Abstract
Background and aims
The rates of fibrosis progression in chronic hepatitis C are significantly different between males and females. The antifibrogenic effect of oestrogen has been proposed, possibly via inhibition of stellate cells. The aim of this study was to evaluate the severity of chronic hepatitis C in women, in relation to the menopause, steatosis and hormone replacement therapy (HRT).
Methods
From November 2003 to October 2004, women with chronic hepatitis C were enrolled prospectively. A questionnaire was completed prospectively and a blood sample was obtained on the day of biopsy. We identified characteristics associated with moderate/severe fibrosis using univariate and multivariate analysis.
Results
251 women were included in the study. 122 women (52%) were menopausal and 65 were receiving HRT. 61 (24%) women with moderate/severe fibrosis (F2–F4, Metavir score) had a longer known duration of infection (>15 years), a higher body mass index and presented with steatosis more frequently than 190 (76%) women with mild fibrosis (F0–F1). Women with F2–F4 were more often menopausal (67% v 47%). The probability of fibrosis F2–F4 was lower for menopausal women receiving HRT (p = 0.012). Steatosis was more frequent and more severe in menopausal women.
Conclusions
Severity of fibrosis was associated with a longer duration of infection (>15 years), a higher body mass index, advanced steatosis and the menopause. Menopausal women receiving HRT presented with a lower stage fibrosis. These results reinforce the hypothesis of a protective role of oestrogens in the progression of fibrosis. Steatosis may be implicated in the progression of fibrosis after the menopause.
Chronic hepatitis C virus (HCV) infection is a major cause of liver diseases and death throughout the world. Most individuals with HCV infection will develop chronic disease. Chronic hepatitis C is usually indolent and asymptomatic. However, some patients can present with a rapidly progressive disease.1,2 Mortality results mainly from the development of liver fibrosis and the subsequent architectural distortion of the liver, occurrence of cirrhosis, with complications such as hepatocellular carcinoma.
The rate of fibrosis progression varies markedly from person to person. Median time from infection to cirrhosis is 30 years but there is important variability between individuals.1,2,3,4 Progression of hepatic fibrosis does not occur in a linear manner and several factors may exert an influence, such as host factors (sex, age, duration of infection and consumption of alcohol).1,2,3,4 Currently, metabolic disorders such as being overweight and diabetes, as well as steatosis, are emerging as cofactors of fibrogenesis.5,6
Clinical observations show that cirrhosis is largely a disease of men and postmenopausal women, with the exception of the classic autoimmune diseases and primary biliary cirrhosis. The severity of fibrosis in chronic hepatitis C is significantly different between males and females.1,2,3 Male sex is an independent risk factor for the severity of liver fibrosis. In females, acceleration seems to occur at approximately 60 years.3
An antifibrogenic effect of oestrogen has been suggested, possibly via inhibition of stellate cells. Studies have shown that oestrogen blocks proliferation and fibrogenesis by these cells.7 Experimental models demonstrated that oestradiol reduced CCL4 induced hepatic fibrosis in rats.8 The antifibrogenic role of oestrogen in the liver may be one reason for the sex associated differences in the progression from hepatic fibrosis to cirrhosis.
The prevalence of the metabolic syndrome increases with the menopause and may partially explain the apparent acceleration in cardiovascular disease after the menopause.9 The transition from pre‐ to postmenopause is associated with the emergence of many features of the metabolic syndrome, including increased intraabdominal body fat, a shift towards a more atherogenic lipid profile and increased glucose and insulin levels. Steatosis is one feature of the metabolic syndrome.
The influence of the menopause and the effect of hormone replacement therapy (HRT) in the evolution of the hepatitis C, particularly on hepatic fibrogenesis, are not well known. Commonly, in women with chronic liver diseases, physicians have a tendency to contraindicate hormonal therapy because of concerns about hepatotoxicity. The aim of this study was to evaluate the severity of chronic hepatitis C in women in relation to steatosis, the menopause and HRT.
Material and methods
A total of 251 women with chronic hepatitis C, seen at the Hepatology Unit, Hospital Beaujon‐Clichy, France, were enrolled prospectively, from November 2003 to October 2004. We included women over 18 years of age with a diagnosis of chronic hepatitis C, defined by the presence of anti‐HCV (ELISA III generation) and detection of HCV‐RNA by polymerase chain reaction (Amplicor; Roche, Nevilly sur Seine, France). All patients had adequate liver biopsy. Patients with markers of human immunodeficiency virus infection, hepatitis B surface antigen, evidence of autoimmune processes, alcohol intake greater than 80 g/day or previous antiviral therapy (interferon alpha with or without ribavirin) were excluded.
A confidential questionnaire was completed by patients on the day of the liver biopsy and the following information was collected: source of infection, estimated duration of infection (the date of the beginning of infection was specified in the case of blood transfusion or intravenous drug use), alcohol consumption (yes or no and amount of alcohol intake), weight, height, body mass index (BMI, calculated as weight (kg) divided by height (m)2). Definitions for overweight (BMI >25 kg/m2 ) and obesity (BMI >30 kg/m2) were used. Furthermore, we recorded the number and dates of gestations, menopause (dichotomous: yes or no), date of the menopause and use of HRT (dichotomous: yes or no; type of treatment, progesterone and/or oestrogens; period of therapy). A fasting blood sample was obtained on the day of liver biopsy for analysis of alanine aminotransferase, aspartate aminotransferase, γ glutamyl transferase, bilirubin, alkaline phosphatase, serum glucose, cholesterol, triglycerides, ferritin and transferrin saturation.
All liver biopsies were read by the same pathologist (DCH) without knowledge of the clinical data. Liver sections were stained with haematoxylin–eosin, trichromus and Perl's. The present analysis focused on the stage of fibrosis, grade of necroinflammation and grade of steatosis. Lesions were evaluated according to the METAVIR scoring system. Necroinflammatory activity was graded on a scale from 0 to 3 (A0 no histological activity; A1 mild activity; A2 moderate activity; and A3 severe activity) and fibrosis was staged on a scale from 0 to 4 (F0 no fibrosis; F1 portal fibrosis without septa; F2 few septa; F3 numerous septa without cirrhosis; and F4 cirrhosis). Steatosis was also evaluated according to the percentage of hepatocytes containing cytoplasmic vacuoles. Steatosis was graded as absent, mild (<10% of hepatocytes), moderate (10–30% of hepatocytes) or marked (⩾30% of hepatocytes).
Statistical analysis
Descriptive analyses were made for the whole sample. Categorical variables were described with frequencies and percentages. Continuous variables were expressed as mean (SD). Percentages correspond to the known data for each category of fibrosis.
The factors analysed were: age (⩽ or >50 years old), duration of infection (⩽ or >15 years), alcohol consumption (< or ⩾40 g/day), body mass index (BMI ⩽ or >25 kg/m2), liver steatosis (absence, <10%, 10–30%, >30%), genotypes (1, 2, 3, 4 and other), menopause (yes or no) and HRT (yes or no).
We identified predictive factors of moderate or severe liver fibrosis (F2, F3 or F4 according to the METAVIR score) using both univariate (Pearson's χ2 test) and multivariate (logistic regression with stepwise procedure) analysis to assess the specific effect of each predictor. Factors included in the multivariate model were those with a significance level p<0.20 in the univariate analysis. Values of p smaller than 5% (p<0.05) were considered significant.
Results
Study population
Between November 2003 and October 2004, 251 women were enrolled in this prospective study. Characteristics are shown in table 1.
Table 1 Characteristics of 251 women with chronic hepatitis C.
| Chronic hepatitis C patients (n = 251) | |
|---|---|
| Age (y)* | 46.7 (10.8) [18.6–72.7] |
| Source of infection† | |
| Blood transfusion | 84 (33.5%) |
| Intravenous drug use | 45 (17.9%) |
| Unknown | 122 (48.6%) |
| Duration of infection (y)* | 24.2 (6.7) [8–43] |
| Body mass index (kg/m2)* | 23.5 (4.5) [16–46] |
| BMI >25 | 58 (29.0%) |
| BMI >30 | 16 (8.0%) |
| Alcohol consumption (g/day)* | 6.5 (16.5) [0–100] |
| 0 | 173 (75.9%) |
| >0 and ⩽20 | 33 (14.5%) |
| >20 and ⩽40 | 11 (4.8%) |
| >40 | 11 (4.8%) |
| HCV genotype† | |
| 1 | 118 (57.3%) |
| 2 | 22 (10.7%) |
| 3 | 42 (20.4%) |
| 4 | 16 (7.8%) |
| Other | 8 (3.9%) |
| Number of pregnancies | |
| None | 50 (24.8%) |
| 1 or 2 | 114 (56.4%) |
| More than 2 | 38 (18.8%) |
| AST (IU/l)* | 59.1 (48.0) [16–333] |
| ALT (IU/l)* | 78.2 (66.4) [12–467] |
| Menopause | 122 (52.1%) |
| Hormone replacement therapy | 65 (31.7%) |
| Liver steatosis (histology) | |
| Presence (v absence) | 104 (42.6%) |
| Liver fibrosis (histology) | |
| F0–F1 | 190 (75.7%) |
| F2–F4 | 61 (24.3%) |
*Results are expressed as mean (SD) [range] or †n (%).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HCV, hepatitis C virus.
In the whole population, mean age was 46.7 (10.8) years (range 18.6–72.7). Transfusion of blood products was identified in 84 (33.5%) patients and intravenous drug use in 45 (17.9%). For patients with a known date of contamination, estimated duration of the infection was 24.2 (6.7) years. Only 24.1% of women consumed alcohol, most in small amounts. Alcohol consumption was as follows: 75.9% of women had no alcohol consumption (0 g/day), 14.5% consumed <20 g/day, 4.8% consumed 20–40 g/day and 4.8% had excessive alcohol consumption (>40 g/day). Among the 251 women with chronic hepatitis C, 71% had a normal BMI (⩽25 kg/m2) and 29% had a BMI >25 kg/m2, of whom 8% had a BMI >30 kg/m2. Mean BMI was 23.5 (4.5) kg/m2.
The distribution of genotypes was as follows: 118 (57.3%) genotype 1; 22 (10.7%) genotype 2; 42 (20.4%) genotype 3; 16 (7.8%) genotype 4; and 8 (3.9%) other genotypes.
Mean ferritin level was 143.2 (173.2) IU/l (normal value <300 IU/l), and mean transferrin saturation was 31.5 (13.6)% (normal value <40%). There was liver iron overload in 7.6% of cases.
A total of 122 women (52.1%) were menopausal for a mean period of 4.05 (6.47) years. In this cohort, mean age of the menopausal women was 55.4 (7.4) years (range 35.6–72.7). Sixty five women (31.7%) received or were receiving HRT for a mean period of 2.3 (3.3) years. HRT consisted of a combination of derivates of progesterone (tablets) and oestrogen (tablets or transdermic patches).
A total of 139 women (55.4%) had undergone liver biopsy before the age of 50 years compared with 112 (44.6%) after the age of 50 years.
Characteristics associated with moderate/severe fibrosis (F2–F4)
In our population, 190 women (75.7%) had mild fibrosis (F0–F1) and 61 (24.3%) had moderate or severe fibrosis (F2–F4). Factors associated with fibrosis in the univariate analysis are indicated in table 2. In the univariate analysis, there was no significant association between the presence of moderate or severe fibrosis and the source of infection, HCV genotype, alcohol consumption, liver iron or number of pregnancies. Four characteristics were significantly associated with moderate or severe fibrosis: estimated duration of infection >15 years, BMI >25 kg/m2, presence of important steatosis (⩾30%) and the menopause. Among menopausal women, those receiving HRT had a lower stage of fibrosis.
Table 2 Factors associated with moderate or severe fibrosis (univariate analysis).
| Fibrosis F0–F1 (n = 190) (%) | Fibrosis F2–F4 (n = 61) (%) | p Value | |
|---|---|---|---|
| Duration of infection >15 y | 89 (89.0) | 28 (100.0) | 0.1201 |
| BMI >25 kg/m2 | 39 (25.7) | 19 (39.6) | 0.0638 |
| BMI >30 kg/m2 | 9 (5.9) | 7 (14.6) | 0.0679 |
| Alcohol consumption (g/day) | 0.7812 | ||
| 0 | 133 (76.4) | 40 (74.1) | |
| >0 and ⩽20 | 25 (14.4) | 8 (14.8) | |
| >20 and ⩽40 | 7 (4.0) | 4 (7.4) | |
| >40 | 9 (5.2) | 2 (0.9) | |
| HCV genotype | 0.6829 | ||
| 1 | 89 (59.3) | 29 (51.8) | |
| 2 | 15 (10.0) | 7 (12.5) | |
| 3 | 31 (20.7) | 11 (19.6) | |
| 4 | 10 (6.7) | 6 (10.7) | |
| Other | 5 (3.3) | 3 (5.4) | |
| Number of pregnancies | 0.88 | ||
| None | 39 (25.2) | 11 (23.4) | |
| 1 or 2 | 88 (56.8) | 26 (55.3) | |
| >2 | 28 (18.1) | 10 (21.3) | |
| Menopause | 82 (47.1) | 40 (66.7) | 0.009 |
| Hormone replacement therapy | 53 (34.2) | 12 (24.0) | 0.1780 |
| Liver steatosis (histology) | 0.0018 | ||
| Absence | 116 (63.0) | 24 (40.0) | |
| Mild (<10%) | 27 (14.6) | 12 (20.0) | |
| Moderate (10–30%) | 5 (2.7) | 4 (6.6) | |
| Marked (> = 30%) | 36 (19.5) | 20 (33.3) |
BMI, body mass index; HCV, hepatitis C virus.
Compared with patients with mild fibrosis, patients with moderate/severe fibrosis (F2–F4) had a longer estimated duration of infection (all had an estimated duration of infection >15 years), a higher BMI (39.6% were overweight and 14.6% were obese), and presented with liver steatosis more frequently and in a more serious form (33.3% with marked steatosis or steatosis ⩾30%). Patients with moderate or severe fibrosis were more often menopausal but were less frequently receiving HRT (table2).
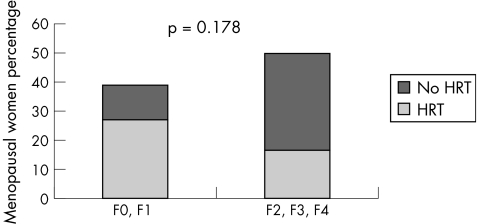
When we analysed 65 women who received HRT, we found a predominance of treated women in the group with mild fibrosis (p = 0.178; fig 1).
Figure 1 Percentage of menopausal women and proportion who were receiving hormone replacement therapy (HRT). Fibrosis was staged according to the METAVIR score.
In the multivariate analysis (table 3), moderate/severe fibrosis was associated with the menopause [odds ratio (OR) 3.73 (95% confidence interval (CI) 1.7–7.8); p = 0.0004]. The probability of high stage of fibrosis was lower in menopausal women receiving HRT (OR 0.35 (95% CI 0.1–0.8); p = 0.012).
Table 3 Factors associated with moderate or severe fibrosis (multivariate analysis; n = 205).
| Modality | OR (95% CI) | p Value | |
|---|---|---|---|
| Menopause | Yes | 3.73 (1.7–7.8) | 0.0004 |
| No | 1 | ||
| Hormone replacement therapy | Yes | 0.35 (0.1–0.8) | 0.0119 |
| No | 1 |
Steatosis and the menopause
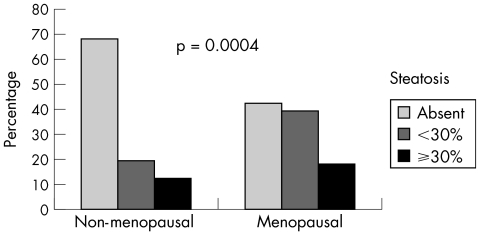
When we evaluated the presence of steatosis, we found a higher frequency and severity of fatty liver in menopausal women (fig 2). Steatosis was absent in 76/112 (67.9%) non‐menopausal women and in 52/122 (42.6%) menopausal women. In menopausal women, we observed mild or moderate steatosis in 48/122 (39.3%) and marked steatosis in 22/122 (18%). In non‐menopausal women, we observed mild or moderate steatosis in 22/112 (19.6%) and marked steatosis in 14/112 (12.5%). This difference was significant (p = 0.0004).
Figure 2 Percentage of steatosis among non‐menopausal and menopausal women.
Serum glucose, cholesterol, triglycerides and weight were not significantly different among menopausal and non menopausal women. BMI was higher among menopausal women although mean BMI was less than 25 kg/m2 in the two groups (table 4).
Table 4 Characteristics of the menopausal and non‐menopausal women.
| Non‐menopausal | Menopausal | p Value | |
|---|---|---|---|
| Age (y) | 37.9 (6.22) [18.6–51.1] | 55.4 (7.42) [35.6–72.6] | 0.0001 |
| Weight (kg) | 60.7 (12.72) | 64.7 (13.17) | 0.08 |
| Body mass index kg/m2 | 22.44 (3.97) | 24.66 (4.92) | 0.002 |
| Glycaemia (mmol/l) | 5.05 (1.02) | 5.54 (1.82) | 0.09 |
| Cholesterol (mmol/l) | 4.91 (1.15) | 5.19 (0.99) | 0.46 |
| Triglycerides (mmol/l) | 0.97 (0.60) | 1.05 (0.48) | 0.68 |
*Results are expressed as mean (SD) [range].
We evaluated steatosis according to age and found that 79.3% of women with no steatosis were aged <55 years and 42% of women with moderate or marked steatosis were aged >55 years (p = 0.0001).
Steatosis was more severe in women infected with genotype 3 than in women with genotype 1 (moderate or marked steatosis in 16/42 (38%) of genotype 3 women; 24/117 (20.5%) in genotype 1; p = 0.034.). There was no difference in genotype distribution in the menopausal and non‐menopausal groups. There was no difference in the frequency or severity of steatosis in relation to HRT (p = 0.275).
Discussion
This prospective study of 251 women with chronic hepatitis C evaluated the severity of liver fibrosis in relation to the menopause and HRT. We found that the menopause was an independent factor associated with higher stage of fibrosis (independent of age and duration of disease). In addition, we found that HRT was associated with lower fibrosis stage in menopausal women.
The progression of fibrosis in chronic hepatitis C determines the ultimate prognosis and thus the need and urgency of therapy. Fibrogenesis is a complex dynamic process which is mediated by necroinflammation and activation of stellate cells. Liver biopsy remains the gold standard to assess fibrosis. The rate at which fibrosis progresses varies markedly between patients. The major factors known to be associated with fibrosis progression are older age at infection, male sex and excessive alcohol consumption.1,2,3,4 Hepatic steatosis, obesity and diabetes may also contribute to more rapid progression of fibrosis.6 Liver biopsy provides the most accurate assessment of the stage of fibrosis and grade of necroinflammation, both of which have prognostic significance.
Ryder et al prospectively studied 214 untreated HCV infected patients with predominantly mild liver disease.10 One third of patients with predominantly mild hepatitis C showed significant fibrosis progression over a median period of 30 months. The overall rate of fibrosis progression in patients with hepatitis C was low but increased in patients who were older or had more marked fibrosis on their index biopsy.
In our study, higher BMI was associated with moderate/severe fibrosis in univariate analysis but this association was not significant in multivariate analysis. An explanation could be that in this patient population, the proportion of women with obesity was low which did not allow an accurate analysis of the relationship between obesity and fibrosis stage.
In multivariate analysis, we did not find a significant association between moderate/severe fibrosis and alcohol consumption. This may be explained by the fact that in this patient population, alcohol consumption was generally low (low proportion of patients with alcohol consumption, and small quantity) and therefore we could not accurately evaluate the relationship between alcohol and fibrosis.
Proportionally, a larger number of women with advanced fibrosis were identified among menopausal women. In addition, a large number of menopausal women were being treated with HRT in the mild fibrosis group. In the multivariate analysis, the probability of severe fibrosis was lower for menopausal women receiving HRT. These results reinforce the hypothesis of a probable antifibrogenic role of oestrogens.
Another interesting result was the higher frequency and greater severity of steatosis among menopausal women. We cannot exclude the fact that HRT may have been prescribed less frequently in women with advanced liver disease. However, most women in the group with advanced fibrosis (F2–F4) had F2 and F3 fibrosis, a minority had cirrhosis (F4) and, in terms of liver function, all women with advanced fibrosis (F4) had compensated liver disease. We did not find a significant difference in serum glucose, cholesterol or triglycerides between menopausal and non‐menopausal women. BMI was higher in menopausal women although mean BMI was <25 kg/m2 in the two groups. The prevalence of the metabolic syndrome increases with the menopause and may partially explain the apparent acceleration in cardiovascular disease after the menopause.9 The transition from pre‐ to postmenopause is associated with the emergence of many features of the metabolic syndrome, including increased intraabdominal body fat; a shift towards a more atherogenic lipid profile and increased glucose and insulin levels. Steatosis is associated with features of the metabolic syndrome.
In this cohort, severe steatosis was more frequent among menopausal women and those infected with HCV genotype 3. Thus we have confirmed the association previously reported between genotype 3 and steatosis.5,11,12 There is some controversy with regard to the influence of steatosis on the progression of fibrosis.5,11,12 Steatosis may be a cofactor for fibrosis in menopausal women.
To our knowledge, the influence of the menopause in other chronic liver diseases has not been studied. Rates of fibrosis progression have been assessed in the most frequent chronic liver diseases: hepatitis C, hepatitis B, delta hepatitis, alcoholic liver disease, primary biliary cirrhosis, autoimmune hepatitis and genetic haemochromatosis.13 There was an acceleration of fibrosis progression with aging. Rates of fibrosis progression were significantly different between men and women. In women, acceleration occurred at approximately 50 years in patients with alcoholic liver disease and hepatitis B virus and at approximately 60 years for genetic haemochromatosis, HCV and primary biliary cirrhosis. The only disease for which there was no acceleration of fibrosis with age was autoimmune hepatitis, suggesting different mechanisms influencing fibrosis progression.
The natural history of chronic hepatitis C infection during pregnancy has been studied. This was not the purpose of our study but some authors have demonstrated a reduction in the levels of transaminases and persistence of viraemia during pregnancy, suggesting a decrease in HCV associated liver cell injury during pregnancy in women with chronic hepatitis C.14 The antifibrogenic role of oestrogen in the liver may be one reason for the sex associated differences in the progression from hepatic fibrosis to cirrhosis. Chronic hepatitis C is not a consequence of direct destruction of hepatic cells by the virus. Hepatic injury is mediated by T cell activity and their cytokines production.15 The decrease in inflammatory activity during pregnancy, observed in two cases of auto‐immune hepatitis could be explained by the phenomenon of fetal–maternal immunotolerance, favoured by oestrogen and progestin production.16 Recently, a study found that the menopause was probably associated with accelerated liver fibrosis progression and that HRT could be of potentially benefit.17
In the USA, the median age of the menopause is 51 years (range 41–59) but ovarian production of oestrogen and progestin begins to decrease years before complete cessation of menses.18 There is evidence that the decline in ovarian function with the menopause is associated with increases in proinflammatory cytokines, such as interleukin (IL)‐1, IL‐6 and tumour necrosis factor α. Oestrogen at physiological concentrations has been shown to inhibit the spontaneous secretion of these proinflammatory cytokines.19 It seems that oestradiol plays an important role as an endogenous fibrosuppressant, accounting for sex associated differences in the progression from hepatitis to cirrhosis and its complications such as hepatocellular carcinoma. It is now evident that hepatic stellate cells, in the space of Disse, undergo transformation into myofibroblast‐like cells, responsible for collagen hypersecretion and fibrosis. Yasuda et al showed that oestradiol inhibits the myofibroblastic transformation of rat hepatic stellate cells and therefore it may have an antifibrosis effect.20 They showed that in rat hepatic stellate cells incubated in primary culture with oestradiol, cell number, type I collagen production and α‐smooth muscle actin expression were reduced. These were dose dependent effects and were reversible after withdrawal of oestradiol.
The acceleration of fibrosis in hepatitis C, viewed after the menopause, suggests participation of circulating levels of oestrogens, as well as hepatic oestrogen receptors, on the progression of this liver disease.19 Studies have shown that oestradiol is a potent endogenous antioxidant that reduces lipid peroxide levels in serum and liver and thus impairs activation of hepatic stellate cells. The beneficial effect of the oestrogen is mediated by hepatic receptors that are reduced in patients with cirrhosis, hepatocellular carcinoma and in postmenopausal women.19
Iron loading has been the subject of intense study in hepatic fibrosis. Hepatic iron concentrations have been found to be elevated in hepatitis C and correlate with necroinflammatory changes and increased stellate cell numbers.21 Iron loading has been suggested as a factor in fibrosis progression in hepatitis C.22 In our patient population, we did not observe iron overload. Our data showed no significant differences in serum ferritin levels between premenopausal and postmenopausal women. Hepatic iron load, assessed on a Perl's stained section, was not correlated with fibrosis stage.
Our results support experimental data and have important clinical implications for the management of postmenopausal women. These results suggest that HRT is safe and potentially beneficial with respect to liver fibrosis.
In conclusion, in our population of women with chronic hepatitis C, fibrosis progression was associated with known risk factors, particularly higher BMI and longer duration of infection. The menopause seems to have a negative role, accelerating fibrosis progression. Furthermore, a large number of menopausal women with HRT presented with mild fibrosis, suggesting a beneficial effect of HRT, particularly oestrogens. Currently, there is no evidence of deleterious hepatic effects associated with HRT among menopausal women with chronic hepatitis C. The benefits of HRT on liver fibrosis need to be confirmed in randomised trials with serial biopsies and balanced against other risks.
In women with mild chronic hepatitis C, therapy can be postponed until more effective/tolerable treatments are available. In untreated patients with mild disease, repeat liver biopsy is, at this time, the more reliable means of assessing the progression of fibrosis and is recommended every 5 years.1,2 As the menopause seems to accelerate fibrosis progression in women after the menopause, fibrosis evaluation may be recommended more frequently (every 3 years) and HRT should be discussed.
Steatosis may be implicated in the progression of fibrosis after the menopause. We need to improve our understanding of the underlying mechanisms and pathogenesis of the association between steatosis and chronic hepatitis C.
Acknowledgements
We thank Dominique Blanc for helpful assistance.
Abbreviations
BMI - body mass index
HCV - hepatitis C virus
HRT - hormone replacement therapy
Footnotes
Conflict of interest: None.
References
- 1.Seeff L B. Natural history of chronic hepatitis C. Hepatology 200236(Suppl 1)S35–S46. [DOI] [PubMed] [Google Scholar]
- 2.Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology 200236(Suppl 1)S47–S56. [DOI] [PubMed] [Google Scholar]
- 3.Massard J, Ratziu V, Thabut D.et al Natural history and predictors of disease severity in chronic hepatitis C. J Hepatol 200644(Suppl 1)S19–S24. [DOI] [PubMed] [Google Scholar]
- 4.Strader D B, Wright T, Thomas D L.et al American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology 2004391147–1171. [DOI] [PubMed] [Google Scholar]
- 5.Asselah T, Rubbia‐Brandt L, Marcellin P.et al Steatosis in chronic hepatitis C: why does it really matter? Gut 200655123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramesh S, Sanyal A J. Evaluation and management of non‐alcoholic steatohepatitis. J Hepatol 200542S2–12. [DOI] [PubMed] [Google Scholar]
- 7.Bissel D M. Sex and hepatic fibrosis. Hepatology 199929988–989. [DOI] [PubMed] [Google Scholar]
- 8.Xu J W, Gong J, Chang X M.et al Estrogen reduces CCL‐4 induced liver fibrosis in rats. World J Gastroenterol 20028883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr M C. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003882404–2411. [DOI] [PubMed] [Google Scholar]
- 10.Ryder S D, Irving W L, Jones D A.et al Trent Hepatitis C Study Group. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study, Gut 200453451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adinolfi L E, Gambardella M, Andreana A.et al Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 2001331358–1364. [DOI] [PubMed] [Google Scholar]
- 12.Asselah T, Boyer N, Guimont M C.et al Liver fibrosis is not associated with steatosis but with necroinflammation in French patients with chronic hepatitis C. Gut 2003521638–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poynard T, Mathurin P, Lai C L.et al A comparison of fibrosis progression in chronic liver diseases. J Hepatol 200338257–265. [DOI] [PubMed] [Google Scholar]
- 14.Gervais A, Bacq Y, Bernuau J.et al Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol 200032293–299. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari C, Urbani S, Penna A.et al Immunopathogenesis of hepatitis C virus infection. J Hepatol 19993131–38. [DOI] [PubMed] [Google Scholar]
- 16.Colle I, Hautekeete M. Remission of autoimmune hepatitis during pregnancy: a report of two cases. Liver 19991955–57. [DOI] [PubMed] [Google Scholar]
- 17.Di Martino V, Lebray P, Myers R P.et al Progression of liver fibrosis in women infected with hepatitis C: long‐term benefit of estrogen exposure. Hepatology 2004401426–1433. [DOI] [PubMed] [Google Scholar]
- 18.U S. Preventive Services Task Force. Postmenopausal hormone replacement therapy for primary prevention of chronic conditions: recommendations and rationale, Ann Intern Med 2002137834–839. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int 20032363–69. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda M, Shimizu I, Shiba M.et al Suppressive effects of estradiol on dimethylnitrosamine‐induced fibrosis of the liver in rats. Hepatology 199929719–727. [DOI] [PubMed] [Google Scholar]
- 21.Rigamonti C, Andorno S, Maduli E.et al Iron, hepatic stellate cells and fibrosis in chronic hepatitis C. Eur J Clin Invest 200232(Suppl 1)28–35. [DOI] [PubMed] [Google Scholar]
- 22.Beinker N K, Voigt M D, Arendse M.et al Threshold effect of liver iron content on hepatic inflammation and fibrosis in hepatitis B and C. J Hepatol 199625633–638. [DOI] [PubMed] [Google Scholar]