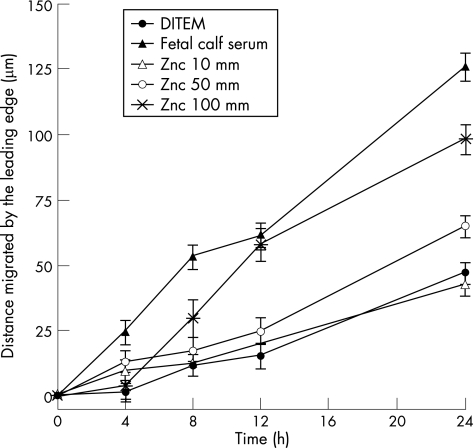
For both HT29 cells and MDCK cells (data not shown), ZnC induced cell migration in a dose‐dependent fashion (fig 2). At concentrations >10 μM, ZnC induced pro‐migratory effects, with maximal response seen at 100 μM (about a threefold increase above baseline rates). Concentrations of ZnC >100 μM did not increase the rate of migration further (data not shown). The equimolar concentrations of zinc sulphate and BSA had no significant effect on the rate of migration (data not shown). As expected, these effects were not dependent on proliferation, as the presence of mitomycin C did not affect the rate of migration induced by ZnC (data not shown).
Figure 2 Effect of zinc carnosine (ZnC) on wound healing as assessed by cell migration. Standard wounds were inflicted on monolayers of human colonic HT29 cells and degree of movement assessed in the presence or absence of various concentrations of ZnC. Negative control was Dulbecco's modified Eagle medium (DMEM) alone, positive control (10% fetal calf serum). Maximal stimulation by ZnC was seen at 100 μM, where p<0.01 versus negative control for all times after 4 h. Addition of equimolar amounts of bovine serum albumin or zinc sulphate did not increase the rate of migration (data not shown). Similar results were seen when ZnC was tested on the canine epithelial cell line MDCK (not shown).
Cell proliferation
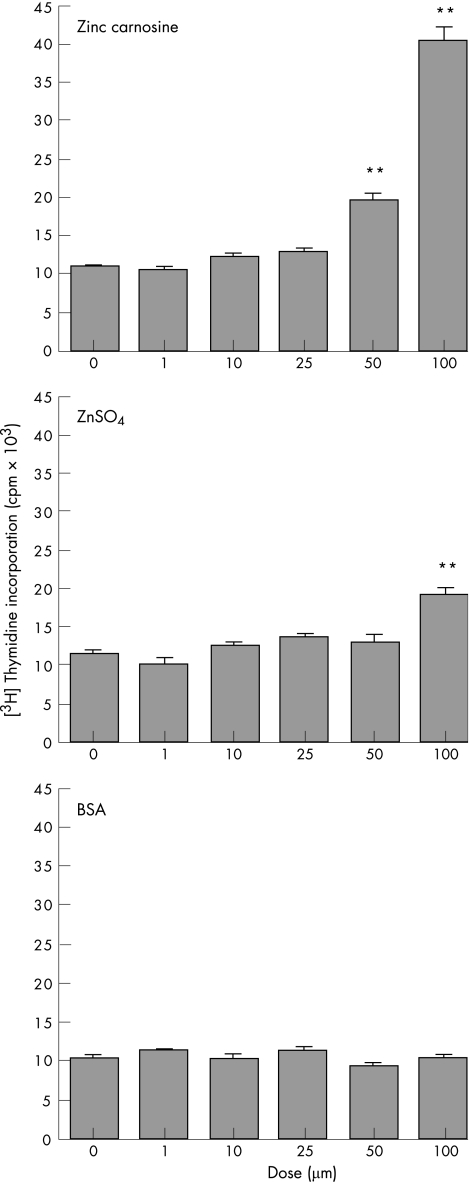
ZnC induced a dose‐dependent increase in [3H]thymidine in HT29 cells (fig 3, top graph). The maximal response was seen at 100 μM ZnC, with higher doses not increasing incorporation further (data not shown). Addition of equimolar zinc sulphate showed that a minor element (about 26%) in this increase in [3H]thymidine may be due to the presence of zinc (fig 3, middle graph). Addition of equimolar BSA did not increase proliferation (fig 3, bottom graph). Similar results were seen in MDCK and RIE cell lines (data not shown).
Figure 3 Effect of zinc carnosine (ZnC) on proliferation. HT29 cells were incubated in the presence of various concentrations of ZnC, zinc sulphate or bovine serum albumin (BSA). Proliferation was assessed using [3H]‐thymidine incorporation. ZnC stimulated proliferation in a dose‐dependent manner, with maximal increase seen at 100 μM. **p<0.01 versus negative control (0 dose). Similar results were seen when rat intestinal epithelial or canine epithelial cells were studied (data not shown). ZnSO4, zinc sulphate.
Study series B: Effect of ZnC on in vivo models of repair
Rat model of gastric damage
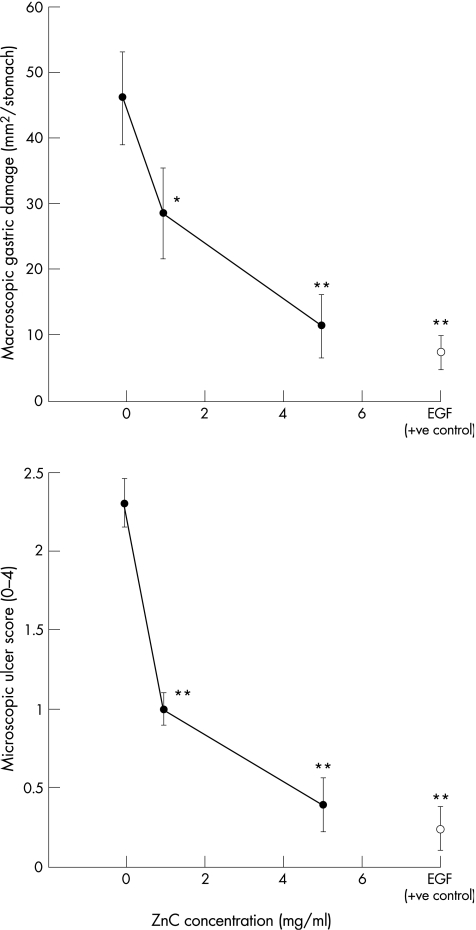
Animals that received indomethacin and restraint and a placebo gavage had a mean (SEM) macroscopic gastric damage score of 46 (7) mm2/stomach. Administration of ZnC caused a dose‐dependent reduction in the amount of gastric injury (fig 4, upper graph). This equated to a 38% reduction in those animals that had received 1 mg/ml of ZnC and a 75% reduction in animals that had received 5 mg/ml of ZnC. Histological assessment using the microscopic scoring system gave results similar to those seen macroscopically (fig 4, lower graph). Administration of the ZnC at 5 mg/ml gave a gastric protective effect that was roughly similar to that seen in animals that had received the potent cytoprotective agent, EGF, at 25 μg/ml (fig 4). Assessment of gastric pH showed that all these effects were independent of change in pH (all gastric pH values were in the range of 1–3).
Figure 4 Effect of zinc carnosine (ZnC) on rat model of gastric damage. Rats (n = 6–8) were given an oral gavage (1 ml) of saline (negative control), epidermal growth factor (EGF) (25 μg/ml, positive control) or ZnC (1 or 5 mg/ml). All solutions also contained 2% HPMC to delay gastric emptying. After 30 min, animals were given indomethacin (20 mg/kg, subcutaneous), placed in restraint cages and left for a further 3 h. At the end of the study, animals were killed, and the extent of macroscopic (top graph) and microscopic (lower graph) damage determined. Data presented as mean (SEM), *p<0.05 and **p<0.01 versus saline control, respectively.
Effect of ZnC on small‐intestinal growth and injury in mice
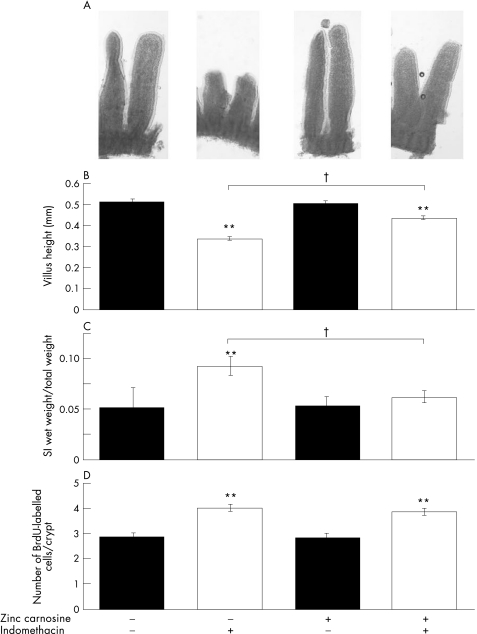
Under non‐damaged (non‐indomethacin treated) conditions, administration of ZnC did not influence growth of small intestine or colon as measured by wet weight, villus height or BrdU incorporation (fig 5).
Figure 5 Effect of zinc carnosine (ZnC) on murine small‐intestinal injury. The effect of ZnC supplementation to the drinking water for 7 days on small‐intestinal morphology (A), morphometry (B), wet weight (C) and proliferation (bromodeoxyuridine (BrdU) staining), (D) was determined under baseline (non‐damaging) circumstances and in mice treated with indomethacin (85 mg/kg subcutaneous) 12 h before killing. ZnC supplementation did not influence parameters under baseline circumstances, but reduced the degree of injury caused by indomethacin. Data expressed as mean (SEM) of n = 6 per group. †p<0.01 versus results from animals not given ZnC or indomethacin. †p<0.01 comparing animals that had received indomethacin with or without ZnC.
Control animals given indomethacin showed the typical morphological response of villus shortening and blunting (fig 5A). Morphological changes in animals that had also received ZnC were far less marked (fig 5A). These results were reflected in the morphometric data, where indomethacin caused a reduction in villus height of about one third (p<0.01), whereas only a minor degree of villus shortening (about 14%) was seen in animals cotreated with ZnC (fig 5B). Similarly, indomethacin increased the wet weight of the small intestine in control animals but not in those given ZnC (fig 5C). Assessment of BrdU staining showed that proliferation of jejunal crypt cells was increased in response to indomethacin in both control and ZnC‐treated animals, and was not influenced by the coadministration of ZnC (fig 5D).
Study series C: Effect of ZnC on indomethacin‐induced changes in gut permeability in humans
Elution profiles
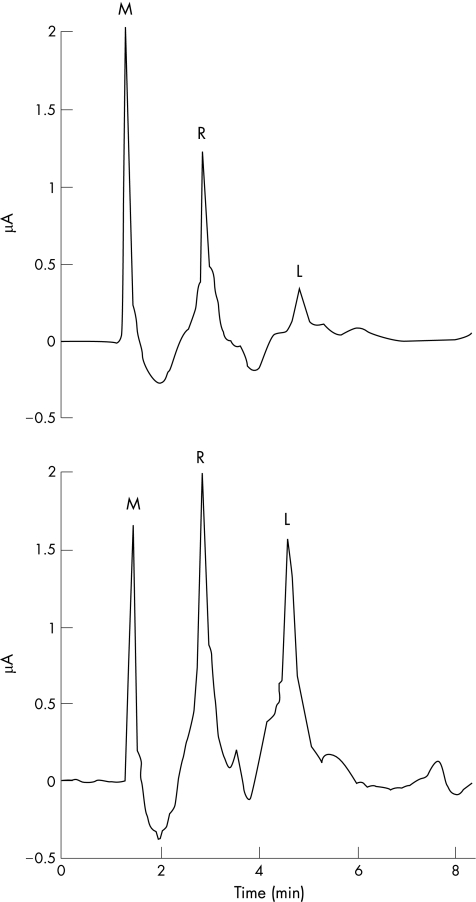
Typically, the mannitol peak eluted from the column at about 1.5 min, rhamnose at 3 min and lactulose at 4.5 min (fig 6). Analysis of single and mixed sugar standards in the concentration range 0.05–50 mg/ml showed good chromatographic separation and proportional changes in the area under the curve (data not shown).
Figure 6 Typical elution profiles of urinary samples from a volunteer who has taken a hypo‐osmolar drink containing test sugars. Samples were run on an isocratic high‐pressure liquid chromatography (HPLC) column and sugars detected using pulsed amphometric detection. The peaks relating to lactulose (L) and rhamnose (R) were always well resolved. Although prominent in these samples, the mannitol (M) peak was occasionally lost within a non‐specific early peak. The top panel shows initial results from the participant and the lower panel shows equivalent results from the same participant who had a repeat test after 5 days of indomethacin treatment (50 mg, three times a day). In the second test, there is a marked increase in the lactulose peak.
Preliminary studies
Administration of ZnC to the participants not taking NSAIDs had no effect on their permeability results (L:R ratios 0.49 (0.18) before ZnC treatment and 0.52 (0.17) at the end of treatment).
Main study
All 10 participants completed the study without protocol violations. Two of ten participants developed mild nondescript upper abdominal discomfort while taking the indomethacin (control) arm, but completed the course. None reported symptoms during the ZnC (plus indomethacin) arm, and no side effects due to ingestion of ZnC were reported.
Baseline permeability values were similar at the beginning of each study arm, and at the first and second (baseline) assessments within a study arm (fig 1, lower panel). Permeability increased about threefold in response to indomethacin during the control arm (p<0.01 v baseline values). By contrast, when the participants were taking ZnC, indomethacin did not cause any significant increase in gut permeability (fig 1, lower panel). The order in which control and ZnC was administered did not influence results (although numbers are too small to perform detailed statistical analysis).
Discussion
We have shown that ZnC, which is currently commercially available in health food stores, stimulates several aspects of gut mucosal integrity. It stimulated cell migration and proliferation in vitro, and reduced the amount of gastric and small‐intestinal injury in rats and mice. We also showed that if volunteers ingested ZnC at the levels suggested by the health food supplement industry, it could prevent the rise in gut permeability caused by standard clinical doses of the NSAID indomethacin.
For the in vitro studies, RIE, human colonic (HT29) and canine epithelial kidney MDCK cells were used to examine effects on cells from different species and regions. Similarly, the in vivo studies examined the potential beneficial effect of ZnC on gastric (indomethacin‐ and restraint‐induced) damage in rats and small‐intestinal (indomethacin‐induced) damage in mice. We have previously validated these models for other bioactive agents such as bovine colostrums,3 and NSAIDs, such as indomethacin, continue to be a major cause of morbidity and mortality in humans.9 Caution always has to be shown, however, in extrapolating results obtained from in vitro cancer cell lines and animal models to the human situation.
Our initial in vivo study showed the protective effects of orally administered ZnC using the rat gastric damage model. As gastric pH was not affected by ZnC administration, it can be described as a “cytoprotective agent”. This result supports and extends the findings of Naiti et al,10 who examined the effect of ZnC on aspirin‐induced gastric injury and also found a protective effect. We then went on to examine the effects of ZnC on small‐intestinal injury, which may have more clinical relevance, and showed a similar protective effect.
In view of the positive result of small bowel protection seen in the in vivo model, we performed a double‐blind, randomised, crossover, controlled clinical trial examining the potential effects of ZnC on indomethacin‐induced increases in gut permeability. Measurement of gut permeability is safe and relatively simple to perform, but has the limitation of being an indirect method of assessing small‐intestinal injury. Assessment of excretion of two molecules of different sizes, such as a monosaccharide and a disaccharide, with HPLC with pulsed amphometric detection provides high sensitivity and allows correction for potential confounding factors such as changes in the rate of gastric emptying and small‐intestinal transit. Although other methods to quantitate small‐intestinal damage are potential alternatives to permeability assessment (eg, direct visualisation enteroscopy, [111In]‐labelled white cells, calprotectin), all have their own drawbacks and limitations.
Our finding that (in the placebo arm) 5 days of treatment with indomethacin caused a threefold rise in gut permeability is in keeping with previous results of ours and of other groups.6 By contrast, when participants also received ZnC, this prevented the rise in permeability caused by indomethacin, strongly suggesting a small‐intestinal protective effect.
Indomethacin causes damage to the gastrointestinal tract by several mechanisms including reduction of mucosal prostaglandin levels, reduction of mucosal blood flow, stimulation of neutrophil activation and possibly also stimulation of apoptosis.11 Further work is required to determine which, if any, of these factors were affected by ZnC in exerting the gut‐stabilising effects shown in this paper. As well as the pro‐migratory and proliferative activity shown here, other groups have reported that ZnC can influence additional protective pathways. For example, Fujii et al12 reported that ZnC decreased indomethacin‐induced apoptosis in rat gastric mucosal RGM1 cells. In their study, most of this effect could also be reproduced by adding zinc sulphate, whereas in our cell proliferation assay this was not the case. ZnC has also been shown to down regulate pro‐inflammatory cytokine expression in gastric epithelial cells exposed to Helicobacter pylori,13 as well as acting as an anti‐oxidant.14 As we showed that ZnC stabilised small bowel permeability, it would also be of interest to examine whether it influenced the expression of molecules that affect intercellular tight junction function such as the protein zonulin.15
NSAIDs are one of the most widely prescribed groups of drugs used worldwide. Point prevalence studies, however, suggest that 10–30% of unselected patients taking NSAIDs have peptic ulceration,16 which can often be asymptomatic.17 In addition, up to 70% of patients taking NSAIDs have some degree of enteropathy associated with low‐grade blood and protein loss,18,19 although it is only of clinical importance in a much smaller percentage of patients. Proton pump inhibitors provide powerful acid suppression and are effective in reducing the risk of gastric and duodenal ulcers, but not of small‐intestinal injury. Prostaglandin analogues provide some protection of the small bowel, but are associated with side effects such as diarrhoea. To our knowledge, this is the first clinical study to show that ZnC protects the small intestine (in either animals or humans) from NSAID‐induced injury and has the additional advantage of being a natural‐based “functional food”.
Currently, there is a resurgence of interest in the use of natural bioactive products by the general public (for both human and veterinary use), with many healthy subjects and patients taking them for the prevention and treatment of multiple conditions, including gastrointestinal disorders and postoperative recovery.20 This results in an annual turnover of around £1.4 billion ($2.4 billion) in the UK and £10 billion ($18 billion) in the USA.20 Unfortunately, current evidence of the scientific validity of most of these traditional and commercial compounds is severely limited, and the level of evidence used in support of their claims often falls well below that acceptable in the medical and scientific community. Therefore, there is a need for a rigorous scientific review of such products in terms of efficacy and, where biological activity is confirmed, also safety.
There is a perception among the general public that natural‐based products equate to a gentle response, in some ways distinct from a pharmaceutical approach.20 It is, therefore, of note that the doses of ZnC used in the study of gastric damage in rats (1 and 5 mg/ml) gave results similar to those seen in animals given the potent cytoprotective agent EGF administered at 25 μg/kg/h. Furthermore, the concentrations of ZnC used in our restitution studies (100 μM, 29.8 μg/ml), and in our model of gastric damage (1–5 mg/ml), are likely to be present in the human gastric juice of participants taking ZnC supplements, as the standard recommended dose is around 37.5 mg once or twice daily. This dose of ZnC (37.5 mg twice daily) was also used in the clinical trial and showed beneficial effects. These results further emphasise that the division between “food products” and “drugs”, when considered in terms of biological activity is far from clear, and that these products should be considered as “nutriceutical” or functional foods.
In conclusion, our studies have shown that ZnC, commercially available as over‐the‐counter “health food” supplements for licensing purposes, possesses biological activity when assessed using several models of gut integrity and repair, and in a clinical trial. Importantly, these effects were seen at concentrations likely to be found in participants taking the product as a health food supplement. Further studies are warranted.
Abbreviations
ANOVA - analysis of variance
BrdU - bromodeoxyuridine
BSA - bovine serum albumin
DMEM - Dulbecco's modified Eagle medium
EGF - epidermal growth factor
HPLC - high‐pressure liquid chromatography
NSAID - non‐steroidal anti‐inflammatory drug
RIE - rat intestinal epithelium
ZnC - zinc carnosine
Footnotes
Funding: This work was partially funded by the Wexham Park Gastrointestinal Trust grant number 2004/6772, a DDF/Belmont Trust Award, Lonza Nutrition, USA and M&M Veterinary Health, USA.
Competing interests: The use of extended health claims for ZnC is currently under consideration by the Federal Drug Administration (USA). RJP provided evidence of relevant data at their hearing.
References
- 1.Boldyrev A A. Protection of proteins from oxidative stress: a new illusion or a novel strategy? Ann N Y Acad Sci 20051057193–205. [DOI] [PubMed] [Google Scholar]
- 2.Playford R J, Marchbank T, Chinery R.et al Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology 1995108108–116. [DOI] [PubMed] [Google Scholar]
- 3.Playford R J, Floyd D N, Macdonald C E.et al Bovine colostrum is a health food supplement which prevents NSAID induced gut damage. Gut 199944653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Playford R J, Marchbank T, Goodlad R A.et al Transgenic mice which overexpress the human trefoil peptide, pS2, have an increased resistance to intestinal damage. Proc Natl Acad Sci 1996932137–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjarnason I, Macpherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology 19951081566–1581. [DOI] [PubMed] [Google Scholar]
- 6.Playford R J, Macdonald C E, Calnan D P.et al Co‐administration of the health food supplement, bovine colostrum reduces the non steroidal anti‐inflammatory drug induced increase in intestinal permeability. Clin Sci 2001100627–633. [PubMed] [Google Scholar]
- 7.Bjarnason I. Intestinal permeability. Gut 199435S18–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy M R, Townsend R R, Lee Y C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem 198817054–62. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald T M, Morant S V, Robinson G C.et al Association of upper gastrointestinal toxicity of non‐steroidal anti‐inflammatory drugs with continued exposure: cohort study. BMJ 19973151333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naiti Y, Yoshikawa T, Yagi N.et al Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and TNF‐alpha expression in rats with aspirin‐induced gastric mucosal injury. Dig Dis Sci 200146845–851. [DOI] [PubMed] [Google Scholar]
- 11.Levi S, Shaw‐Smith C. Non‐steroidal anti inflammatory drugs; how do they damage the gut? Br J Rheumatol 199433605–612. [DOI] [PubMed] [Google Scholar]
- 12.Fujii Y, Matsura T, Kai M.et al Protection by polaprezinc, an anti‐ulcer drug, against indomethacin‐induced apoptosis in rat gastric mucosal cells. Jpn J Pharmacol 20008463–70. [DOI] [PubMed] [Google Scholar]
- 13.Handa O, Yoshida N, Tanaka Y.et al Inhibitory effect of polaprezinc on the inflammatory response to Helicobacter pylori. Can J Gastroenterol 200211785–789. [DOI] [PubMed] [Google Scholar]
- 14.Hiraishi H, Sasai T, Oinuma T.et al Polaprezinc protects gastric mucosal cells from noxious agents through antioxidant properties in vitro. Aliment Pharmacol Ther 199913261–269. [DOI] [PubMed] [Google Scholar]
- 15.Watts T, Berti I, Sapone A.et al Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic‐prone rats. Proc Natl Acad Sci USA 20051022916–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy D. Non‐steroidal anti‐inflammatory drug‐induced ulcers: management by traditional therapies. Gastroenterology 198996662–674. [DOI] [PubMed] [Google Scholar]
- 17.Larkai E N, Smith J L, Lidsky M D.et al Gastroduodenal mucosa and dyspeptic symptoms in arthritic patients during chronic non‐steroidal anti‐inflammatory drug use. Am J Gastroenterol 1987821153–1158. [PubMed] [Google Scholar]
- 18.Morris A J, Wasson L A, MacKenzie J F. Small bowel enteroscopy in undiagnosed gastrointestinal blood loss. Gut 199233887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjarnason I, Hayllar J, Macpherson A J.et al Side effects of nonsteroidal anti‐inflammatory drugs on the small and large intestine in humans. Gastroenterology 19931041832–1847. [DOI] [PubMed] [Google Scholar]
- 20.Sloan A E. The top 10 functional food trends. Next Generation Food Technol 20025632–57. [Google Scholar]