Inflammatory bowel diseases (IBDs) such as Crohn's disease and ulcerative colitis are the result of an imbalanced mucosal T cell response. Despite the identification of a genetic susceptibility region in the NOD2/CARD15 (nucleotide‐binding oligomerisation domain 2/caspase recruitment domain 15) gene, the aetiology is still unclear. Thus, the hunt for disease‐initiating factors such as defects in the mucosal barrier or pathogenic microorganisms is ongoing. By contrast, the immunopathogenesis in IBDs is better understood. The identification of cytokines that are involved in T cell and monocyte signalling led to specific therapeutic concepts. Recent data have clearly shown that the most powerful therapeutic approaches inhibit T cell survival by inducing apoptosis. The efficacy of anti‐tumour necrosis factor (TNF) strategies was proved to be at least partially due to its ability to induce apoptosis in T cells and monocytes. Furthermore, other powerful anticytokine strategies—namely, anti‐interleukin (IL)12 and anti‐IL6 antibodies, which are currently tested in clinical trials—also inhibit antiapoptotic pathways in T cells. Recently, the well‐established immunosuppressive drug azathioprine was identified as blocking antiapoptotic pathways in T cells. Data from these studies underline the pivotal role of lymphocyte apoptosis in the regulation of mucosal immune balance.
Background
A role for apoptosis or programmed cell death is well established in the context of inflammation or cancer. Decades ago, cell death was described as a phenomenon in the normal development of vertebrates.1 In 1965, Kerr and colleagues observed the cell death of hepatocytes after portal branch ligation. In this experimental setting, cell death was attributed to necrosis with concomitant inflammatory processes, but a different type of cell death also occurred in scattered individual cells with shrunken nuclei. In contrast with necrosis, evidence suggested no lysosomal rupture or inflammation.2 Some years later, the nuclear masses were found to be membrane‐enclosed bodies containing pieces of condensed DNA (chromatin) and well‐preserved organelles.3 In the 1970s, the terms “apoptosis” and, later on, “programmed cell death” were defined in the context of growth.4
Apoptosis selectively reduces cell populations. For example, the removal of interdigit tissues to form fingers and toes is regulated by apoptosis.5,6 Even in the developing central nervous system, several structures are only temporary phenomena that go into regression during subsequent stages. Thereby, programmed cell death enables normal development.7,8 Finally, in the fully formed organism, programmed cell death regulates the maintenance of tissue homeostasis, including the removal of epithelial cells from the gastrointestinal mucosa.9 Furthermore, some research groups focused on lymphoid tissue where the degradation of chromatin into fragments was also observed.10 In the 1980s and early 1990s, it became clear that apoptosis was an important mechanism to regulate T cell repertoire selection in the thymus. Without this regulatory tool, uncontrolled development of T cells would lead to autoreactivity.11 Thymic tolerance thus depends on programmed cell death.12,13 Moreover, in the beginning of the 1990s, the dogma that apoptosis can also be initiated in mature peripheral T cells was established.14,15
On the other hand, if foreign epitopes are detected on the cell surface, as observed in viral infections and carcinogenesis, apoptosis of the target cell can be selectively induced by natural killer T cells.16 Furthermore, tumour cells can escape from immunosurveillance by inducing apoptosis in tumour cell‐attacking T cells.17,18 To avoid overwhelming immune responses and to limit damage to healthy tissue, antigen‐activated T cells die as a result of apoptosis during shutdown of such immune responses.19
Apoptosis has an essential role in T cell regulation. In the early development of the human immune system, thymocytes expressing non‐functional or autoreactive T cell receptors are eliminated by programmed cell death. Furthermore, in mature T cells, apoptosis leads to the deletion of expanded effector T cells during immune responses. The dysregulation of apoptosis in the immune system results in autoimmunity, tumorigenesis and also immunodeficiency.
Morphological and biochemical characteristics of apoptosis
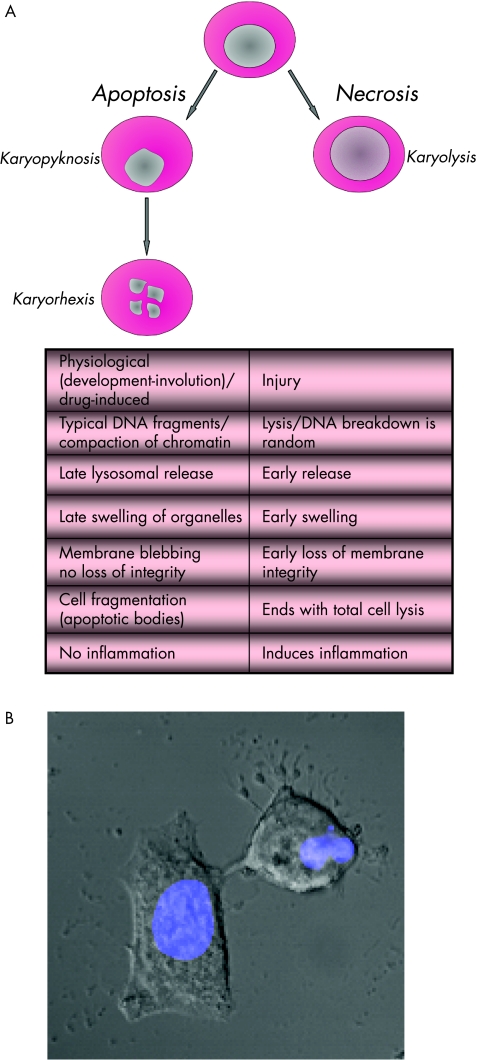
The mechanisms leading to necrosis are always pathological and caused by injury. By contrast, apoptosis is a physiological phenomenon in health and disease. There is no marked inflammatory reaction and organelle swelling in apoptotic cells, whereas necrosis is accompanied by inflammatory reactions of the surrounding tissue. Earlier nuclear compaction is observed, followed by breakdown of the nucleus into several fragments. This phenomenon is called “karyorhexis”, and is accompanied by cytoskeletal degradation leading to blebbing of the cell membrane (Fig 1A, B). At later stages, complete cell fragmentation is observed. Those cell fragments form apoptotic bodies or vesicles in which cytoplasmic organelles appear to be intact, and most of the apoptotic bodies have a nuclear component. Epithelial cells or cells forming tissues are characterised by early loss of cell adhesion. In tissues, apoptosis occurs in single cells, whereas necrosis usually hits a group of cells.
Figure 1 (A) Characteristics of cell death: in the case of apoptosis, the morphological sign of a nuclear breakdown is the shrunken nucleus (karyopyknosis). In the late state, nuclei break down into discrete fragments (karyohexis). The table shows further characteristics. (B) Image of a viable cell (left side) and an apoptotic cell (right side) under a microscope. The nucleus (blue) breaks down into several fragments (karyohexis). Some small apoptotic bodies resulting from cytoskeletal changes, and consecutive membrane blebbing can be seen near the apoptotic cell. (Photo contributed by Dr Dennis Strand, First Department of Internal Medicine, Johannes Gutenberg University, Mainz, Germany.)
A well‐defined biochemical event in apoptosis involves nuclear DNA. Cleavage of double‐stranded DNA is observed at linker regions between nucleosomes. The results of this cleavage are DNA fragments consisting of approximately 200‐bp units. These fragments of 200, 400 or 600 bp can be shown by agarose gel electrophoresis of the DNA, and show a typical ladder pattern. In necrosis, however, the DNA breakdown is random and seen as a smear after electrophoresis.
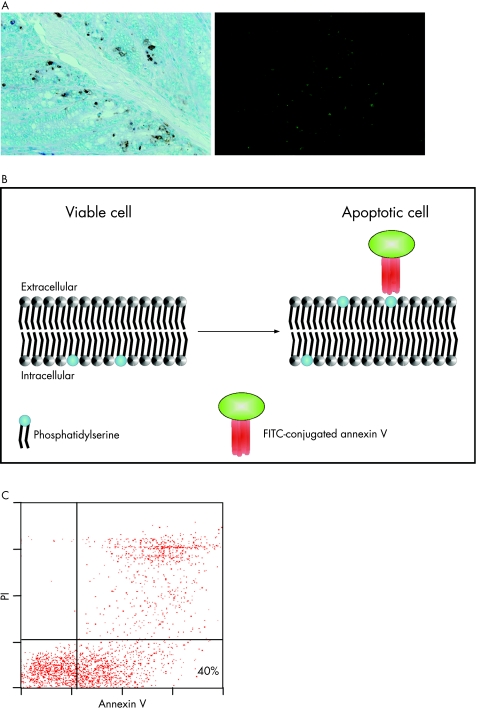
A state‐of‐the‐art detection of apoptotic single cells is the TdT‐mediated X‐dUTP nick end labelling assay, whereby apoptotic cells are detected by labelling of DNA strand breaks in individual cells by flow cytometry or microscopy. The typical feature of late apoptotic cells is DNA degradation occurring as double‐strand breaks. Those DNA strand breaks can be detected by enzymatic labelling of the free 3′‐OH terminals with modified oligonucleotides—for example, fluroscein‐conjugated deoxyuridine triphosphate. The necessary enzyme is deoxynucleotidyl transferase (fig 2A).
Figure 2 (A) TdT‐mediated X‐dUTP nick end labelling assay on murine colon cryosections. Apototic cells are stained either brown (immunohistochemistry, left) or green (immunofluorescence, right). (B) Model of cell membrane double layer. In viable cells, phosphatidylserine is located in the inner membrane. A few hours after induction of apoptosis, phosphatidylserine molecules flip outside and appear on the outer membrane. Fluorescence‐conjugated annexin V can now bind to phosphatidylserine and can be detected via flow cytometry (C) as an early marker of apoptosis. (C) Flow cytometry. Annexin V‐positive and propidium iodide (PI)‐negative cells are shown in the right lower quadrant.
Another method is the detection of loss of cell membrane integrity in apoptotic cells. In the early phases of apoptosis, certain molecules that are localised in the inner membrane of the cell membrane double layer flip outside and appear on the outer membrane. Annexin V can bind to these phosphatidylserine molecules. It cannot bind to intact cells (fig 2B). However, as necrotic cells have leaky membranes, annexin V gains access to the inner membrane where it can also bind to phosphatidylserine. Therefore, apoptotic cells can be differentiated from necrotic cells. Thus, an additional dye—for example, propidium iodide—that intercalates with DNA is used together with annexin V. Propidium iodide has access to only necrotic cells with leaky membranes. Exclusion of propidium iodide, coupled with annexin V, indicates an apoptotic cell. Propidium iodide exhibits a bright red colour on binding to DNA, whereas annexin V is conjugated to a fluorescent dye (fig 2C).
Furthermore, substrates of apoptosis can be detected. Caspase assays serve to quantify in vitro caspase activity. Anti‐PARP assays recognise poly‐ADP‐ribose‐polymerase (PARP), a protein that binds to DNA strand breaks. Furthermore, PARP is a substrate for effector caspases activated during the early stages of apoptosis. PARP is cleaved by caspases and the detection of one of the fragments with anti‐PARP thus serves as a marker of early apoptosis.
Control of apoptosis in the healthy organism
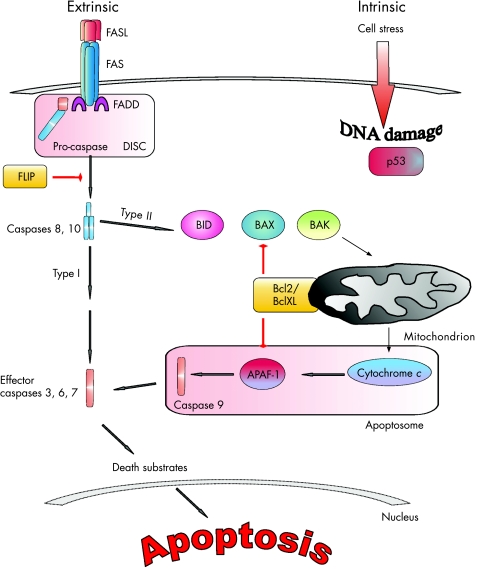
Two major pathways lead to apoptosis: the intrinsic cell death pathway controlled by the Bcl2 family involving mitochondria, and the extrinsic cell death pathway controlled by death receptor signalling.20 These two pathways work together to regulate the development and function of T lymphocytes. On the other hand, cytokines and growth factors contribute to T cell survival11,21,22 (fig 3).
Figure 3 Apoptotic pathways. The extrinsic pathway is initiated by death receptor–ligand interaction (FAS/CD95 and FASL, tumour necrosis factor (TNF) receptor 1 (TNFR1) and TNF, TRAIL‐R and TRAIL, and others). On receptor–ligand binding, the intracellular death domain of the death receptor attracts the intracellular adaptor molecule, Fas‐associated death domain (FADD). The adaptor molecule recruits caspases 8 and 10, forming the death‐inducing signal complex (DISC), where they are cleaved and activated. In some cells, these initiator caspases are sufficient to activate effector caspases 3, 6 and 7 (type I cells). Some other cell types require the mitochondrial pathway to amplify their death signals (type II cells). In this case, caspase 8 or 10 activates the Bcl2‐interacting domain (BID), which translocates to mitochondria and induces release of cytochrome c. Cytochrome c itself interacts with apoptotic protease‐activating factor 1 (APAF1) and the inactive form of caspase 9, termed the apoptosome. This complex cleaves and activates caspase 9 in an adenosine triphosphate‐dependent manner. Consecutively, effector caspases 3, 6 and 7 are activated. Important counter‐regulatory factors of apoptosis are antiapoptotic members of the Bcl family (Bcl2 and BclXL) and the FLICE inhibitory protein (FLIP). The FLICE inhibitory protein inhibits the activation of initiator caspases 8 and 10. The intrinsic pathway is classically mediated by mitochondria. Pro‐apoptotic signals cause the perturbation of the mitochondrial membrane, with consecutive release of cytochrome c into the cytoplasm. The activation of the intrinsic apoptotic pathway is held in balance between pro‐apoptotic factors such as BAX and BAK, and inhibitory factors such as Bcl2 and BclXL. The unbalanced expression of either pro‐apoptotic or apoptotic factors thus perturbs the equilibrium of cell homeostasis towards apoptosis or enhanced survival. An important regulator molecule is p53, which is the guard of cell integrity. On detection of genomic DNA damage or high cell stress, p53 translocates from the cytoplasm to the nucleus to initiate transcription of pro‐apoptotic factors.
The extrinsic apoptotic cell death pathway is initiated by death receptor–ligand interaction (FAS, also called CD95, and FASL, tumour necrosis factor receptor 1 (TNFR1) and TNF, TRAIL‐R1 or TRAIL‐R2 and TRAIL, and others). On receptor–ligand binding, the intracellular death domain of the death receptor attracts the intracellular adaptor molecule Fas‐associated death domain. The adaptor molecule in turn recruits caspases 8 and 10, thereby forming the death‐inducing signal complex, where they are cleaved and become activated. In some cells, these initiator caspases are sufficient to activate effector caspases 3, 6 and 7, finally activating death substrates inducing DNA degradation and changes in the cytoskeleton (type I cells). Some other cell types require the mitochondrial pathway to amplify their death signals (type II cells)11,21 (fig 3). In this case, caspase 8 or 10 activates Bcl2‐interacting domain, which translocates to mitochondria and induces release of cytochrome c.23 Cytochrome c itself interacts with apoptotic protease‐activating factor 1 and the inactive form of caspase 9, termed the apoptosome. This complex cleaves and activates caspase 9 in an adenosine triphosphate‐dependent manner. Consecutively, effector caspases 3, 6 and 7 are activated. Important counter‐regulatory factors of apoptosis are antiapoptotic members of the Bcl family (Bcl2 and BclXL) and FLICE inhibitory protein (FLIP). FLIP inhibits the activation of initiator caspases 8 and 10.
The intrinsic pathway is classically mediated by mitochondria. Pro‐apoptotic signals cause the perturbation of the mitochondrial membrane, with consecutive release of cytochrome c into the cytoplasm. Activation of the intrinsic apoptotic pathway is held in balance between pro‐apoptotic factors such as BAX and BAK, and inhibitory factors such as Bcl2 and BclXL. The imbalanced expression of either pro‐apoptotic or antiapoptotic factors thus perturbs the equilibrium of cell homeostasis towards apoptosis or enhanced cell survival.18 An important regulator molecule is p53, which is the guard of cell integrity. On detection of genomic DNA damage or high cell stress, p53 translocates from the cytoplasm to the nucleus to initiate transcription of pro‐apoptotic factors, initiating cell death16 (fig 3).
IBD pathogenesis: the idea of resistance against apoptosis in the regulation of T cell survival
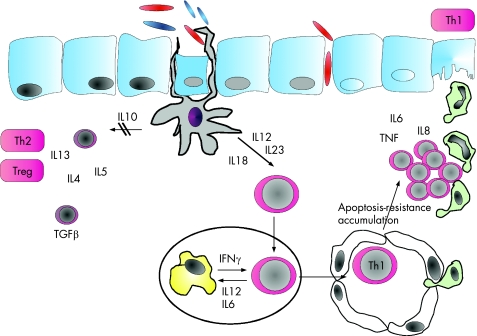
The current understanding of IBD pathogenesis is based on findings several years old. Experimental data suggested that mucosal inflammation is the result of abnormal T cell‐mediated immune reactivity towards bacterial antigens in the gut.24,25,26 This misled immune response can occur in genetically susceptible people27 (fig 4). Susceptibility genes were described in IBD1, IBD2, IBD3 and IBD4 loci. A breakthrough occurred in May 2001, when two groups simultaneously reported that Crohn's disease was associated with mutations in the NOD2/CARD15 gene.28,29 Mutations in this gene explain about 20% of the genetic susceptibility in Crohn's disease.30,31,32 The gene product NOD2 protein acts as an intracellular receptor for bacterial wall components that were inoculated from antigen‐presenting cells, thus representing a link between innate and acquired immunity in patients with IBD.33 However, it is still unclear what exactly induces pro‐inflammatory responses on the epithelial barrier when antigen‐presenting cells are exposed to bacterial antigens. The identification of naturally occurring antibiotic products secreted by mucosal cells, the so‐called defensins, also suggested the importance of the mucosal barrier.34,35 Other aspects of the pathomechanism of the disease (fig 4) were clarified by several therapeutic concepts targeting specific elements of signal transduction—for example, anticytokines, such as anti‐TNF or anti‐IL12 receptor antibodies or antibodies to homing or costimulatory receptors on the surface of T cells. Unfortunately, some of the later biologicals had severe side effects: natalizumab was associated with some cases of progressive multifocal leucoencephalopathy, and the clinical phase I trial of an anti‐CD28 superagonistic antibody was also stopped.36,37,38,39,40 The most powerful biologicals seem to be those inducing apoptosis in monocytes and T cells—for example, antibodies to TNF, IL12 or to the IL6 receptor.38,41,42,43,44,45,46
Figure 4 Pathogenesis of Crohn's disease. Antigen‐presenting cells are activated by luminal antigens and subsequently produce pro‐inflammatory cytokines, initiating or at least contributing to uncontrolled T cell response. Anti‐inflammatory T cell cytokine responses are shut down. Both interleukin (IL)12/IL23 and IL18 contribute to T helper cell 1 (Th1) differentiation of CD4 lamina propria T cells. On priming in lymphatic organs, Th1 effector cells stimulate macrophages to secrete further pro‐inflammatory cytokines, inducing T cell apoptosis resistance, which is conveyed by cytokine‐dependent activation of transcription factors—namely, signal transducer and activator of transcription (STAT)3 and nuclear factor (NF)κB. Activated Th1 lymphocytes accumulate in the lamina propria where they secrete antiapoptotic cytokines, maintaining resistance to apoptosis and further contributing to accumulation of T cells. The chemoattractant IL8 contributes to the influx of granulocytes and local stroma cells, finally mediating local tissue damage by the release of matrix metalloproteinases.
In IBD, apoptosis was studied first in the late 1990s, when Boirivant et al47 found that stimulated human lamina propria T cells undergo enhanced FAS (CD95)‐mediated apoptosis. They showed that unstimulated lamina propria T cells from healthy people, as compared with unstimulated peripheral blood T cells from the same people, show an increased level of apoptosis. This mechanism was postulated to be important to regulate T cell activity and expansion in the healthy gut. Moreover, it was shown that apoptosis was further increased via stimulation of the CD2 pathway. A comparison of T cells isolated from patients with IBD and controls showed that lamina propria lymphocytes from inflamed tissues express the same amount of cell surface FAS (CD95) but were less sensitive to FAS (CD95)‐mediated apoptosis than cells from controls. T cells from patients with Crohn's disease were characterised by increased survival when stimulated via the CD2 pathway. Thus, T cells isolated from patients with IBD manifest decreased CD2 pathway‐induced apoptosis compared with healthy controls. Furthermore, the tumour‐suppressor gene p53 seems to act as a negative regulator of the intestinal immune system by slowing down T cell cycling and subsequently preventing uncontrolled T cell replication.48 By contrast, T cells of patients with Crohn's disease cycle faster, thus having an increased capacity for cellular expansion compared with mucosal T cells from healthy controls.49 A further checkpoint of cell death regulation might be caspase regulation, because lamina propria T cells are highly sensitive to apoptosis induced by caspase 8. Compared with peripheral lymphocytes, lamina propria T cells undergo apoptosis on a slight activation of caspase 8.50 T cells in patients with ulcerative colitis were also shown to have a markedly higher expression of the caspase 8 inhibitor FLIP, thus blocking death receptor‐induced cell death (fig 3). In the light of the mitochondrial pathway, a disturbed ratio between pro‐apoptotic and antiapoptotic factors was also shown to mediate apoptosis resistance in patients with Crohn's disease.51 Obviously, an increased Bcl2:BAX ratio enhances apoptosis resistance in lamina propria T cells from patients with Crohn's disease. The above‐mentioned apoptosis control mechanisms clearly show that, on the one hand, the ability to undergo death receptor‐induced apoptosis contributes to lymphocyte control. Costimulatory pathways such as CD2 as well as CD28 and CD45v752 signalling also have an important role in lymphocyte homeostasis. On the other hand, these mechanisms might be secondary effects in IBD, because no genetic predisposition leading to impaired expression of apoptosis‐regulating factors has been described so far. Most of these mechanisms mainly seem to affect lymphocytes from the lamina propria rather than T cells from the peripheral blood.
IL6 trans‐signalling: a well‐defined antiapoptotic signal transduction pathway
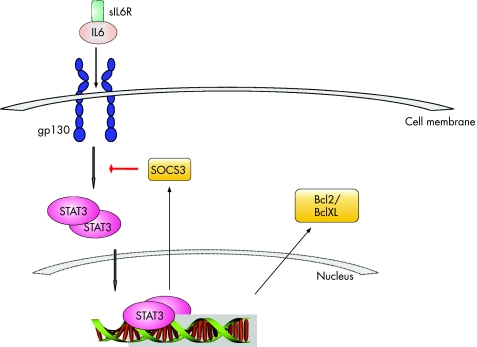
IL6 is a central cytokine in IBD, which contributes to enhanced T cell survival and apoptosis resistance in the lamina propria at the inflamed site.43,53,54,55,56 Consecutively, CD4 T cells can accumulate in the lamina propria, leading to perpetuation of inflammation. The role of the soluble IL6 receptor in the pathogenesis of IBD was elucidated on isolated lamina propria mononuclear cells obtained from surgical colon specimens from patients with Crohn's disease and ulcerative colitis.43 In this study, macrophages and T cells from the lamina propria were separated from total lamina propria mononuclear cells. Compared with controls, both CD4 T cells and macrophages produced increased amounts of IL6. Soluble IL6 receptor was released via shedding from the surface of macrophages rather than via alternative splicing.57,58 In a process called IL6 trans‐signalling, the IL6–soluble IL6 receptor complex can activate gp130‐positive T cells from the lamina propria of patients with IBD, which normally do not express the membrane‐bound IL6 receptor. IL6 trans‐signalling further induces STAT3 activation in patients with Crohn's disease and in those with ulcerative colitis.59,60,61 STAT3 itself mediates resistance against apoptosis through induction of anti‐apoptotic genes such as Bcl2 and BclXL51 (fig 5).
Figure 5 Lamina propria T cells in the inflamed gut produce interleukin (IL)6. The cells cannot respond to IL6 alone, as they express only gp130 but not the membrane‐bound IL6 receptor. Soluble IL6 receptor (sIL6R) can bind to IL6 and in turn activate lamina propria T cells through gp130 surface molecules (IL6 trans‐signalling). Activation of T cells through IL6 causes dimerisation and translocation of signal transducer and activator of transcription (STAT)3 into the nucleus, with consecutive induction of antiapoptotic genes such as Bcl2 and BclXL. STAT3 also induces trancription of its natural inhibitor SOCS3.
The antiapoptotic IL6 pathway is also well characterised in experimental colitis and results found in experimental colitis and human patients with IBD are largely consistent.43,62 However, a critical control point seems to be STAT3 activation, which exerts antiapoptotic and pro‐inflammatory effects in cells. For instance, STAT3 is also involved in anti‐inflammatory IL10 signalling in macrophages, because mice devoid of STAT3 in macrophages and neutrophils develop spontaneous enterocolitis.63 Furthermore, specific STAT3 deletion in bone marrow cells in mice causes death of these mice within 4–6 weeks after birth, with Crohn's disease‐like pathogenesis.64 Thus, unspecific targeting STAT3 in IBD might not be useful because of blocking anti‐inflammatory mechanisms. Interestingly, a recently published work exhibited anti‐inflammatory effects of growth hormone in experimental colitis. This mechanism was mediated as a result of inhibition of gp130 and consecutive STAT3 activation in T cells65 (fig 5). Taken together, IL6 exerts pleiotropic and pro‐inflammatory effects, but also targets different cell populations where it may induce STAT3 activation.66 In any case, targeting of IL6 signalling by antibodies is an attractive therapeutic approach, as it was shown to reduce disease activity in patients with Crohn's disease in a recent phase II study.45
IL12‐induced T cell survival
IL12 and IL23 are important cytokines that respectively promote T cell differentiation and activation. In the pathogenesis of Crohn's disease, IL12 is a major player in driving Th1 T cell differentiation.39 Like IL12, IL23 is a heterodimer, comprising the IL12 p40 subunit and IL23‐specific p19 subunit. IL23 uses many of the same signal transduction components as IL12, including IL12Rβ1, Janus kinase 2, Tyk2, STAT1, STAT3, STAT4 and STAT5.67,68
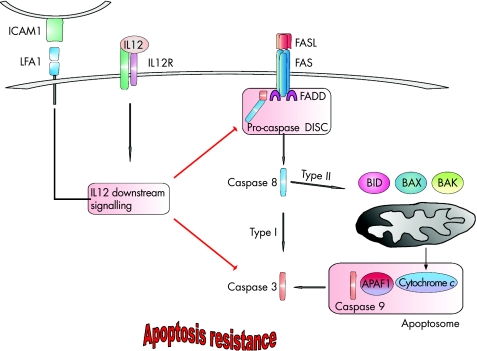
As mentioned before, during intestinal inflammation, lamina propria T cells from patients are resistant to FAS‐induced apoptosis compared with lamina propria T cells from controls.69 Data from murine experiments showed that IL12 inhibits FAS‐dependent apoptosis via inhibition of caspase 3 activity. Thus, increased IL12 production in patients with Crohn's disease might contribute to enhanced T cell survival.70 In experimental trinitrobenzene sulphonic acid colitis, the application of antibodies to IL12 led to an increased rate of apoptotic lamina propria lymphocytes. This apoptosis is mediated by the FAS pathway because MRL/MpJ‐lpr(fas) mice lacking the FAS function developed colitis that responded poorly to treatment with anti‐IL12 antibodies.71 Another research group identified a possible mechanism of antiapoptotic effects of IL12. They proposed that IL12 inhibited FAS‐dependent apoptosis by inhibiting activation of casapases 3 and 9. This mechanism was investigated in naive T cells stimulated via the intercellular adhesion molecule 1/leucocyte function‐associated antigen 1 pathway. This experimental setting might also be important in Crohn's disease because expression of the intercellular adhesion molecule 1 is up regulated in patients with IBD72,73 (fig 6).
Figure 6 Interleukin (IL)12‐mediated apoptosis. On IL12 receptor (IL12R)–ligand binding, the antiapoptotic signal cascade becomes activated. Stimulation of the adhesion molecule leucocyte function‐associated antigen 1 (LFA1) on the surface of lymphocytes might have a synergystic effect, leading to inhibition of the caspase cascade, thus inhibiting the capacity of the cell to undergo FAS‐mediated apoptosis. APAF1, apoptotic protease‐activating factor 1; BID, Bcl2‐interacting domain; DISC, death‐inducing signal complex; FADD, Fas‐associated death domain; FASL, FAS ligand; ICAM1, intercellular adhesion molecule 1.
The pro‐apoptotic effect of anti‐IL12 treatment was also observed by another group. A newly designed antagonistic fusion protein consisting of the dimeric IL12 p40 subunit of IL12 and the constant region of immunoglobulin (Ig)G2b ameliorated intestinal inflammation in experimental colitis. Furthermore, antibody treatment of human lamina propria mononuclear cells at low concentrations induced apoptosis.38 In a pilot study, patients with Crohn's disease were treated with monoclonal antibodies to IL12. After 7 weeks of application, 75% of patients treated with anti‐IL12 showed clinical responses (defined by a reduction in the score for Crohn's disease activity index of 100 points) compared with 25% of patients receiving placebo. However, at 18 weeks of follow‐up, the difference in response was no longer notable.39 Taken together, these data suggest that IL12 increases T cell survival in patients with Crohn's disease via inhibition of pro‐apoptotic pathways.
TNF signalling: an antiapoptotic pathway in Crohn's disease
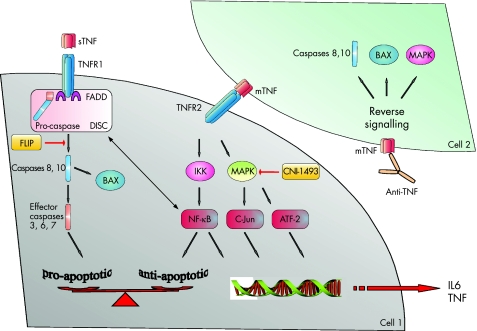
Human TNF is a polypeptide that exists as either transmembrane TNF or soluble TNF. The membrane‐bound form can be cleaved and shed from the cell surface by TNFα‐converting enzyme. Secreted TNF can bind to its two cell surface receptors TNFR1 (p55) and TNFR2 (p75). Membrane‐bound TNF can also activate target cells via cell–cell contact. This mechanism works mainly via TNFR2.74 On binding of transmembrane TNF to TNFR2, signals can be transmitted in a bidirectional way (fig 7).
Figure 7 Tumour necrosis factor (TNF) appears in two forms: soluble TNF (sTNF) is shed from the cell surface on enzymatic cleavage of the membrane‐bound form (mTNF). sTNF has a high affinity for the TNF receptor (TNFR)1 and induces pro‐apoptotic signals on ligation. TNFR1 assembles a death‐inducing signal complex (DISC) similar to that of FAS/CD95 and exerts its effect via activation of caspases. On the other hand, mTNF has a high affinity for TNFR2 that rather induces antiapoptotic signals via activation of the nuclear factor (NF)κβ pathway and the mitogen‐activated protein kinase (MAPK) cascade. NFκβ might also exert an antiapoptotic function via inhibition of the FAS‐associated death domain (FADD) and formation of the DISC. In some cells, NFκβ up regulates the FLICE inhibitory protein (FLIP). Some data also suggest that NFκβ is induced by TNFR1, resulting in a complex cross‐talk of both TNF receptors. MAPKs such as extracellular signal‐regulated kinase1/2, c‐Jun N‐terminal kinase and p38 can activate transcription factor targets (c‐Jun, ATF2), turning on genes that regulate growth and apoptosis. These factors also contribute to initiation of transcription of pro‐inflammatory and antiapoptotic cytokines such as interleukin (IL)6 and TNF. CNI‐1493 is a composite that inhibits phosphorylation and activation of several MAPKs. A different mechanism is shown in cell 2: infliximab might induce reverse signalling on binding to mTNF. In turn, activation of pro‐apoptotic pathways including activation of upstream caspases and the mitochondrial pathway via BAX induces apoptosis of T cells and monocytes.
Antibodies to TNF are an important option to induce remission in steroid refractory patients with Crohn's disease. Fistulising disease is an indication to start infliximab. Besides these main indications, there are some other constellations for infliximab application. Anti‐TNF antibodies are being tested in clinical trials: adalimumab75 and certolizumab.76 Another anti‐TNF biological is etanercept, a TNFR2–Fc fusion protein that blocks only soluble TNF but not membrane‐bound TNF.
Interestingly, etanercept is successfully used in the treatment of rheumatoid arthritis but is not effective in the treatment of Crohn's disease. However, both diseases can be treated with infliximab. These observations suggest that inflammation in rheumatoid arthritis and Crohn's disease is mediated in a different manner.77 In rheumatoid arthritis, etanercept down regulates inflammation via inhibition of soluble TNF. By contrast, inflammation in Crohn's disease seems to depend more on signalling via the membrane‐bound TNF.
A striking difference beyond the involvement of different receptors is the functional relevance of the different pathways. Several studies have clearly shown that treatment with anti‐TNF antibodies suppresses Crohn's disease activity as a result of antibody‐induced monocytes42 and T cell apoptosis in the gut.78,79 By contrast, etanercept, which blocks soluble TNF but not membrane‐bound TNF, fails to induce apoptosis of T cells, suggesting that infliximab might be effective in Crohn's disease because of its pro‐apoptotic effects.80
Lügering et al42 showed in detail that peripheral monocytes isolated from patients with active Crohn's disease underwent apoptosis on treatment with infliximab. The induction of apoptosis depended on the activation of members of the caspase family, as activation of caspases 8, 9 and 3 could be determined. Furthermore, mitochondrial release of cytochrome c and transcripts for the pro‐apoptotic factors BAX and BAK were detected on treatment with infliximab. ten Hove et al78 analysed lamina propria T cells at the site of inflammation. Biopsy samples from patients with Crohn's disease were taken 24 h after a single infusion of infliximab, and the rate of apoptotic T cells was determined via TdT‐mediated X‐dUTP nick end labelling assay. On treatment, the number of apoptotic lamina propria T cells was markedly increased. By contrast, infliximab did not alter properties of peripheral T cells. In cell culture, infliximab induced apoptosis in CD3/CD28‐stimulated Jurkat T cells but not in unstimulated Jurkat T cells. Also, solely stimulated Jurkat T cells showed raised levels of BAX on anti‐TNF treatment, resulting in an increased BAX:Bcl2 ratio and subsequent apoptosis.78
Another research group published similar data: patients with Crohn's disease underwent treatment with infliximab according to a standard protocol, with infusions at 0, 2 and 6 weeks. Infliximab induced sustaining apoptosis in lamina propria T cells, which was still evident 4 weeks after the last application. In vitro, infliximab induced apoptosis of lamina propria T cells in patients with Crohn's disease, independent of the FAS pathway. These lamina propria T cells showed a higher susceptibility to infliximab‐induced apoptosis than T cells from the peripheral blood in patients with Crohn's disease.81 These data suggest that infliximab selectively induces apoptosis in activated T cells at the site of inflammation but not in resting T cells in the peripheral blood.
Moreover, one of the “new” anti‐TNF antibodies, adalimumab, seems to induce apoptosis at least in monocytes.82 Further studies on lamina propria T cells are needed. The mechanism of certolizumab is still unclear. Fossati et al found that as compared with infliximab and adalimumab, certolizumab cannot induce apoptosis in the human NS0 cell line. In their experimental setting, etanercept induced apoptosis in a manner similar to infliximab and adalimumab (unpublished data). These data are in conflict with results from other groups, who uniquely observed that etanercept does not induce apoptosis in monocytes or T cells. Therefore, the data on certolizumab should be confirmed under other experimental conditions using human T cells and monocytes. Elucidating the mechanism of action of anti‐TNF is important because therapeutic failure might be predicted in the future. One possibility is to identify polymorphisms in TNF‐dependent genes encoding pro‐apoptotic factors that tip the balance of proper regulation of apoptosis.83
To summarise, the mechanism of action of anti‐TNF antibodies is not fully understood. Evidence suggests that the anti‐inflammatory effect of anti‐TNF strategies in IBD is mainly mediated by its ability to inhibit membrane‐bound TNF. Membrane‐bound TNF has a high affinity for TNFR2 that rather induces antiapoptotic mechanisms via activation of the NFκβ pathway and the mitogen‐activated protein kinase (MAPK) cascade (fig 7). Particularly, TNFR2 is upregulated during the inflammatory processes.84,85 By contrast, soluble TNF prefers TNFR1 that mediates pro‐apoptotic signals similar to death receptors such as FAS.85 Targeting the membrane‐bound TNF/TNFR2 pathway via inhibition of NFκβ86 or MAPKs87 uncovers the pro‐apoptotic effects of TNF and, thus, drives the cell towards apoptosis.88,89 Inhibitors of MAPKs such as CNI‐1493 are in clinical trials.90 Another theory for the mechanism of action of infliximab is based on reverse signalling, postulating that binding of anti‐TNF antibodies on membrane‐bound TNF has intrinsic effects and directly mediates apoptosis by induction of caspases and pro‐apoptotic members of the Bcl‐2 family such as BAX and BAK.91 However, both TNF receptors are involved in the pro‐apoptotic and the antiapoptotic cross‐talk (fig 7).
Established immunosuppressants: pro‐apoptotic mechanism of action of thiopurine drugs and 5‐aminosalicylic acid
Azathioprine has become an important drug for the maintenance of remission in IBDs. The therapeutic effect of azathioprine is due to the pro‐apoptotic effect of its metabolite 6‐thioguanine, which becomes phosphorylated. The end product 6‐thio‐guanosine triphosphate is an inhibitor of Rac1 and can bind to Rac1 instead of binding to guanosine triphosphate.92 As Rac1 is involved in activation of the MAPK cascade, and the NFκβ and Bcl2 pathways, inhibition of Rac1 leads to reduced activation of antiapoptotic factors, and therefore tips the balance towards mitochondria‐mediated apoptosis. Doering et al93 recently showed that sulfasalazine induced T cell apoptosis in lamina propria and peripheral T cells isolated from patients with Crohn's disease, ulcerative colitis and also controls. Interestingly, only the intact molecule exhibited pro‐apoptotic properties, whereas the metabolites 5‐aminosalicylic acid or sulphapyridine failed to induce lymphocyte apoptosis.93 The apoptotic effect of sulfasalazine was independent of the FAS pathway, but involved mitochondria‐mediated apoptosis. Analysis of pro‐apoptotic and antiapoptotic factors showed no changes in BAX and BAK expression, but marked down regulation of the antiapoptotic molecules Bcl2 and BclXL. This imbalance resulted in activation of caspase 9 and apoptosis. Beyond the mechanisms elucidated in IBD, other immunosuppressive drugs such as mycophenolate mofetil may also induce lymphocyte apoptosis.94
In summary, many established immunosuppressive drugs or “new” biologicals seem to work partly by the induction of T cell apoptosis in patients with IBD. In the light of current studies, it would be of great interest to differentiate between primary intrinsic defects in apoptosis in IBD, particularly on the basis of genetic phenotypes and secondary phenomena—for example, cytokine‐induced activation of antiapoptotic pathways and the growth cycle. In any case, selective targeting of apoptosis emerges as a novel approach for effective treatment of IBD.
Acknowledgements
We thank our colleagues Susanne and Dennis Strand, Raja Atreya, Brigitte Bartsch, Ralf Kiesslich, Martin Sprinzl, Wulf O Boecher and Matthias Kittler for helpful comments.
Abbreviations
FLIP - FLICE inhibitory protein
IBD - inflammatory bowel disease
MAPK - mitogen‐activated protein kinase
NOD - nucleotide‐binding oligomerisation domain
PARP - poly‐ADP‐ribose‐polymerase
STAT - signal transducer and activator of transcription
TLR - toll‐like receptor
TNF - tumour necrosis factor
TNFR - tumour necrosis factor receptor
Footnotes
Funding: This work was funded by the Stiftung Rheinland‐Pfalz für Innovation.
Competing interests: None.
References
- 1.Saunders J W., Jr Death in embryonic systems. Science 1966154604–612. [DOI] [PubMed] [Google Scholar]
- 2.Kerr J F. A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol 196590419–435. [DOI] [PubMed] [Google Scholar]
- 3.Kerr J F. Shrinkage necrosis: a distinct mode of cellular death. J Pathol 197110513–20. [DOI] [PubMed] [Google Scholar]
- 4.Gerschenson L E, Rotello R J. Apoptosis: a different type of cell death. FASEB J 199262450–2455. [DOI] [PubMed] [Google Scholar]
- 5.Danial N N, Korsmeyer S J. Cell death: critical control points. Cell 2004116205–219. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson M D, Weil M, Raff M C. Programmed cell death in animal development. Cell 199788347–354. [DOI] [PubMed] [Google Scholar]
- 7.Keyser A. Basic aspects of development and maturation of the brain: embryological contributions to neuroendocrinology. Psychoneuroendocrinology 19838157–181. [DOI] [PubMed] [Google Scholar]
- 8.Nijhawan D, Honarpour N, Wang X. Apoptosis in neural development and disease. Annu Rev Neurosci 20002373–87. [DOI] [PubMed] [Google Scholar]
- 9.Ciccocioppo R, Di Sabatino A, Gasbarrini G.et al Apoptosis and gastrointestinal tract. Ital J Gastroenterol Hepatol 199931162–172. [PubMed] [Google Scholar]
- 10.Skalka M, Matyasova J, Cejkova M. DNA in chromatin of irradiated lymphoid tissues degrades in vivo into regular fragments. FEBS Lett 197672271–274. [DOI] [PubMed] [Google Scholar]
- 11.Fas S C, Fritzsching B, Suri‐Payer E.et al Death receptor signaling and its function in the immune system. Curr Dir Autoimmun 200691–17. [DOI] [PubMed] [Google Scholar]
- 12.Owen J J, Jenkinson E J. Apoptosis and T‐cell repertoire selection in the thymus. Ann N Y Acad Sci 1992663305–310. [DOI] [PubMed] [Google Scholar]
- 13.King L B, Ashwell J D. Signaling for death of lymphoid cells. Curr Opin Immunol 19935368–373. [DOI] [PubMed] [Google Scholar]
- 14.Kabelitz D, Pohl T, Pechhold K. Activation‐induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today 199314338–339. [DOI] [PubMed] [Google Scholar]
- 15.Marrack P, Hugo P, McCormack J.et al Death and T cells. Immunol Rev 1993133119–129. [DOI] [PubMed] [Google Scholar]
- 16.Vousden K H, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer 20022594–604. [DOI] [PubMed] [Google Scholar]
- 17.Strand S, Hofmann W J, Hug H.et al Lymphocyte apoptosis induced by CD95 (APO‐1/Fas) ligand‐expressing tumor cells—a mechanism of immune evasion? Nat Med 199621361–1366. [DOI] [PubMed] [Google Scholar]
- 18.Igney F H, Krammer P H. Death and anti‐death: tumour resistance to apoptosis. Nat Rev Cancer 20022277–288. [DOI] [PubMed] [Google Scholar]
- 19.Strasser A, Pellegrini M. T‐lymphocyte death during shutdown of an immune response. Trends Immunol 200425610–615. [DOI] [PubMed] [Google Scholar]
- 20.Opferman J T, Korsmeyer S J. Apoptosis in the development and maintenance of the immune system. Nat Immunol 20034410–415. [DOI] [PubMed] [Google Scholar]
- 21.Scaffidi C, Fulda S, Srinivasan A.et al Two CD95 (APO‐1/Fas) signaling pathways. EMBO J 1998171675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaffidi C, Kirchhoff S, Krammer P H.et al Apoptosis signaling in lymphocytes. Curr Opin Immunol 199911277–285. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Zhu H, Xu C J.et al Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 199894491–501. [DOI] [PubMed] [Google Scholar]
- 24.Duchmann R, Kaiser I, Hermann E.et al Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995102448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartor R B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 20041261620–1633. [DOI] [PubMed] [Google Scholar]
- 26.Elson C O. Commensal bacteria as targets in Crohn's disease. Gastroenterology 2000119254–257. [DOI] [PubMed] [Google Scholar]
- 27.Shanahan F. Crohn's disease. Lancet 200235962–69. [DOI] [PubMed] [Google Scholar]
- 28.Ogura Y, Bonen D K, Inohara N.et al A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001411603–606. [DOI] [PubMed] [Google Scholar]
- 29.Hugot J P, Chamaillard M, Zouali H.et al Association of NOD2 leucine‐rich repeat variants with susceptibility to Crohn's disease. Nature 2001411599–603. [DOI] [PubMed] [Google Scholar]
- 30.Hugot J P, Zouali H, Lesage S. Lessons to be learned from the NOD2 gene in Crohn's disease. Eur J Gastroenterol Hepatol 200315593–597. [DOI] [PubMed] [Google Scholar]
- 31.Torok H P, Glas J, Lohse P.et al Alterations of the CARD15/NOD2 gene and the impact on management and treatment of Crohn's disease patients. Dig Dis 200321339–345. [DOI] [PubMed] [Google Scholar]
- 32.Hampe J, Cuthbert A, Croucher P J.et al Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet 20013571925–1928. [DOI] [PubMed] [Google Scholar]
- 33.Girardin S E, Boneca I G, Viala J.et al Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 20032788869–8872. [DOI] [PubMed] [Google Scholar]
- 34.Fellermann K, Wehkamp J, Herrlinger K R.et al Crohn's disease: a defensin deficiency syndrome? Eur J Gastroenterol Hepatol 200315627–634. [DOI] [PubMed] [Google Scholar]
- 35.Wehkamp J, Fellermann K, Herrlinger K R.et al Mechanisms of disease: defensins in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol 20052406–415. [DOI] [PubMed] [Google Scholar]
- 36.Hanauer S B, Feagan B G, Lichtenstein G R.et al Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 20023591541–1549. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt C, Marth T, Wittig B M.et al Interleukin‐12 antagonists as new therapeutic agents in inflammatory bowel disease. Pathobiology 200270177–183. [DOI] [PubMed] [Google Scholar]
- 38.Stallmach A, Marth T, Weiss B.et al An interleukin 12 p40‐IgG2b fusion protein abrogates T cell mediated inflammation: anti‐inflammatory activity in Crohn's disease and experimental colitis in vivo. Gut 200453339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mannon P J, Fuss I J, Mayer L.et al Anti‐interleukin‐12 antibody for active Crohn's disease. N Engl J Med 20043512069–2079. [DOI] [PubMed] [Google Scholar]
- 40.Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs 2005652253–2286. [DOI] [PubMed] [Google Scholar]
- 41.Neurath M F, Finotto S, Fuss I.et al Regulation of T‐cell apoptosis in inflammatory bowel disease: to die or not to die, that is the mucosal question. Trends Immunol 20012221–26. [DOI] [PubMed] [Google Scholar]
- 42.Lügering A, Schmidt M, Lugering N.et al Infliximab induces apoptosis in monocytes from patients with chronic active Crohn's disease by using a caspase‐dependent pathway. Gastroenterology 20011211145–1157. [DOI] [PubMed] [Google Scholar]
- 43.Atreya R, Mudter J, Finotto S.et al Blockade of interleukin 6 trans signaling suppresses T‐cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med 20006583–588. [DOI] [PubMed] [Google Scholar]
- 44.Mudter J, Wirtz S, Galle P R.et al A new model of chronic colitis in SCID mice induced by adoptive transfer of CD62L+ CD4+ T cells: insights into the regulatory role of interleukin‐6 on apoptosis. Pathobiology 200270170–176. [DOI] [PubMed] [Google Scholar]
- 45.Ito H. Treatment of Crohn's disease with anti‐IL‐6 receptor antibody. J Gastroenterol 200540(Suppl 16)32–34. [DOI] [PubMed] [Google Scholar]
- 46.Gordon J N, Di Sabatino A, Macdonald T T. The pathophysiologic rationale for biological therapies in inflammatory bowel disease. Curr Opin Gastroenterol 200521431–437. [PubMed] [Google Scholar]
- 47.Boirivant M, Pica R, DeMaria R.et al Stimulated human lamina propria T cells manifest enhanced Fas‐mediated apoptosis. J Clin Invest 1996982616–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sturm A, Itoh J, Jacobberger J W.et al p53 negatively regulates intestinal immunity by delaying mucosal T cell cycling. J Clin Invest 20021091481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sturm A, Leite A Z, Danese S.et al Divergent cell cycle kinetics underlie the distinct functional capacity of mucosal T cells in Crohn's disease and ulcerative colitis. Gut 2004531624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sturm A, Mohr S, Fiocchi C. Critical role of caspases in the regulation of apoptosis and proliferation of mucosal T cells. Gastroenterology 20021221334–1345. [DOI] [PubMed] [Google Scholar]
- 51.Ina K, Itoh J, Fukushima K.et al Resistance of Crohn's disease T cells to multiple apoptotic signals is associated with a Bcl‐2/Bax mucosal imbalance. J Immunol 19991631081–1090. [PubMed] [Google Scholar]
- 52.Wittig B M, Johansson B, Zoller M.et al Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7). J Exp Med 20001912053–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hosokawa T, Kusugami K, Ina K.et al Interleukin‐6 and soluble interleukin‐6 receptor in the colonic mucosa of inflammatory bowel disease. J Gastroenterol Hepatol 199914987–996. [DOI] [PubMed] [Google Scholar]
- 54.Jones S A, Richards P J, Scheller J.et al IL‐6 transsignaling: the in vivo consequences. J Interferon Cytokine Res 200525241–253. [DOI] [PubMed] [Google Scholar]
- 55.Ito H. IL‐6 and Crohn's disease. Curr Drug Targets Inflamm Allergy 20032125–130. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M, Yoshizaki K, Kishimoto T.et al IL‐6 is required for the development of Th1 cell‐mediated murine colitis. J Immunol 20001644878–4882. [DOI] [PubMed] [Google Scholar]
- 57.Jones S A, Novick D, Horiuchi S.et al C‐reactive protein: a physiological activator of interleukin 6 receptor shedding. J Exp Med 1999189599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullberg J, Schooltink H, Stoyan T.et al The soluble interleukin‐6 receptor is generated by shedding. Eur J Immunol 199323473–480. [DOI] [PubMed] [Google Scholar]
- 59.Shirogane T, Fukada T, Muller J M.et al Synergistic roles for Pim‐1 and c‐Myc in STAT3‐mediated cell cycle progression and antiapoptosis. Immunity 199911709–719. [DOI] [PubMed] [Google Scholar]
- 60.Mudter J, Weigmann B, Bartsch B.et al Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol 200510064–72. [DOI] [PubMed] [Google Scholar]
- 61.Musso A, Dentelli P, Carlino A.et al Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis 20051191–98. [DOI] [PubMed] [Google Scholar]
- 62.Ito H, Hirotani T, Yamamoto M.et al Anti‐IL‐6 receptor monoclonal antibody inhibits leukocyte recruitment and promotes T‐cell apoptosis in a murine model of Crohn's disease. J Gastroenterol 200237(Suppl 14)56–61. [DOI] [PubMed] [Google Scholar]
- 63.Takeda K, Clausen B E, Kaisho T.et al Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 19991039–49. [DOI] [PubMed] [Google Scholar]
- 64.Welte T, Zhang S S, Wang T.et al STAT3 deletion during hematopoiesis causes Crohn's disease‐like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA 20031001879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han X, Sosnowska D, Bonkowski E L.et al Growth hormone inhibits signal transducer and activator of transcription 3 activation and reduces disease activity in murine colitis. Gastroenterology 2005129185–203. [DOI] [PubMed] [Google Scholar]
- 66.Oberg H H, Wesch D, Grussel S.et al Differential expression of CD126 and CD130 mediates different STAT‐3 phosphorylation in CD4+CD25− and CD25high regulatory T cells. Int Immunol 200618555–563. [DOI] [PubMed] [Google Scholar]
- 67.Lankford C S, Frucht D M. A unique role for IL‐23 in promoting cellular immunity. J Leukoc Biol 20037349–56. [DOI] [PubMed] [Google Scholar]
- 68.Jacobson N G, Szabo S J, Weber‐Nordt R M.et al Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med 19951811755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boirivant M, Marini M, Di Felice G.et al Lamina propria T cells in Crohn's disease and other gastrointestinal inflammation show defective CD2 pathway‐induced apoptosis. Gastroenterology 1999116557–565. [DOI] [PubMed] [Google Scholar]
- 70.Marth T, Zeitz M, Ludviksson B R.et al Extinction of IL‐12 signaling promotes Fas‐mediated apoptosis of antigen‐specific T cells. J Immunol 19991627233–7240. [PubMed] [Google Scholar]
- 71.Fuss I J, Marth T, Neurath M F.et al Anti‐interleukin 12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology 19991171078–1088. [DOI] [PubMed] [Google Scholar]
- 72.Palmer E M, Farrokh‐Siar L, van Seventer M J.et al IL‐12 decreases activation‐induced cell death in human naive Th cells costimulated by intercellular adhesion molecule‐1. I. IL‐12 alters caspase processing and inhibits enzyme function. J Immunol 2001167749–758. [DOI] [PubMed] [Google Scholar]
- 73.Mudter J, Neurath M F. Mucosal T‐cells: mediators or guardians of inflammatory bowel disease? Curr Opin Gastroenterol 200319343–349. [DOI] [PubMed] [Google Scholar]
- 74.Grell M, Douni E, Wajant H.et al The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 199583793–802. [DOI] [PubMed] [Google Scholar]
- 75.Hanauer S B, Sandborn W J, Rutgeerts P.et al Human anti‐tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC‐I trial. Gastroenterology 2006130323–333. [DOI] [PubMed] [Google Scholar]
- 76.Schreiber S, Rutgeerts P, Fedorak R N.et al A randomized, placebo‐controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology 2005129807–818. [DOI] [PubMed] [Google Scholar]
- 77.van den Brande J, Hommes D W, Peppelenbosch M P. Infliximab induced T lymphocyte apoptosis in Crohn's disease. J Rheumatol Suppl 20057426–30. [PubMed] [Google Scholar]
- 78.ten Hove T, van Montfrans C, Peppelenbosch M P.et al Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut 200250206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Deventer S J. Transmembrane TNF‐alpha, induction of apoptosis, and the efficacy of TNF‐targeting therapies in Crohn's disease. Gastroenterology 20011211242–1246. [DOI] [PubMed] [Google Scholar]
- 80.Van den Brande J M, Braat H, van den Brink G R.et al Infliximab but not etanercept induces apoptosis in lamina propria T‐lymphocytes from patients with Crohn's disease. Gastroenterology 20031241774–1785. [DOI] [PubMed] [Google Scholar]
- 81.Di Sabatino A, Ciccocioppo R, Cinque B.et al Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn's disease. Gut 20045370–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen C, Assche G V, Colpaert S.et al Adalimumab induces apoptosis of human monocytes: a comparative study with infliximab and etanercept. Aliment Pharmacol Ther 200521251–258. [DOI] [PubMed] [Google Scholar]
- 83.Hlavaty T, Pierik M, Henckaerts L.et al Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn's disease. Aliment Pharmacol Ther 200522613–626. [DOI] [PubMed] [Google Scholar]
- 84.Holtmann M H, Douni E, Schutz M.et al Tumor necrosis factor‐receptor 2 is up‐regulated on lamina propria T cells in Crohn's disease and promotes experimental colitis in vivo. Eur J Immunol 2002323142–3151. [DOI] [PubMed] [Google Scholar]
- 85.Varfolomeev E E, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell 2004116491–497. [DOI] [PubMed] [Google Scholar]
- 86.Neurath M F, Pettersson S, Meyer zum Buschenfelde K H.et al Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF‐kappa B abrogates established experimental colitis in mice. Nat Med 19962998–1004. [DOI] [PubMed] [Google Scholar]
- 87.Lowenberg M, Verhaar A, van den Blink B.et al Specific inhibition of c‐Raf activity by semapimod induces clinical remission in severe Crohn's disease. J Immunol 20051752293–2300. [DOI] [PubMed] [Google Scholar]
- 88.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 20031045–65. [DOI] [PubMed] [Google Scholar]
- 89.Lamb J A, Ventura J J, Hess P.et al JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell 2003111479–1489. [DOI] [PubMed] [Google Scholar]
- 90.Hommes D W, Peppelenbosch M P, van Deventer S J. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti‐inflammatory targets. Gut 200352144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waetzig G H, Schreiber S. Mechanisms of infliximab: the reverse side of a drug effect. Inflamm Bowel Dis 200410(Suppl 1)S38–S43. [DOI] [PubMed] [Google Scholar]
- 92.Tiede I, Fritz G, Strand S.et al CD28‐dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 20031111133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doering J, Begue B, Lentze M J.et al Induction of T lymphocyte apoptosis by sulphasalazine in patients with Crohn's disease. Gut 2004531632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allison A C. Mechanisms of action of mycophenolate mofetil. Lupus 200514(Suppl 1)S2–S8. [DOI] [PubMed] [Google Scholar]