Abstract
Background and aims
Intestinal inflammation alters neuronal and enteroendocrine signalling, leading to functional adaptations in the inflamed bowel. Human studies have reported functional alterations at sites distant from active inflammation. Our aims were to determine whether neuronal and enteroendocrine signalling are altered in the uninflamed colon during ileitis.
Methods
We used neurophysiological, immunohistochemical, biochemical and Ussing chamber techniques to examine the effect of 2,4,6‐trinitrobenzene sulphonic acid (TNBS)‐induced ileitis on the properties of submucosal neurones, enteroendocrine cells and epithelial physiology of the distal colon of guinea pigs.
Results
Three days after TNBS administration, when inflammation was restricted to the ileum, the properties of colonic enteric neurones were altered. Submucosal AH neurones were hyperexcitable and had reduced afterhyperpolarisations. S neurones received larger fast and slow excitatory postsynaptic potentials, due to an increase in non‐cholinergic synaptic transmission. Despite the absence of inflammation in the colon, we found increased colonic prostaglandin E2 content in animals with ileitis. Ileitis also increased the number of colonic 5‐hydroxytryptamine (5‐HT)‐ and GLP‐2‐immunoreactive enteroendocrine cells. This was accompanied by an increase in stimulated 5‐HT release. Functional alterations in epithelial physiology occurred such that basal short circuit current was increased and veratridine‐stimulated ion transport was reduced in the colon of animals with ileitis.
Conclusion
Our data suggest that inflammation at one site in the gut alters the cellular components of enteric reflex circuits in non‐inflamed regions in ways similar to those at sites of active inflammation. These changes underlie altered function in non‐involved regions during episodes of intestinal inflammation.
Keywords: enteric nervous system, intestinal inflammation, ion transport, serotonin, submucosal plexus
Inflammatory bowel diseases (IBD) are associated with disturbances in intestinal motility and secretion. The abnormalities in gut motor and secretory patterns that accompany intestinal inflammation contribute to the development of symptoms, including abdominal pain and diarrhoea. Previous studies have demonstrated that properties of key regulatory elements involved in reflex control of intestinal function are altered at sites of inflammation: enterochromaffin (EC) cell activity is altered and the excitability of primary afferent neurones of the enteric nervous system (ENS) is enhanced.1,2,3,4,5 Furthermore, inflammatory changes to motor neurones, epithelium and smooth muscle alter the response properties of the effector tissues of the gut.6,7,8,9 Taken together, these data indicate that alterations to key components of enteric reflex circuits contribute to functional abnormalities at sites of inflammation.
It is now well established that there are functional and structural abnormalities throughout the gastrointestinal (GI) tract, even at distant, non‐inflamed sites in the bowel during intestinal inflammation. For example, gastric emptying, orocaecal transit and small bowel transit times are reduced in patients with ulcerative colitis.10,11 Immunohistochemical analysis of tissue samples taken from normal patients and those with IBD has uncovered alterations in the neurochemical coding of enteric neurones in regions that are actively inflamed, as well as in regions that have no evidence of inflammation.12,13 Studies in animal models of IBD have described a similar phenomenon in which 2,4,6‐trinitrobenzene sulphonic acid (TNBS)‐induced colitis in rats results in alterations in the motility of non‐inflamed regions of the small intestine.14,15 Jacobson et al showed that TNBS‐induced colitis was associated with a reduced noradrenaline release, both in the inflamed colon and the non‐inflamed ileum. A more recent study concluded that changes in ileal motility in colitis may be due to over‐expression of presynaptic inhibitory α2 adrenoceptors.16 Inflammation in the small intestine was also reported to lead to smooth muscle cell and neuronal dysfunction in the non‐inflamed gastric fundus.17 These data point to the possibility that inflammation of one region of the gastrointestinal (GI) tract can lead to neurological alterations in the ENS which can result in GI dysfunction in uninflamed regions.
Because of the importance of enteroendocrine cell signalling and the ENS in regulating gut function, we have examined these parameters in the colon of animals with ileitis in order to establish if these essential regulatory sites contribute to the mechanism of altered function at distant sites in the bowel. We have tested the hypothesis that altered neuronal and enteroendocrine signalling contributes to functional alterations in secretion in non‐inflamed regions of the GI tract.
Methods
Male albino guinea pigs (Charles River, Montreal, Canada) weighing 200–300 g were housed in a temperature‐controlled room. The animals were maintained on a normal 12:12‐h light‐dark cycle and were allowed access to food and water ad libitum. All methods used in this study were approved by the University of Calgary Animal Care Committee and were carried out in accordance with the guidelines of the Canadian Council on Animal Care.
TNBS (0.5 ml; Sigma‐Aldrich; 30 mg/ml in 30% ethanol) ileitis was induced in guinea pigs as previously described.3 Two different control groups were examined: the first group of controls were similarly treated with the exception that 0.5 ml physiological saline (0.9% NaCl) was injected into the distal ileum and the second group of animals remained naive. Vehicle controls have previously been assessed by injecting 0.5 ml of 30% ethanol into the distal ileum.3 Three days after surgery the animals were anaesthetised with an overdose of sodium pentobarbital and exsanguinated. The ileum and colon were then removed and used for experimental studies.
Assessment of inflammation
The severity of ileitis was assessed by measuring changes in the weight of the animals and examining the macroscopic damage to the mucosa. Animals were weighed prior to administration of TNBS or saline and daily after surgery. After animals were euthanised, the ileum and colon were removed, opened along the mesenteric border and examined macroscopically. The criteria used for scoring gross morphologic damage have been described in detail previously.3 Briefly, the total score of mucosal damage included: the presence and severity of adhesions (score 0–1), the maximum thickness of the bowel wall (in mm), the presence of diarrhoea (score 0–1) and the extent of ulceration and hyperaemia (score 0–10).
Myeloperoxidase assay
Myeloperoxidase (MPO), an enzyme found in cells of myeloid origin, is used as a marker of neutrophil infiltration. MPO activity was measured in samples of colon that were weighed, snap frozen in liquid nitrogen and stored at −80°C until assay as described previously.18 Values are expressed as units of MPO activity per gram of tissue sample, where 1 U of MPO is defined as that which degrades 1 μmol of hydrogen peroxide per minute.
Enteric neurophysiology
The distal colon was removed, the oral end marked, and placed in Krebs solution (mM: NaCl, 117; KCl, 4.8; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and d‐glucose, 11; aerated with 95% O2/5% CO2) containing nicardipine (3 μM) and scopolamine (1 μM). Whole‐mount preparations of submucosal plexus were prepared for electrophysiological characterisation as previously described.19 Neurones were impaled with microelectrodes fabricated from 1‐mm outer diameter borosilicate glass (World Precision Instruments, Sarasota, FL) that were filled with 1% biocytin in 1‐M KCl. Electrode resistances were 70–120 MΩ. Recordings of membrane potential were made using a Multiclamp 700A amplifier in current clamp mode (Axon Instruments, Foster City, CA). Signals were digitised at 5–50 kHz (Digidata 1322A; Axon instruments) and stored using PC‐based data acquisition and analysis software (pClamp 9.2 suite; Axon Instruments). Electrophysiological variables were measured off line using Clampfit 9.2 (Axon Instruments).
Neuronal electrical properties were determined after allowing the impalements to stabilise for 5 min without applying intracellular holding current. At this time, the ability of the cell to fire an action potential (AP) upon intracellular current injection was assessed. Only neurones that were able to fire APs that overshot 0 mV and had resting membrane potentials more negative than −40 mV were included in the electrophysiological analyses.
Excitability was measured by injecting 500‐ms depolarising and hyperpolarising current pulses whose amplitude increased in 20‐pA increments. This protocol revealed input resistance, whether the neurone fired anode break APs, the number of APs at rheobase, twice rheobase, and the maximum number of APs that each neurone could fire. Synaptic inputs and antidromic APs of neurones were stimulated using bipolar silver chloride electrodes (10–50‐μm tip diameter), insulated except at the tip, that were carefully placed on interganglionic nerve bundles. Stimulus pulses of 0.5‐ms duration and 5–15‐V intensity were delivered via a Grass SD9 stimulator (Grass Medical Instruments, Quincy, MA); stimulation electrode location and stimulation intensity were adjusted to evoke maximal synaptic potential amplitudes for each neurone in both experimental groups. Neurones were hyperpolarised to −80 mV for measurement of fast excitatory postsynaptic potential (EPSP) amplitude to prevent APs and to minimise changes in the electrochemical driving force. The average amplitude of at least three fast EPSPs at −80 mV was recorded for each neurone under control conditions and after superfusion with hexamethonium (100 μM). To determine whether the impaled neurones received slow synaptic input, 1‐s trains of pulses at 20 Hz were applied to internodal strands. Slow EPSP amplitudes were measured from slow EPSPs evoked at the resting membrane potential of each neurone.
Measurement of PGE2 content in the colon
Segments of distal colon were removed, weighed and immediately snap frozen in liquid nitrogen. PGE2 content was measured as previously described.20 Briefly, the isolated segments of colon were homogenised in 1 ml PBS (pH 7.4) containing 1 mM EDTA and 100 μM ibuprofen. Samples were centrifuged at 13 000 rpm for 20 min at 4°C. The supernatant was diluted 1:100 in immunoassay buffer and PGE2 levels were measured by enzyme immunoassay according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI).
Immunohistochemistry
Colonic segments to be used for immunohistochemistry were fixed overnight at 4°C in Zamboni's fixative (2% paraformaldehyde, 0.2% picric acid; pH 7.4). Samples were then transferred to 20% sucrose in PBS overnight at 4°C. Transverse segments from each animal were embedded in OCT compound (Miles, Elkhardt, IN). Sections of colon (10 μm) were cut on a cryostat and mounted onto poly‐d‐lysine coated slides.
We have previously demonstrated that saline‐treated and naive controls did not differ in enteroendocrine cell numbers3; therefore, in the present study, cell numbers were assessed in naive controls only. Tissue sections were washed with PBS containing 0.1% Triton X‐100 (3×10 min), followed by incubation with rabbit anti‐5‐hydroxytryptamine (5‐HT; 1:5000, Incstar, Stillwater, MN), rabbit anti‐glucagon‐like peptide‐2 (GLP‐2; 1:500, Biogenesis, Kingston, NH), or rabbit anti‐somatostatin (1:1000, Peninsula, Belmont, CA) for 48 h at 4°C. After a second wash, sections were incubated with Cy3‐conjugated donkey anti‐rabbit IgG (1:100; Jackson, West Grove, PA) secondary antiserum for 2 h at room temperature. Slides were examined with a Zeiss Axioplan microscope and micrographs were taken using a digital imaging system consisting of a digital camera (Sensys; Photometrics, Tucson, Arizona) and image analysis software (V for Windows; Digital Optics, Auckland, New Zealand). The number of enteroendocrine cells was expressed as a function of length of colon. Controls consisting of liquid phase preabsorption of primary antisera with cognate peptides or 5‐HT (10 nmol/ml diluted antisera) and omission of the primary antisera have previously been shown to abolish any immunoreactivity.3
Measurement of mucosal serotonin release in the colon
The colon was opened along the mesentery and cut into two segments (1×0.5 cm). The segments were pinned flat, mucosal side up, in a Sylgard‐coated six‐well dish containing 37°C HEPES solution (NaCl: 110 mM; KCl: 5.4 mM; CaCl2 2H2O: 1.8 mM; MgCl 6H2O: 1 mM; sucrose: 60 mM; glucose: 5 mM; HEPES: 20 mM). To mechanically stimulate the mucosa, segments of colon were gently stroked in a circumferential direction with a rounded glass probe (diameter∼2 mm) at a rate of 8 strokes/min for a total of 15 min.1 Basal levels of serotonin release were determined by leaving preparations undisturbed in the bathing solution for 15 min. The serotonin released into the bathing solution was measured with an enzyme immunoassay kit used according to the manufacturer's instructions (Beckman Coulter, Fullerton, CA).
Electrolyte transport studies
Segments of distal colon were removed and immediately placed in ice‐cold Krebs solution (as described above). Segments were cut open along the mesenteric border and the muscularis externa, including the myenteric plexus, was removed by blunt dissection. The submucosa/mucosa preparations were mounted in standard Ussing chambers (WPI, Sarasota, FL) with 0.6 cm2 exposed surface area. The mucosal and serosal surfaces were bathed with 10 ml Krebs solution. Chambers were maintained at 37°C and aerated with 95% O2/5% CO2. The transepithelial potential difference (PD) across the tissue was voltage clamped at 0 mV by applying a short circuit current (Isc) using a voltage clamp apparatus (DVC 4000; WPI).
After a 15–20‐min equilibration period, the baseline Isc and PD (in mV) were measured and the resistance of the tissue was calculated using Ohm's law. The Isc was monitored as a measure of net electrolyte transport across the tissue. The maximum change in Isc within 10 min of the addition of various drugs was measured. The drugs applied were: veratridine (30 μM), bethanechol (10 μM) or forskolin (10 μM). In addition, bethanechol and forskolin were applied in the presence of tetrodotoxin (TTX; 300 nM), a potent neurotoxin, to measure the maximum change in Isc in response to direct stimulation of chloride secretion by the enterocytes. All drugs were added to the serosal compartment.
Drugs
Veratridine (dissolved in 80% ethanol), forskolin (dissolved in DMSO), nicardipine, scopolamine, hexamethonium and bethanechol (dissolved in distilled water) were obtained from Sigma‐Aldrich, St. Louis, MO. TTX (dissolved in distilled water) was obtained from Tocris, Ellisville, MO.
Data analysis
The data are presented as means (SE) for n animals and statistical comparisons were conducted with GraphPad Prism software (Version 3.03; GraphPad Software, San Diego, CA). Comparisons between two groups were analysed with a two‐way Student's unpaired t test. Comparisons between three or more groups were done with a one‐way ANOVA followed by a Dunnett's multiple comparison test. Data with a non‐parametric distribution were analysed by a Kruskal‐Wallis test followed by a Dunn's multiple comparison test. p<0.05 was considered statistically significant. Isc responses were expressed as the maximum change in Isc from baseline (μA/cm2).
Results
TNBS‐induced inflammation
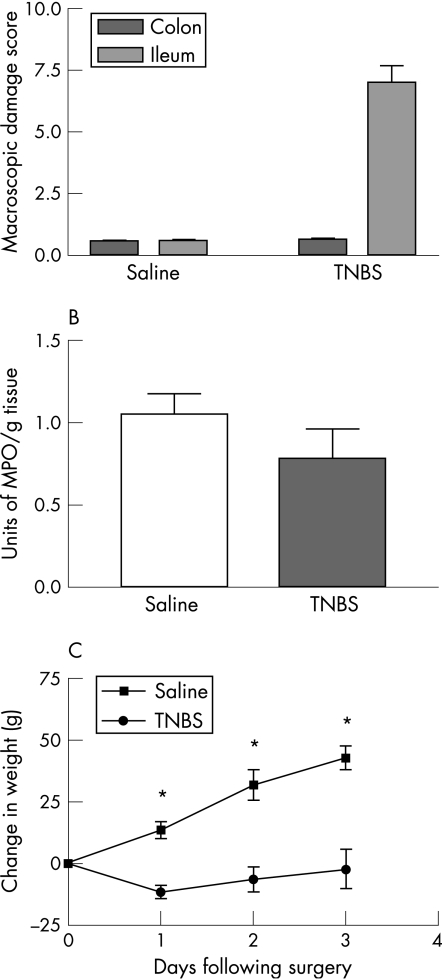
Administration of TNBS into the lumen of the ileum caused localised inflammation characterised by ulceration, adhesions, hyperaemia and changes to mucosal architecture that were similar to those in a previous study.3 The macroscopic damage score for the ileum 3 days after administration of TNBS was significantly increased when compared to the ileum of naive or saline‐treated controls (fig 1A: p<0.01; naive controls, n = 6; saline controls, n = 13; TNBS, n = 17). In contrast, macroscopic evaluation of the colon from guinea pigs with an inflamed ileum revealed no significant difference from the colon of naive or saline controls. MPO activity was similar in the colon of guinea pigs with TNBS ileitis when compared to control animals (fig 1B: saline controls, n = 5; TNBS, n = 5). In addition, TNBS‐treated guinea pigs gained significantly less weight than the saline‐treated controls (fig 1C: p<0.05; saline controls, n = 7; TNBS, n = 13).
Figure 1 (A) Macroscopic damage scores for the ileum were significantly higher in guinea pigs treated with TNBS 3 days previously compared to saline‐treated controls. *p<0.05 (mean (SE)). However, there was no detectable change in damage score of the colons from animals with TNBS‐induced ileitis. (B) MPO activity, a measure of neutrophil infiltration, was similar in the colon of animals with TNBS‐induced ileitis when compared to the saline‐treated controls. (C) TNBS ileitis animals initially lost weight and regained weight at a slower rate than controls. *p<0.05 (mean (SE)).
Enteric neurophysiology
Colonic function is governed in large part by the properties of enteric neurones. Submucosal neurones can be divided into several functional classes including intrinsic primary afferent neurones and different classes of secretomotor and vasomotor neurones. These functional classes of submucosal nerves have distinct electrophysiological characteristics: intrinsic primary afferents are classified as AH neurones, whereas the cholinergic and non‐cholinergic secretomotor/vasomotor neurones are typically classified as S neurones. In contrast to myenteric neurones,21,22 there is currently no evidence that the subclasses of submucosal S neurones have distinct electrophysiological characteristics; therefore, we have grouped S neurones together for the purpose of determining the effect of ileitis on colonic submucosal motor neurones. A further class of neurone, the low‐threshold (LT) neurone, has also been identified in the submucosal plexus;19 however, LT neurones where not encountered in the present study. We measured the properties of both major classes (AH and S) of enteric neurones in the submucosal plexus. Electrophysiological recordings were made from 35 neurones from animals with TNBS‐induced ileitis (24 S type and 11 AH type) and compared with data obtained from saline‐treated (12 S neurones and 7 AH neurones) and naive controls (12 S neurones). A variety of neurophysiological parameters were assayed, as described in Methods section. There were no differences between saline‐treated and naive control S neurones and the data from these two populations were pooled. When electrophysiological parameters from control animals were compared to the data from TNBS animals, a number of statistically significant differences were evident (tables 1 and 2).
Table 1 Electrophysiological characteristics of colonic S neurones.
| Control | Ileitis | |
|---|---|---|
| RMP (mV) | −49.2 (1.6) | −48.9 (1.5) |
| Input resistance (MΩ) | 220 (19) | 198 (25) |
| AP threshold (pA) | 108 (20) | 122 (23) |
| APs at rheobase | 1.6 (0.2) | 1.2 (0.3) |
| APs at 2×rheobase | 6.2 (1.2) | 5.6 (1.0) |
| Maximum APs | 13.6 (2.5) | 12.3 (2.4) |
| AP amplitude (mV) | 59 (1.9) | 65 (4) |
| APD50 (ms) | 1.53 (0.05) | 1.70 (0.09) |
| Fast EPSP amplitude (mV) | 21.6 (1.5) | 28.2 (1.12)* |
| Hexamethonium‐resistant | 1.7 (0.5) | 9.8 (1.3)* |
| fast EPSP amplitude (mV) | ||
| Slow EPSP amplitude (mV) | 4.8 (0.9) | 10.95 (2.0)* |
Mean (SE) data are shown (*p<0.05). n = 24 for control neurones and n = 24 ileitis neurones.
Table 2 Electrophysiological characteristics of colonic AH neurones.
| Control | Ileitis | |
|---|---|---|
| RMP (mV) | −60.3 (1.4) | −58.8 (1.5) |
| Input resistance (MΩ) | 222 (59) | 197 (23) |
| AP threshold (pA) | 114 (45) | 103 (28) |
| APs at rheobase | 1.14 (0.15) | 1.45 (0.21) |
| AP amplitude (mV) | 82 (2.6) | 76 (3.0) |
| APD50 (ms) | 2.0 (0.1) | 1.9 (0.1) |
| Slow EPSP amplitude (mV) | 12.6 (4.1) (n = 4) | 11.1 (1.5) (n = 4) |
| APs at 2×rheobase | 2.0 (0.23) | 3.5 (0.5)* |
| Maximum number of APs | 3.7 (0.9) | 11.3 (2.6)* |
| Afterhyperpolarisation | 5.7 (0.8) | 3.2 (0.7)* |
| amplitude (mV) |
Mean (SE) data are shown (*p<0.05). Data are from 11 neurones from ileitis animals and seven from controls unless otherwise noted.
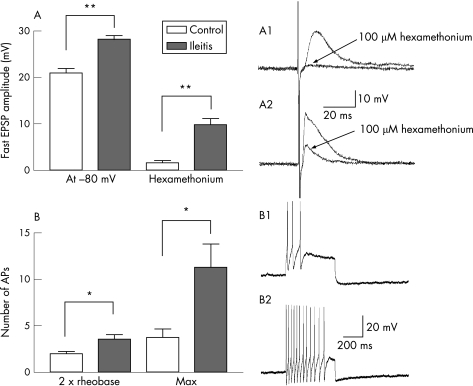
The amplitude of fast EPSPs evoked at −80 mV and the amplitude of hexamethonium‐resistant fast EPSPs was increased in S neurones from TNBS animals (fig 2A, table 1). Slow EPSPs were also larger in S neurones from animals with ileitis (n = 11) compared to controls (n = 13) (table 1). The number of action potentials at rheobase in AH neurones was not changed in the colon of ileitis animals, likely due to the fact that AH neurones from both normal and inflamed colons rarely fire more than a single action potential at rheobase.5 Nevertheless, there was a significant increase in the number of action potentials fired at twice rheobase, as well as the maximum number of action potentials that AH neurones can fire, demonstrating an enhanced excitability of colonic AH neurones taken from animals with TNBS ileitis when compared to controls (fig 2B, table 2). This was associated with a decrease in the amplitude of the afterhyperpolarisation that characteristically follows action potentials in these neurones (table 2).
Figure 2 Neurophysiological alterations in the submucosal plexus of the colon during ileitis. (A) The amplitude of fast EPSPs recorded at −80 mV was significantly larger in animals with ileitis compared to controls. In addition, blockade of nicotinic receptors with 100 μM hexamethonium abolished fast EPSPs in control animals but ileitis neurones had a substantial hexamethonium‐resistant component. A1 and A2 are typical examples of fast EPSPs in S neurones from control animals (A1) and TNBS ileitis animals (A2) and their sensitivity to hexamethonium. (B) The number of action potentials at twice rheobase, as well as the maximum number of action potentials, were significantly lower in AH neurones from saline‐treated controls (example of maximum number of action potentials in B1) compared to ileitis animals (example in B2). *p<0.05; **p<0.001.
Colonic PGE2 levels
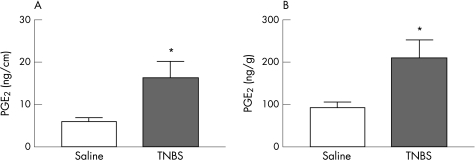
Eicosanoid levels, including PGE2, are increased in the actively inflamed colon and have been reported to mediate electrophysiological changes observed during inflammation.20 We assessed the level of this eicosanoid in colonic homogenates from animals with ileitis. The PGE2 content of full wall thickness samples of colon was expressed as function of wet weight of colon, as well as a function of length of colon. In both parameters, colonic PGE2 content from animals with an inflamed small intestine was significantly increased when compared to the colon of saline‐treated controls (fig 3A,B: saline controls, n = 10; TNBS, n = 10).
Figure 3 PGE2 content in the colon of control animals and animals with TNBS‐induced ileitis was expressed as a function of (A) length of colon and as a function of (B) wet weight of colon. In both parameters, animals with an inflamed small intestine demonstrated significantly increased colonic PGE2 content when compared to the colons of saline‐treated controls. *p<0.05 (mean (SE)).
Enteroendocrine cell numbers
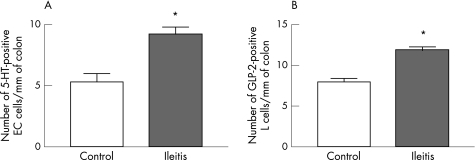
Enteroendocrine cells are an essential element of signalling from the colonic lumen.23 We quantified 5‐HT, GLP‐2 or somatostatin‐immunoreactive enteroendocrine cells in sections of colon from control animals or animals with TNBS‐induced ileitis. Three days after administration of TNBS into the ileum, the number of 5‐HT‐immunoreactive EC cells was significantly increased in the colon when compared to the colon of naive controls (fig 4A: p<0.05; naive controls, n = 7; TNBS, n = 7). The number of GLP‐2‐immunoreactive L cells was also significantly increased in the TNBS‐treated animals when compared to the naive controls (fig 4B: p<0.05; saline controls, n = 8; TNBS; n = 6). In contrast, there was no significant difference in the number of somatostatin‐immunoreactive D cells in the colon of TNBS‐treated guinea pigs versus naive controls (data not shown).
Figure 4 Ileitis changes the numbers of colonic mucosal enteroendocrine cell populations. Following ileitis, the number of (A) 5‐HT‐immunoreactive enterochromaffin (EC) cells and (B) GLP‐2‐immunoreactive L cells were increased compared to controls. *p<0.05 (mean (SE)).
5‐HT release
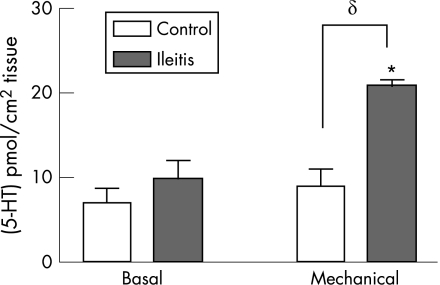
To determine if the increase in EC cell numbers was associated with functional changes in the secretion of 5‐HT, we assessed the release of 5‐HT from isolated segments of colon under basal and stimulated conditions. Under basal conditions, the amount of 5‐HT released from the colon of guinea pigs with ileitis did not differ from the colon of naive controls. However, 5‐HT release in response to mechanical stimulation of the colonic mucosa was significantly increased in the animals with TNBS‐induced ileitis when compared to the naive controls (fig 5: δ p<0.05; naive controls, n = 6; TNBS, n = 4).
Figure 5 Mucosal 5‐HT release in the colon during ileitis. Ileitis leads to an increase in the amount of 5‐HT released following mechanical stimulation of the mucosa. However, there was no change in the basal amount of release. *p<0.05 v basal release; δp<0.05 v saline controls.
Mucosal electrolyte transport
Basal electrical parameters
Having assessed the major signalling components of the colon, we next examined functional properties of the colon. Basal electrical parameters of the guinea pig distal colon are shown in table 3. Variations in basal Isc occurred such that animals consisted of two groups: group 1 demonstrated a positive basal Isc, whereas group 2 had a negative basal Isc. The pooled basal Isc in the colon of guinea pigs with ileitis was significantly greater than average basal Isc in the colon of controls. Basal mucosal resistance calculated using Ohm's law, was significantly increased in the colon from TNBS‐treated animals when compared to the saline‐treated controls (table 3; p<0.05).
Table 3 Basal Isc and resistance in saline‐treated and TNBS‐treated animals.
| Basal Isc (μA/cm2) | Pooled basal Isc (μA/cm2) | Resistance (Ω/cm2) | ||
|---|---|---|---|---|
| Group 1 | Group 2 | |||
| Saline | 32.96 (7.69); n = 7 | −20.50 (6.68); n = 5 | 10.69 (9.42); n = 12 | 115.5 (9.990); n = 12 |
| TNBS | 101.7 (16.28)*; n = 10 | −21.67; n = 1 | 90.45 (18.51)*; n = 11 | 150.0 (13.26)*; n = 11 |
Data are mean (SE) (*p<0.05).
Isc response to veratridine
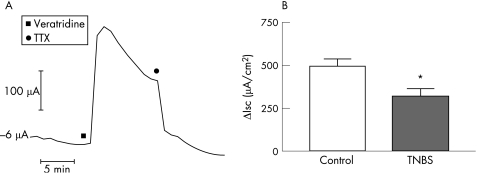
Addition of veratridine to the serosal bath caused a TTX‐sensitive increase in Isc (fig 6A). The Isc response to veratridine was significantly reduced in the colon of guinea pigs with TNBS‐induced ileitis when compared to the saline‐treated controls (fig 6B: p<0.05; saline controls, n = 7; TNBS, n = 8).
Figure 6 Veratridine‐stimulated electrogenic secretion in the colon during ileitis. (A) Representative trace depicting the Isc response to application of veratridine (30 μM) to the serosal compartment of the Ussing chamber. The response slowly desensitises and is abolished by the addition of TTX (300 nM) to the serosal bath. (B) Comparison of Isc responses of control and ileitis colonic mucosa to addition of veratridine. Ileitis results in a significantly reduced effect of veratridine in the colon. *p<0.05 (mean (SE)).
Isc response to bethanechol and forskolin
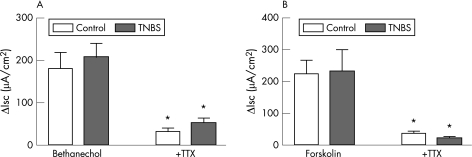
The Isc response to bethanechol (fig 7A: saline controls, n = 6; TNBS, n = 6) or forskolin (fig 7B: saline controls, n = 9; TNBS, n = 9) in the colon of guinea pigs with ileitis was not significantly different from saline‐treated controls. The increases in Isc elicited by both bethanechol and forskolin were significantly attenuated in the presence of TTX (fig 7A,B: p<0.05). However, there was no significant difference in the response to either drug in the colon of guinea pigs with TNBS‐induced ileitis versus saline‐treated controls. Similar to previous studies,24 the addition of TTX alone to the serosal bath caused a reduction in basal Isc; however, the effect of TTX on basal Isc was not significantly different in the colon of guinea pigs with TNBS‐induced ileitis when compared to the saline‐treated controls (data not shown).
Figure 7 Effects of bethanecol and forskolin on Isc during ileitis. TNBS ileitis has no effect on calcium‐mediated or cAMP‐mediated secretion in colonic mucosa (A and B). TTX added to the serosal bath prior to addition of bethanechol or forskolin significantly attenuated both the calcium‐dependent and cAMP‐dependent secretion in the colon of control and TNBS‐treated animals. *p<0.05 (mean (SE)).
Discussion
In the present study we show that ileitis alters enteroendocrine cell signalling and enteric neurophysiology in the non‐inflamed colon. This occurs despite the lack of overt inflammation in the colon, although an increased PGE2 content was observed in the colon of animals with ileitis. Previous studies have found that inflammation of the colon can lead to functional impairment in the ileum,14,16 and others have shown that colitis can upregulate TTX‐resistant Na+ channels in the dorsal root ganglion neurones that innervate the urinary bladder.25 These findings are consistent with an ability of inflammation in a discrete region of the bowel to cause neural dysfunction in other non‐inflamed regions.
Enteric neurophysiology
We have previously examined the effect of colitis on the electrophysiological characteristics of submucosal neurones in the inflamed colon. During colitis, the excitability of AH neurones is enhanced, due to a reduced afterhyperpolarising potential (AHP). This was possibly due to changes in the Ca2+ component of the AP as there was a decrease in the action potential duration at half amplitude (APD50).5 Ileitis caused similar changes in this class of neurone in the colon, although alterations to the Ca2+ component of the action potential are unlikely to underlie these changes as the APD50 is unchanged in the colon of ileitis animals when compared to controls. This change is likely due to a decrease in the activation of a Ca2+‐activated potassium conductance or an increase in a hyperpolarisation‐activated non‐selective cation conductance (IH) in these neurones.4,5
Our previous work, similar to the present study, demonstrates a decrease in the amplitude of the AHP without accompanying changes in membrane potential and input resistance in enteric AH neurones during TNBS colitis.4,5 These findings seem counterintuitive as the Ca2+‐activated potassium conductance that mediates the AHP also contributes to resting input resistance and membrane potential (RMP). An increase in the IH current would also suppress the AHP; however, an increase in this current would be associated with a decrease in input resistance and a depolarisation. Although the properties of IH and the Ca2+‐activated potassium current have been extensively characterised in AH neurones, it is likely that other background conductances also contribute to the RMP and input resistance of AH neurones. For instance, it is highly likely that the Na+‐Ca2+ exchanger and the Na+/K+ ATPase contribute inward current at rest. In addition, background K+ currents such as those recently described in dorsal root ganglion neurones26 will contribute outward current. A possible consequence of this complex balancing act of inward and outward currents is that TNBS colitis could significantly alter one or more ion channels that determine AH neurone excitability without substantially altering either RMP or input resistance.
In myenteric AH neurones from inflamed colon, the excitability of AH neurones was increased due to the upstream release of a cyclooxygenase (COX‐2)‐derived prostaglandin.20 It is unclear what the sequence of events downstream of COX‐2 activation were, but it is likely that the release of prostaglandins leads to changes in the activity of the ENS which then alters ion channel function in neurones in the inflamed region. In support of this, Manning and coworkers reported that chronic application (48 h) of PGE2 leads to increased excitability of AH neurones with increased detection of spontaneous action potentials.27 In the present study, it is possible that prostaglandins participate in the altered AH neurone electrophysiology as PGE2 content was significantly increased in the colon of animals with ileitis. Furthermore, in vitro incubation with PGE2 has been reported to cause a concentration‐dependent increase in Fos expression, indicative of neural activation, in ileal submucosal neurones.28 It will be important to determine whether increased neuronal activity emanating from the ileum during ileitis, or exposure to COX‐2‐derived prostaglandins in the colon following ileitis is the cause of the changes in AH neurone electrophysiology observed in the present study.
Motor or interneurones of the submucosal plexus (S neurones) were also altered in the colon of animals with TNBS colitis. The amplitudes of evoked fast and slow excitatory synaptic potentials were increased in submucosal S neurones. In the case of both synaptic events, the increased amplitude appeared to be due to an increased participation of non‐cholinergic transmitters.5 The results of the present study indicate that ileitis has very similar effects to colitis on synaptic transmission to colonic submucosal S neurones. Fast and slow EPSPs were larger during ileitis, and the fast EPSPs were more resistant to blockade of nicotinic receptors by hexamethonium. In the colon of animals with colitis, the hexamethonium‐resistant component of fast synaptic transmission was due to purinergic and serotonergic synaptic transmission during inflammation,5 and based on the present data it is likely that a similar scenario occurs during ileitis.
A recent study demonstrated that inflammation of the hindpaw of rats augments colonic nociception.29 Carageenan‐induced inflammation of the hindpaws, but not the forepaws, of rats leads to hyperalgesia to colorectal distension. The authors concluded that this effect was due to the terminals of primary afferent neurones converging on similar regions of the dorsal horn, and that increased traffic in the somatic pathway led to increased efficacy of synaptic transmission in the visceral pathway due to increased release of glutamate. It is possible that a similar mechanism accounts for our observations. Increased neural activity in spinal afferent neurones and sympathetic pathways that innervate the inflamed region might be feasible pathways for the spread of increased neural activity from an inflamed region of gut to non‐inflamed regions.
Enteroendocrine cell signalling
Enteroendocrine cells, which are specialised epithelial cells that release their contents from their basolateral membrane into the lamina propria, are important components in afferent signalling within the GI tract.23,30 5‐HT‐containing EC cells have been shown to play a key role in transducing luminal chemical and mechanical stimuli and activating the nerve terminals of enteric, vagal and spinal afferent neurones.30,31,32 Several studies on biopsies from patients with IBD and irritable bowel syndrome, as well as in animal models of IBD, have highlighted changes in this cell population during inflammation.1,2,3,33
During TNBS colitis in the guinea pig, serotonin availability in the colon is altered due to increased numbers of 5‐HT‐immunoreactive EC cells and a reduction in the expression of SERT.1 These changes resulted in increased basal and mechanically stimulated mucosal release of serotonin. In the present study, ileitis caused an increase in the number of EC cells in the colon, as well as an increase in stimulated 5‐HT release. Thus, ileitis and colitis are associated with similar changes to the colonic mucosal serotonin signalling system, although the effects of ileitis are not quite as extensive as during colitis. However, given the important role of 5‐HT in intestinal secretion and motility, the increased release of the amine may contribute to altered gut function at non‐inflamed regions of the gastrointestinal tract.
The number of GLP‐2 immunoreactive L cells in the epithelium of the colon was also increased during ileitis. In human studies, circulating levels of GLP‐2 were increased in patients with IBD.34 GLP‐2 has been shown to have a number of roles in gastrointestinal physiology, including potent trophic effects on the intestinal mucosa and enhanced nutrient absorption.35 Furthermore, administration of a human GLP‐2 analogue was protective in a murine model of dextran sodium sulfate‐induced colitis.36 It can be speculated that the increase in GLP‐2‐positive enteroendocrine cell numbers in the colon is an adaptive response to the injury and inflammation in the small intestine. The increased GLP‐2 levels could facilitate repair of damage to the epithelium and increase mucosal surface area for absorption of nutrients, although a direct role in sensory transduction has yet to be described. The increase in 5‐HT and GLP‐2‐immunoreactive cell numbers was not due to an indiscriminate increase in all enteroendocrine cell populations in the colon during ileitis, as the number of somatostatin‐immunoreactive D cells was unaltered by ileitis.
Colonic function in ileitis: electrogenic chloride secretion
Submucosal nerves are primarily involved in secretory reflexes and thus it is possible that the altered neurophysiology in the colon of animals with ileitis may lead to abnormal secretion. Previous studies have demonstrated profound effects of inflammation on mucosal secretory function. In animal models of colitis, the colonic epithelium is hyporesponsive to stimulation with the phosphodiesterase inhibitor, IBMX6,37 and the muscarinic agonist, carbachol.37,38 Similarly, the secretory response to capsaicin and carbachol is reduced in both the ileum and jejunum of animals with TNBS‐induced ileitis.39 A more recent study examined the proximal colon of rats after administration of TNBS into the distal colon.40 Inflammation in these animals was limited to the distal colon, yet the non‐inflamed proximal colon was hyporesponsive to the calcium‐dependent secretagogues, carbachol and histamine.
In the present study, calcium‐dependent and cAMP‐dependent secretion evoked by bethanechol or forskolin, respectively, remained unchanged in the colon of guinea pigs with ileitis. The absence of change in the secretory response to these secretagogues in the present study could be due to a number of factors. For example, the previous reports of a reduced response to carbachol examined areas proximal to the site of inflammation 7 days after the administration of TNBS,39,40 whereas the present study assessed regions that were distal to the site of inflammation at 3 days.
In contrast to the absence of change in carbachol‐evoked and forskolin‐evoked secretion, our results demonstrate that ileitis is associated with a reduced secretory response to veratridine in the distal colon. There was also considerable variation in the basal Isc measurements whereby approximately 58% of the control animals had positive basal Isc. Such variation has been observed previously when examining guinea pig tissue in Ussing chamber studies.41 In contrast to the control animals, virtually all of the TNBS‐treated animals had a positive basal Isc. It has been reported that both positive and negative basal Isc in the guinea pig colon are associated with net sodium absorptive flux, with little or no difference in chloride fluxes.41 Thus, it is possible that the increase in basal Isc in the colon of guinea pigs with ileitis represents an increase in sodium absorption that acts as a compensatory mechanism for the loss of absorptive capacity in the inflamed distal small intestine. Alternatively, it has previously been reported that prostaglandins evoke a dose‐dependent increase in basal Isc.28,42,43 Moreover, in the guinea pig colon and ileum, the PGE2‐evoked secretion is partially mediated by submucosal neurones.43 Therefore, the increased level of PGE2 in the colon of animals with ileitis may contribute to the increased basal Isc that is observed in the TNBS‐treated animals.
Veratridine, an activator of voltage‐dependent sodium channels that causes the repetitive firing of action potentials and depolarisation of enteric neurones,44 has been used to non‐selectively stimulate submucosal neurones resulting in an increase in Isc that is attributed to chloride secretion.45,46 In the present study, it is unlikely that the hyporesponsiveness to veratridine is attributable to a loss of epithelial integrity because baseline resistance is increased in the colon of guinea pigs with TNBS‐induced ileitis. This suggests that not only is the epithelial barrier intact but there is an enhanced barrier function. This may also be a compensatory mechanism to the injury and inflammation in the distal ileum. Furthermore, inflammation of the distal colon was not a factor because macroscopic damage scores and MPO activity were similar in TNBS‐treated and control animals. The mechanism behind the reduced response in the colon of animals with ileitis is unknown; however, the absence of inflammation and an intact epithelial barrier, as well as the altered submucosal neurophysiology in the distal colon, suggests that the enteric nervous system may be involved.
Conclusions
We show that inflammation in one region of the gut has marked effects on cell types at various levels of the enteric neural reflex circuitry located at a distant, non‐inflamed site. The mucosal sensory transducers, the EC cells, are more numerous and release more serotonin, enteric neurones are more excitable and the pharmacology of synaptic transmission is altered, and the electrical properties of the secretory epithelium are changed. An increase in colonic PGE2 content, in a colon that appears normal, is a likely mediator of these changes. These data indicate that the effects of inflammation on function in distant uninvolved regions of the GI tract may be due to alterations in neural signalling at these distant sites. These findings help to explain the clinical observations in patients with IBD who have altered gut function in uninflamed parts of the GI tract.
Acknowledgements
We thank Cathy MacNaughton and Dr Niall Hyland for their assistance with these studies, and Dr Randy Blakely for providing the SERT antiserum.
Abbreviations
AHP - afterhyperpolarising potential
AP - action potential
APD50 - action potential duration at half maximal amplitude
EC - enterochromaffin cell
ENS - enteric nervous system
EPSPs - excitatory postsynaptic potentials
GI - gastrointestinal
GLP‐2 - glucagon‐like peptide‐2
5‐HT - 5‐hydroxytryptaminel
IH - hyperpolarisation‐activated non‐selective cation conductance
IBD - inflammatory bowel disease
Isc - short circuit current
LT - low‐threshold
MPO - myeloperoxidase
PD - potential difference
PGE2 - prostaglandin E2
RMP - resting membrane potential
TNBS - 2,4,6‐trinitrobenzene sulphonic acid
TTX - tetrodotoxin
Footnotes
Grant support: This work was supported by an operating grant from the Crohn's and Colitis Foundation of Canada (CCFC) (KS and GM), and by NIH grant DK62267 (GM). KS is an Alberta Heritage Foundation for Medical Research (AHFMR) Medical Scientist and the CCFC Chair in Inflammatory Bowel Disease Research. AL is the recipient of a Canadian Association of Gastroenterology/Astra Zeneca/Canadian Institutes of Health Research postdoctoral fellowship. JO'H is an AHFMR graduate scholar.
Competing interests: None.
References
- 1.Linden D R, Chen J X, Gershon M D.et al Serotonin availability is increased in mucosa of guinea pigs with TNBS‐induced colitis. Am J Physiol Gastrointest Liver Physiol 2003285G207–G216. [DOI] [PubMed] [Google Scholar]
- 2.Coates M D, Mahoney C R, Linden D R.et al Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 20041261657–1664. [DOI] [PubMed] [Google Scholar]
- 3.O'Hara J R, Ho W, Linden D R.et al Enteroendocrine cells and 5‐HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol 2004287G998–1007. [DOI] [PubMed] [Google Scholar]
- 4.Linden D R, Sharkey K A, Mawe G M. Enhanced excitability of myenteric AH neurones in the inflamed guinea‐pig distal colon. J Physiol 2003547589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lomax A E, Mawe G M, Sharkey K A. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea‐pig. J Physiol 2005564863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell C J, Gall D G, Wallace J L. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol Gastrointest Liver Physiol 1995268G622–G630. [DOI] [PubMed] [Google Scholar]
- 7.Collins S M. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 19961111683–1699. [DOI] [PubMed] [Google Scholar]
- 8.Mawe G M, Collins S M, Shea‐Donohue T. Changes in enteric neural circuitry and smooth muscle in the inflamed and infected gut. Neurogastroenterol Motil 200416(Suppl 1)133–136. [DOI] [PubMed] [Google Scholar]
- 9.Lomax A E, Fernandez E, Sharkey K A. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil 2005174–15. [DOI] [PubMed] [Google Scholar]
- 10.Rao S S, Read N W, Brown C.et al Studies on the mechanism of bowel disturbance in ulcerative colitis. Gastroenterology 198793934–940. [DOI] [PubMed] [Google Scholar]
- 11.Manousos O N, Salem S N. Abnormal motility of the small intestine in ulcerative colitis. Gastroenterologia 1965104249–257. [DOI] [PubMed] [Google Scholar]
- 12.Neunlist M, Aubert P, Toquet C.et al Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut 20035284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider J, Jehle E C, Starlinger M J.et al Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non‐inflamed rectum of patients with Crohn's disease. Neurogastroenterol Motil 200113255–264. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson K, McHugh K, Collins S M. Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology 1995109718–722. [DOI] [PubMed] [Google Scholar]
- 15.Aube A C, Cherbut C, Barbier M.et al Altered myoelectrical activity in noninflamed ileum of rats with colitis induced by trinitrobenzene sulphonic acid. Neurogastroenterol Motil 19991155–62. [DOI] [PubMed] [Google Scholar]
- 16.Blandizzi C, Fornai M, Colucci R.et al Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by alpha2‐adrenoceptors in the presence of experimental colitis. Br J Pharmacol 2003139309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreels T G, De Man J G, De Winter B Y.et al Effect of 2,4,6‐trinitrobenzenesulphonic acid (TNBS)‐induced ileitis on the motor function of non‐inflamed rat gastric fundus. Neurogastroenterol Motil 200113339–352. [DOI] [PubMed] [Google Scholar]
- 18.McCafferty D M, Wallace J L, Sharkey K A. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am J Physiol Gastrointest Liver Physiol 1997272G272–G280. [DOI] [PubMed] [Google Scholar]
- 19.Lomax A E, Bertrand P P, Furness J B. Electrophysiological characteristics distinguish three classes of neuron in submucosal ganglia of the guinea‐pig distal colon. Neuroscience 2001103245–255. [DOI] [PubMed] [Google Scholar]
- 20.Linden D R, Sharkey K A, Ho W.et al Cyclooxygenase‐2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol 2004557191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Z M, Brookes S J, Ramsay G A.et al Characterization of myenteric interneurons with somatostatin immunoreactivity in the guinea‐pig small intestine. Neuroscience 199780907–923. [DOI] [PubMed] [Google Scholar]
- 22.Spencer N J, Smith T K. Mechanosensory S‐neurons rather than AH‐neurons appear to generate a rhythmic motor pattern in guinea‐pig distal colon. J Physiol 2004558577–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raybould H E, Cooke H J, Christofi F L. Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil 200416(Suppl 1)60–63. [DOI] [PubMed] [Google Scholar]
- 24.Cooke H J, Xue J, Yu J G.et al Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol 20044691–15. [DOI] [PubMed] [Google Scholar]
- 25.Malykhina A P, Qin C, Foreman R D.et al Colonic inflammation increases Na+ currents in bladder sensory neurons. Neuroreport 2004152601–2605. [DOI] [PubMed] [Google Scholar]
- 26.Kang D, Kim D. TREK‐2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol 2006291C138–C146. [DOI] [PubMed] [Google Scholar]
- 27.Manning B P, Sharkey K A, Mawe G M. Effects of PGE2 in guinea pig colonic myenteric ganglia. Am J Physiol Gastrointest Liver Physiol 2002283G1388–G1397. [DOI] [PubMed] [Google Scholar]
- 28.Dekkers J A, Kroese A B, Keenan C M.et al Prostaglandin E2 activation of VIP secretomotor neurons in the guinea pig ileum. J Auton Nerv Syst 199766131–137. [DOI] [PubMed] [Google Scholar]
- 29.Peles S, Miranda A, Shaker R.et al Acute nociceptive somatic stimulus sensitizes neurones in the spinal cord to colonic distension in the rat. J Physiol 2004560291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gershon M D. Review article: roles played by 5‐hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 199913(Suppl 2)15–30. [PubMed] [Google Scholar]
- 31.Bulbring E, Crema A. Observations concerning the action of 5‐hydroxytryptamine on the peristaltic reflex. Br J Pharmacol Chemother 195813444–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertrand P P, Kunze W A, Furness J B.et al The terminals of myenteric intrinsic primary afferent neurons of the guinea‐pig ileum are excited by 5‐hydroxytryptamine acting at 5‐hydroxytryptamine‐3 receptors. Neuroscience 2000101459–469. [DOI] [PubMed] [Google Scholar]
- 33.Linden D R, Foley K F, McQuoid C.et al Serotonin transporter function and expression are reduced in mice with TNBS‐induced colitis. Neurogastroenterol Motil 200517565–574. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Q, Boushey R P, Cino M.et al Circulating levels of glucagon‐like peptide‐2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol 2000278R1057–R1063. [DOI] [PubMed] [Google Scholar]
- 35.Drucker D J. Glucagon‐like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 200317161–171. [DOI] [PubMed] [Google Scholar]
- 36.Drucker D J, Yusta B, Boushey R P.et al Human [Gly2]GLP‐2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol Gastrointest Liver Physiol 1999276G79–G91. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez de Medina F, Perez R, Martinez‐Augustin O.et al Disturbances of colonic ion secretion in inflammation: role of the enteric nervous system and cAMP. Pflugers Arch 2002444378–388. [DOI] [PubMed] [Google Scholar]
- 38.MacNaughton W K, Lowe S S, Cushing K. Role of nitric oxide in inflammation‐induced suppression of secretion in a mouse model of acute colitis. Am J Physiol Gastrointest Liver Physiol 1998275G1353–G1360. [DOI] [PubMed] [Google Scholar]
- 39.Miceli P, Morris G P, MacNaughton W K.et al Alterations in capsaicin‐evoked electrolyte transport during the evolution of guinea pig TNBS ileitis. Am J Physiol Gastrointest Liver Physiol 2002282G972–G980. [DOI] [PubMed] [Google Scholar]
- 40.Perez‐Navarro R, Martinez‐Augustin O, Ballester I.et al Experimental inflammation of the rat distal colon inhibits ion secretion in the proximal colon by affecting the enteric nervous system. Naunyn Schmiedebergs Arch Pharmacol 2005371114–121. [DOI] [PubMed] [Google Scholar]
- 41.Kuwahara A, Bowen S, Wang J.et al Epithelial responses evoked by stimulation of submucosal neurons in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 1987252G667–G674. [DOI] [PubMed] [Google Scholar]
- 42.Goldhill J M, Burakoff R, Donovan V.et al Defective modulation of colonic secretomotor neurons in a rabbit model of colitis. Am J Physiol Gastrointest Liver Physiol 1993264G671–G677. [DOI] [PubMed] [Google Scholar]
- 43.Frieling T, Rupprecht C, Dobreva G.et al Differential effects of inflammatory mediators on ion secretion in the guinea‐pig colon. Comp Biochem Physiol A Physiol 1997118341–343. [DOI] [PubMed] [Google Scholar]
- 44.Mawe G M, Gershon M D. Functional heterogeneity in the myenteric plexus: demonstration using cytochrome oxidase as a verified cytochemical probe of the activity of individual enteric neurons. J Comp Neurol 1986249381–391. [DOI] [PubMed] [Google Scholar]
- 45.Sheldon R J, Malarchik M E, Burks T F.et al Effects of nerve stimulation on ion transport in mouse jejunum: responses to Veratrum alkaloids. J Pharmacol Exp Ther 1990252636–642. [PubMed] [Google Scholar]
- 46.Hyland N P, Cox H M. The regulation of veratridine‐stimulated electrogenic ion transport in mouse colon by neuropeptide Y (NPY), Y(1) and Y(2) receptors. Br J Pharmacol 2005146712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]