Abstract
Background
Even though inflammation is a traditional tool for the induction of hyperalgesia in many tissues, recent observations suggest that not all inflammatory processes produce this change. Tolerance to colorectal distension (CRD) is reduced in patients with acute ulcerative colitis but is increased in patients with chronic inflammatory bowel disease. This suggests that the nature of the inflammatory infiltrate influences visceral perception.
Aim
To test this hypothesis by assessing responses to CRD in mice with mild, acute or chronic colitis.
Methods
CRD responses were measured in mice with mild non‐specific colitis, and dextran sodium sulphate (DSS)‐induced acute and chronic colitis. Responses were compared with tissue infiltrate and damage, interleukin (IL)1β and myeloperoxidase (MPO) activity and substance P, β‐endorphin and μ opioid receptor (MOR) expression.
Results
Mild and acute colitis were associated with increased responsiveness to CRD. In contrast, CRD responses were not increased in mice with chronic colitis and this difference was not due to altered colonic wall compliance. MPO and IL1β levels were greater in acute than in chronic colitis. Larger increases in tissue substance P were seen in acute than in chronic DSS, whereas CD4 T cells, β‐endorphin and MOR expression were evident only in chronic colitis. An inverse correlation was seen between substance P and MOR in these tissues.
Conclusions
Acute colitis increased responsiveness to CRD and is accompanied by an acute inflammatory infiltrate and increased tissue substance P. Chronic DSS is accompanied by an increase in β‐endorphin and MOR expression, and CD4 T cells, but no change in compliance or CRD responses. We conclude that acute inflammation generates hyperalgesia, whereas chronic inflammation involves infiltration by lymphocytes accompanied by MOR and β‐endorphin up regulation, and this provides an antinociceptive input that restores normal visceral perception.
Traditionally, experimental models of visceral or somatic hyperalgesia have involved the use of a range of inflammatory stimuli, including zymosan,1,2 acetic acid3,4,5 and mustard oil.6,7 Invariably, studies are conducted shortly after the induction of inflammation, and are thus the assessments of the effect of acute inflammation on sensory pathways.
Studies on visceral pain responses in patients with inflammatory bowel disease (IBD) have generated conflicting results. Patients with active colitis exhibit a reduction in tolerance to balloon distension of the rectum whereas patients with chronic or quiescent disease exhibit normal or increased tolerance to distension.8,9,10,11 These differences have been attributed to changes in descending spinal modulation of sensory input from the periphery during chronic inflammation,11 although no experimental evidence has been generated to support this.
Recent studies from our laboratory have clearly showed the antinociceptive properties of T lymphocytes in the gut.12 Specifically, we showed that reconstitution with CD4 T cells in hyperalgesic severe combined immune deficient (SCID) mice normalised pain perception. The analgesic effect of T cells was accompanied by an increase in β‐endorphin expression in the myenteric plexus. We consider that adaptive immune cell reconstitution initiates long‐term neuroplastic changes in the enteric nervous system (ENS), which ultimately leads to peripherally derived antinociception. In contrast, increased acute inflammatory cell activity induces visceral hyperalgesia in mice and is associated with increased neural substance P expression.13 Taken together, these results imply that visceral perception is critically influenced by the dominant cellular constituent in the gut, and that immune cells may modulate pain perception through changes in sensory neuropeptides.
In this study, we extend this observation to determine whether the nature of the inflammatory response influences nociception in the acute and chronically inflamed colon. We postulate that observed variations in visceral perception seen in patients with acute and chronic IBD are reflections of differences in the inflammatory cell infiltrate in the tissue generating different nociceptive influences. To deal with this, we examined visceromotor responses to colorectal distension (CRD) in mice with mild non‐specific colitis (NSC), and acute and chronic colitis induced by dextran sodium sulphate (DSS). We compared pain thresholds with levels of tissue infiltrate and damage, inflammatory activity, pro‐nociceptive cytokines and sensory neuropeptides. We hypothesised that an increased presence of acute inflammatory cells would induce visceral hyperalgesia, while a predominance of adaptive immune cells would maintain normal sensitivity. In addition, we theorised hypersensitivity and normal sensitivity would be associated with up‐regulated substance P and β‐endorphin, respectively. Our results showed that hypersensitivity resulting from acute inflammatory cell infiltrate could be down regulated by CD4 T cells. Local neuropeptide expression was sensitive to changes in inflammatory cells, and thus hypersensitivity was normalised by a peripheral endogenous mechanism of antinociception involving immune cell modulation of sensory neurones. This implies that the immune cell environment in the gut is a critical determinant of how inflammation influences visceral pain perception.
Materials and methods
Animal housing and handling
Male Balb/c mice (6–8 weeks of age) were purchased from Harlan (Indianapolis, Indiana, USA). Mice were kept under specific pathogen‐free conditions at McMaster University Central Animal Care Facility. On arrival at our facility, mice were quarantined for 2 weeks before the start of experiments. Cages, bedding and food were autoclaved as per standard procedure in McMaster University Central Animal Care Facility. All experiments were approved by the McMaster University Animal Care Committee and the Canadian Council on Animal Care. All mice were only exposed to HEPA (high‐efficiency particulate air filter)‐filtered air and in preparation for experimental testing mice were handled inside a level B hood and kept in custom‐made sterile HEPA filtered cages during CRD recordings. Fistulas and electromyographic (EMG) cables were sterilised at the Central Sterilization Unit at McMaster University.
Overall design
CRD was performed on day 0, before any treatment, to obtain a baseline response. Mice were then segregated into four groups: control non‐inflamed mice, mice with mild NSC and mice administered DSS for acute and chronic durations. CRD was performed on completion of the first DSS cycle (day 5 of treatment). Three mice were killed at this time point to obtain tissue samples while the rest of the group completed three DSS cycles. CRD was performed in all mouse groups 60 days after the completion of the third DSS cycle. A long recovery time after DSS treatment was chosen to allow induction of chronic inflammation. Mice from the remaining groups (control, NSC and chronic colitis) were then killed on the same day (24 h after CRD on day 100) and colon tissue samples were obtained.
Control mice
Mice were handled under a “sterile protocol” during experimental testing, which involved sterilisation of Bollman restrainers, intrarectal balloons and instruments using CIDEX OPA and rinsing with sterile distilled water before use.
Induction of mild non‐specific inflammation (NSC)
Mice were handled under non‐sterile conditions during experimental testing which specifically included using Bollman restrainers, intrarectal balloons and instruments that were not sterilised before use; however, they were exposed only to HEPA filtered air.
Induction of acute and chronic inflammation
Mice were administered DSS (5%) with a molecular weight of 40 000 (ICN Biomedicals, Aurora, California, USA) in drinking water for 5 days to induce acute inflammation. For chronic inflammation, mice were treated with three cycles of DSS (5%) for 5 days/cycle and 15 days of normal drinking water in between each cycle.
Sham mice
Tissues were also taken from age‐matched Balb/c mice that were not used for CRD testing, but were kept under the same conditions as the mice that were tested. We used this group as an additional control for immunostaining comparisons to determine if balloon distension alone affected neuropeptide expression in the colon.
Colorectal distension
EMG electrodes were surgically implanted under sterile conditions in the anterior abdominal muscle wall of mice anaesthetised with ketamine (Ketalean, Bimeda‐MTC, Cambridge, Ontario, Canada, 90 mg/kg) and xylazine (Rompun, Bayer, Toronto, Ontario, Canada, 20 mg/kg) intraperitoneal and a chronic fistula was exteriorised. Mice were then allowed to recover for a period of at least 7 days.
Mice were briefly anaesthetised with enflurane (Enflurane USP, Abbott Laboratories, Saint‐Laurent, Quebec, Canada) and a custom‐made balloon catheter (20×10 mm) was gently inserted into the distal colon. A recording cable was connected to the chronic fistula and mice were placed into Bollman restrainers. After connecting the catheters and cables to the barostat and EMG acquisition system, respectively, the mice were allowed a 5‐min rest. CRD was then performed in a stepwise fashion. Each 10‐s distension was followed by a 5‐min resting period. Distension at 30 mm Hg was repeated three times. EMG activity of the abdominal muscle was continuously recorded using customised software (Acquire V.5.0, A. Bayatti). The area under the curve was calculated for 10 s before and after the beginning of each distension period using customised software (GrafView V.4.1, A. Bayatti). The median value for each distension at 30 mm Hg per mouse was calculated; this value is described as the visceromotor response.
Colitis assessment
The colon was removed and opened longitudinally, and macroscopic damage was assessed immediately. Tissue was obtained from the distal colon and fixed in 10% formalin and stained with H&E for subsequent histological examination. Macroscopic and histological scores were performed using a previously described scoring system for DSS colitis14 slightly modified to score separately for rectal and gross colonic bleeding.
Myeloperoxidase assay
Myeloperoxidase (MPO) activity was used as an index of polymorphonuclear cell infiltration as described previously.15 Colonic tissue was frozen in liquid nitrogen and stored at 70°C until assayed. MPO was expressed in units per milligram of tissue, where 1 unit corresponds to the activity required to degrade 1 mmol of hydrogen peroxide in 1 min at 24°C.
Cytokine measurements
Tissues for cytokine measurements were taken from the mid to distal region of the colon. Tissue samples were homogenised in a solution of 100 µM phenylmethanesulphonylfluoride and 10 mg/ml aprotinin using a Brinkman Polytron (Lucerne, Switzerland). Supernatants were collected and used for cytokine analysis. Concentrations of interleukin (IL)‐1β and were measured by enzyme‐linked immunosorbent assay technique using commercially available kits purchased from R&D Systems (Minneapolis, Minnesota, USA). Results were corrected for protein concentration that was measured by a DC Protein Assay kit (Bio‐Rad Laboratories, Hercules, California, USA).
CD4 T cell immunohistochemistry
Tissue for immunohistochemistry was taken from the distal colon. Frozen sections were incubated with a rat antimouse CD4 (L3t4) primary antibody (Fitzgerald, Massachusetts, USA) for 1 h at room temperature followed by a donkey antirat immunoglobulin G horseradish peroxidase‐conjugated secondary antibody (Fitzgerald) for 1 h at room temperature. 3,3′‐diaminobenzidine (Sigma‐Aldrich, Oakville, Ontario, Canada) was used for colour development and modified Mayers' haematoxylin was used to counterstain sections. Omission of the primary antibody was used as a control.
Neuropeptide immunohistochemistry
Tissue for immunohistochemistry was taken from the distal colon. Paraffin‐wax‐embedded sections were incubated with rabbit antimouse primary antibody for either β‐endorphin (Research Diagnostics, Flanders, New Jersey, USA), substance P or μ opioid receptor (MOR) (Chemicon International) for 18 h at 4°C after deparaffinisation, peroxidase blocking and protein blocking. Envision, horseradish peroxidase‐coupled antirabbit secondary reagent (DakoCytomation, Carpinteria, California, USA) was incubated with the sections for 30 min at room temperature. 3,3′‐diaminobenzidine (Sigma‐Aldrich) was used for colour development and modified Mayers' haematoxylin was used to counterstain sections. Controls used included omission of the primary antibody and antibody preabsorption with either β‐endorphin peptide (Sigma‐Aldrich) or μ (third extracellular loop) opioid receptor peptide (Chemicon International).
Quantification of immunostaining
Peptide staining was measured by immunostaining‐based semi‐quantification. Five positions of each cross‐section (using a 20× objective) were photographed by a digital camera (Olympus Q‐Color, Olympus America Inc., Center Valley, Philadelphia, USA), and the stained areas were measured by a blind observer using ImageJ (v1.37) software. Only the submucosal and myenteric plexus regions of the colon were quantified. Results are expressed as percentage of positive stained tissue of total area as five pictures per colon allowed the total tissue area to be measured. The results for CD4 staining are expressed as average cell number per field.
Data presentation and statistical analysis
Parametric data are presented as mean+standard deviations (SDs) and non‐parametric data as box plots (box median; 25%, 75% percentiles; whiskers 5th and 95th centiles. EMG responses to CRD are expressed as percentage increase in area under the curve from day 0 (day 0 = 100%) to 30 mm Hg. Parametric data were analysed using two‐sample t tests (two tailed) for unpaired data with p⩽0.05 considered to be significant. Mann–Whitney U test was used for the analysis of non‐parametric data with p⩽0.05 considered to be significant.
Results
Effect of inflammation on visceral sensitivity
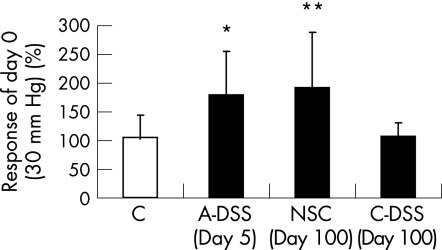
Visceral sensitivity to CRD at 30 mm Hg increased in mice with acute DSS treatment than in control mice (fig 1). A 70% increase in CRD response was observed on 5 days of exposure to DSS (one cycle) compared with day 0. In contrast, mice that were treated with three cycles of DSS did not show an increase in CRD response at 30 mm Hg 60 days after DSS treatment compared with day 0 and were not different from control mice (fig 1). Mice with mild NSC tested at the same time point, day 100, did show a 90% increase in CRD response to 30 mm Hg and was significantly increased compared with both the control and chronic DSS group.
Figure 1 Visceral sensitivity to colorectal distension to 30 mm Hg in control (C) mice (n = 5) and acute dextran sodium sulphate‐treated (A‐DSS) mice (n = 9) on day 5 of treatment and mice with mild non‐specific colitis (NSC, n = 8), and mice with chronic DSS administration (C‐DSS, n = 6) on day 100 (60 days after DSS treatment). Results are shown as percentage response of day 0 for each group.*p = 0.050 versus C. **p = 0.050 versus C and C‐DSS. Data are mean+SD.
Effect of inflammation on compliance
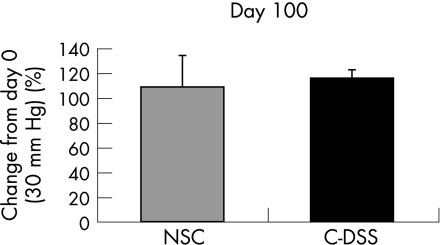
To determine if chronic inflammation induced by DSS had an effect on colonic compliance, next, we compared volumes in balloons during distension at 30 mm Hg on day 100 to volumes at the same pressure on day 0 and no difference was found in volumes due to chronic DSS (fig 2).
Figure 2 Volumes measured in balloons during colorectal distension to 30 mm Hg (n = 6–8/group). Results are shown as percentage change on day 100 from day 0. Data are mean+SD. C‐DSS, chronic dextran sodium sulphate; NSC, non‐specific colitis.
Assessment of inflammation
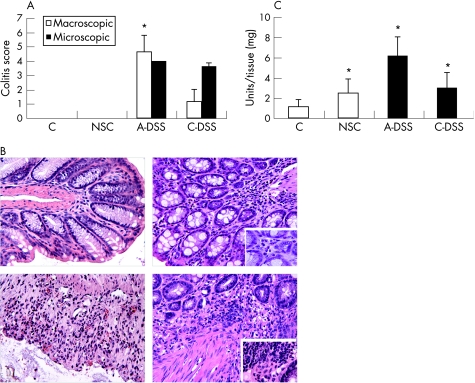
Macroscopic assessment of colitis showed a higher degree of damage in mice treated with acute DSS than mice with post‐chronic DSS treatment (fig 3A). Rectal and colon bleeding, as well as diarrhoea, were severe in the acute DSS group, while only mild diarrhoea and colon bleeding were noted in post‐chronic DSS. Microscopic assessment, however, was similar (fig 3A) as both groups showed signs of architectural damage, erosions and immune cell infiltrate. No signs of colitis were seen in mice that were not treated with DSS; however, microscopic examination of mice handled under non‐sterile conditions did show considerable infiltrate of polymorphonuclear cells, but not in mice handled under sterile conditions (fig 3B). Activity of these cells, as measured by the MPO assay, was significantly increased in all groups with colitis compared with control (fig 3C). While the highest activity was seen in the acute DSS group, a lower, but similar level of MPO was observed in the post‐chronic DSS group and mice with NSC.
Figure 3 (A) Assessment of colitis using macroscopic and microscopic parameters. Acute dextran sodium sulphate (A‐DSS; n = 3), chronic DSS (C‐DSS; n = 8); *p = 0.042 compared with C‐DSS. (B) H&E stain of colon sections (×20, insets ×63) illustrate increased polymorphonuclear (PMN) cell infiltrate in non‐specific colitis (NSC) and A‐DSS, and increased PMN cell and lymphocyte infiltrate in C‐DSS. (C) MPO activity in colon. *p = 0.05 (NSC; n = 7), *p = 0.049 (A‐DSS; n = 3), *p = 0.016 (C‐DSS; n = 8) compared with control (C; n = 4). Data are mean+SD.
IL1β measurement in colonic tissue
The acute pro‐inflammatory and pro‐nociceptive cytokine,16,17 IL1β, was significantly increased in all colitis groups compared with control. However, the mean IL1β concentration in the acute DSS group was more remarkable as it was approximately 35 times higher than control (mean (SD) 70.67 (6.29) vs 2.7(0.36); p = 0.003), whereas IL1β was only 2–3 times higher in the chronic DSS group (6.59 (2.37); p = 0.006) and mice with mild colitis (4.44 (1.55); p = 0.026) compared with control.
CD4 T cell infiltrate analysis in colon
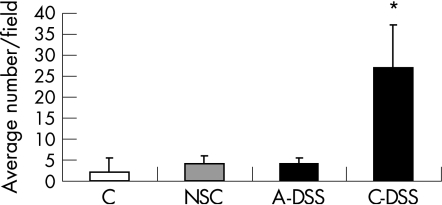
CD4 T cells counted in the colon showed an increase in CD4 immunostaining only in the chronic DSS group (fig 4). CD4 T cells were seen in lymphoid follicles and aggregates in all groups; however, only those cells that were seen infiltrating the mucosal and submucosal tissue were counted. CD4 T cells were not observed in the deeper muscular layer or in the region of the myenteric plexus.
Figure 4 CD4 T cell infiltrate in colon. (n = 3–8/group); *p<0.001. Results are expressed as average number of cells per field counted for each sample. Data are mean+SD. A‐DSS, acute dextran sodium sulphate; C, control; C‐DSS, chronic dextran sodium sulphate; NSC, non‐specific colitis.
Effect of inflammation on sensory neuropeptides
β‐endorphin and substance P immunostaining in neurones was observed in all groups. Some immune cells positive for β‐endorphin were seen in colon samples from mice with mild colitis and more so in the chronic DSS group, while cellular substance P immunostaining was seen in all inflammatory groups, most notably in the acute DSS group. As our focus was on the neural expression of these neuropeptides, only positive staining in the submucosal plexus and myenteric plexus, in addition to fibres in the circular muscle, were measured. β‐endorphin was more prominent in the myenteric plexus and was significantly increased in the chronic DSS group than in sham and mice with NSC and acute colitis (table 1). Expression in control mice was variable and thus did not reach significance. Substance P immunostaining was more evenly distributed by the submucosal and myenteric plexus, and although it was significantly increased in all colitis groups compared with sham, immunostaining in chronic colitis was significantly lower than that in acute colitis and mild colitis, and was not different from control (table 1). Thus, the ratio of β‐endorphin to substance P expression is significantly increased in mice with chronic DSS treatment than in sham (1.32 (0.32) vs 0.69 (0.13), p<0.05).
Table 1 Semi‐quantitative analysis of immunolabelling of β‐endorphin, substance P and μ opioid receptor in the submucosal and myenteric plexus.
| Sham | C | NSC | A‐DSS | C‐DSS | |
|---|---|---|---|---|---|
| β‐endorphin | 0.23 (0.18–0.47) | 0.66 (0.27–0.88) | 0.41 (0.31–0.47) | 0.18 (0.07–0.21) | 0.57* (0.48–0.82) |
| SP | 0.19 (0.17–0.19) | 0.35 (0.18–0.35) | 0.45**,† (0.34–0.72) | 0.49***,† (0.41–0.52) | 0.25*** (0.22–0.5) |
| MOR | 0.51 (0.29–0.8) | 0.3 (0.18–0.53) | 0.43 (0.3–0.54) | 0.39 (0.31–0.39) | 1.19‡ (1.00–1.44) |
A‐DSS, acute dextran sodium sulphate (DSS) treated; C, control; MOR, μ opioid receptor; NSC, non‐specific colitis; SP, substance P;
Results are expressed as percentage positive stained tissue of total area. Data are median (interquartile range).
*p<0.05 versus sham, NSC and A‐DSS (β‐endorphin); **p<0.05 versus sham and C; ***p<0.05 versus sham (SP).
†p<0.05 versus C‐DSS.
‡p<0.05 versus sham, C, NSC and A‐DSS (MOR).
MOR immunostaining was positive in all groups and was observed in neurones and on immune cells. Again, we focused on neural expression of the receptor and its expression was mostly visible in the myenteric plexus and on fibres in the circular muscle; this was significantly increased compared with all other groups only in the chronic DSS mice (table 1).
Sensory neuropeptide expression relative to MOR expression
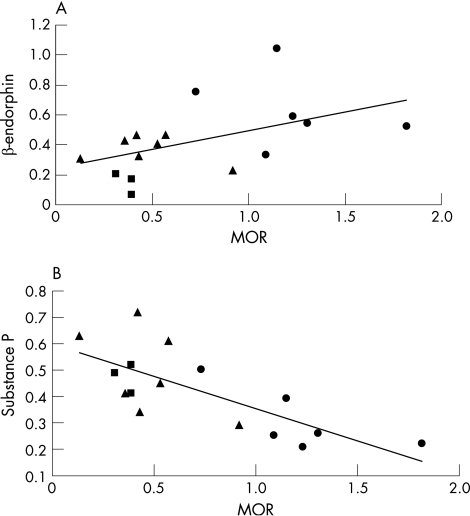
As the ratio of β‐endorphin to substance P expression was related to visceral sensitivity, we examined if MOR expression was linked to these neuropeptides in mice with mild, acute and chronic colitis. β‐endorphin and MOR were positively associated as β‐endorphin values increased with increasing MOR expression (fig 5A). In contrast, substance P values decreased with increasing MOR expression, and therefore were negatively associated (fig 5B). Both correlations were associated with visceral sensitivity as mice exhibiting hypersensitivity (mild and acute colitis) displayed high substance P and low β‐endorphin and MOR expression, while mice exhibiting normal sensitivity (chronic colitis) displayed low substance P and high β‐endorphin and MOR expression.
Figure 5 (A) Correlation of β‐endorphin expression versus μ opioid receptor (MOR) expression in myenteric plexus, Pearson's correlation = (0.5); p = 0.05. (B) Correlation of substance P expression versus MOR expression in the myenteric plexus, Pearson's correlation = (−0.8); p<0.001. Triangles, non‐specific colitis; squares, acute dextran sodium sulphate (DSS) treated; circles, control DSS.
Discussion
The purpose of this study was to investigate the effect of mild, acute and chronic inflammation on visceral pain. Animal work often describes increased sensitivity associated with acute and chronic inflammation, whereas clinical data fail to consistently show increased sensitivity in patients with IBD. Thus, our aim was to determine if visceral sensitivity could be differentially modulated by varying degrees of intestinal inflammation, and link changes in CRD responsiveness to the dominant immune cell type present in the gut. Our results show that mild non‐specific and acute colitis induced visceral hyperalgesia, whereas chronic colitis did not affect visceral sensitivity. Each state of inflammation was associated with diverse intestinal environments determined by the dominant cell infiltrates, and we believe these cellular variations are accountable for the differences in visceral sensitivity.
We showed acute DSS administration resulted in increased response to CRD; this was associated with severe signs of colitis, as well as a significant increase in acute inflammatory cell activity and IL1β. Hypersensitivity seen in this group is consistent with other models of acute inflammation. In contrast, a recent study by Larsson et al18 failed to find any effect of acute DSS treatment on visceral sensitivity. However, they measured pain responses approximately 1 week after treatment and the sample size (n = 2) was too low, in our experience, to detect any differences using the CRD method; inflammatory markers were not increased during the later time points tested. Thus, we believe that in our study, hypersensitivity was due to the increased inflammatory cell activity still present during CRD testing.
Interestingly, visceral hyperalgesia was comparable between mice with mild inflammation and with acute inflammation. We have previously shown that mice, handled in our laboratory under a protocol that is not sterile, develop spontaneous increases in sensitivity over time due to subtle increases in inflammatory cell activity.13 Therefore, here we confirmed this finding by showing that even in the absence of histological changes, mild increases in inflammatory cell infiltrate, MPO activity and IL1β production in comparison with changes seen in acute colitis, were enough to induce a notable increase in CRD responsiveness. Thus, subclinical inflammation reminiscent of that seen in subsets of patients with irritable bowel syndrome19,20,21 is associated with visceral hyperalgesia.
Acute inflammatory changes seen with mild colitis were equivalent to those observed in chronic colitis, including acute cell infiltrate, increased MPO and IL1β; yet, chronic inflammation did not induce hyperalgesia. The key finding here was the marked infiltration of CD4 T cells. Approximately six times more T cells were counted in mice with chronic colitis than in mice with mild and acute colitis. We have previously described analgesic properties of CD4 T cells in the gut as they normalised hyperalgesia seen in SCID mice lacking T cells.12 Here we showed in immunocompetent mice, that the increased presence of CD4 T cells in the gut is able to surmount pro‐nociceptive activity of acute inflammatory cells and maintain normal visceral sensitivity. Together, these results indicate that, in the absence of overt inflammation, CD4 T cells confer normal visceral pain perception, and that this mechanism can be exaggerated during inflammation to counteract hyperalgesia.
Our results imply that hyperalgesia seen in active colitis versus normal visceral sensation during quiescent disease, could be due to a shift in the proportion of acute inflammatory and T cells occurring between flare ups and remission. A weakness of the initial studies showing pain thresholds in patients with IBD was the use of fixed volumes for rectal distension,10 and therefore possible compliance changes were not considered in the analysis. In our study, we used fixed pressure distension and monitored possible volume fluctuations and did not find any changes with chronic inflammation. In animal models of trinitrobenzene sulphonic acid‐induced chronic inflammation showing hyperalgesia,22 changes in compliance was a likely factor due to the inflammatory stimulus used, yet this was not considered and therefore may explain the discrepancy in results.
In addition to cell infiltrate, changes in sensory neuropeptides subsequent to changes in immune cells may explain variations in pain perception. Acute inflammatory cell‐induced hyperalgesia has been associated with increased neural expression of the pro‐nociceptive neuropeptide, substance P.13 In functional dyspepsia, pain was found to correlate with substance P and not inflammation.23 Our previous work showed an association of increased β‐endorphin expression with reduced visceral sensitivity.12 Furthermore, patients with IBD exhibited abnormal expression of these neuropeptides in the rectal mucosa.24 Thus, it may be that immune cells influence visceral perception through modulation of sensory neuropeptide expression in enteric neurones.
We observed up‐regulated substance P expression in all mice with colitis than in sham (undistended mice). In contrast, β‐endorphin was considerably up regulated only during chronic colitis, although control mice showed a trend of increased expression. Although both neuropeptides are influenced by inflammation, distension alone can alter central expression of sensory neuropeptides.25 Presumably, these changes also occur locally even in the absence of inflammation and may explain the difference in β‐endorphin expression between undistended (sham) and distended (control) mice. Therefore, in response to distension, a decreased ratio of β‐endorphin to substance P was associated with hyperalgesia, whereas an increased ratio was associated with normal visceral sensitivity. These results imply that β‐endorphin and substance P are coexpressed in sensory neurones, most likely in the myenteric plexus as this was an area of considerable up regulation. As immune cells were not observed in the myenteric plexus region, it is probable that immune mediators regulate neuropeptides via receptors on primary afferent terminals innervating the lamina propria. Inflammation increases expression of MOR,26 a high‐affinity receptor for β‐endorphin expressed on immune cells and enteric neurones. We observed a striking neural up regulation in mice with chronic colitis. T cell‐mediated analgesia is reversed by naloxone methiodide, a peripheral opioid receptor antagonist with high affinity for MOR. Therefore, we suspect that increased MOR is involved in nociception as β‐endorphin, via MOR, inhibits substance P release27,28 and ensuing pain perception.
T cells are a novel source of opioids29,30 and our earlier work provided a link between β‐endorphin release by T cells and its expression in the ENS,12 suggesting a positive regulation of opioid expression. We believe that a consequence of increased CD4 T cell infiltrate is increased β‐endorphin–MOR interaction leading to up regulation of neural β‐endorphin. Accordingly, we found a positive correlation between β‐endorphin and MOR among the groups with colitis. Thus, inflammation results in increased local opioid peptide and receptor as well as better availability of the ligand to the receptor,31,32 enhancing the potential for analgesia during chronic colitis. Interestingly, we also found that MOR expression was negatively associated with substance P and that this association was linked to visceral sensitivity. It is intriguing to speculate that MOR is expressed on sensory neurones containing both substance P and β‐endorphin, and activation of MOR up regulates β‐endorphin and down regulates substance P expression to induce analgesia. Although our results strongly suggest the existence of this endogenous antinociceptive mechanism, colocalisation studies of enteric neurones need to be done to confirm this conjecture.
We have shown that visceral sensitivity is not related to the presence of intestinal inflammation alone. Instead, the dominant immune cell population and sensory neuropeptide expressed in the gut during inflammation is associated with visceral sensitivity. Acute inflammation‐induced hyperalgesia is modified by subsequent infiltration of adaptive immune cells and accompanying ENS changes, the end result of which is normalised visceral sensitivity. Our study supports the notion that adaptive immune cell‐mediated analgesia is an active process involving modulation of sensory neuropeptides rather than simply decreasing pro‐nociceptive activity of acute inflammation. This is the first animal study to show a differential effect of inflammation on visceral sensitivity and corresponds with results obtained in the clinical setting. On the basis of our findings, we propose increased intestinal infiltrate of adaptive immune cells in IBD as a possible mechanism for normal pain thresholds observed in these patients. This does not exclude a role for increased descending inhibition as proposed by Chang et al;9 several mechanisms may contribute including this recently described antinociceptive effect of infiltrating lymphocytes.
Abbreviations
CRD - colorectal distension
DSS - dextran sodium sulphate
EMG - electromyograph
ENS - enteric nervous system
IBD - inflammatory bowel disease
MPO - myeloperoxidase
MOR - μ opioid receptor
NSC - non‐specific colitis
SCID - severe combined immune deficient
Footnotes
Funding: This work has been supported by a grant from the Canadian Institutes of Health Research (CIHR) to SMC and by a CIHR/Canadian Digestive Disease Foundation Doctoral Research Award to MVG.
Competing interests: None.
References
- 1.Honore P, Kamp E H, Rogers S D.et al Activation of lamina I spinal cord neurons that express the substance P receptor in visceral nociception and hyperalgesia. J Pain 200233–11. [DOI] [PubMed] [Google Scholar]
- 2.Traub R J, Hutchcroft K, Gebhart G F. The peptide content of colonic afferents decreases following colonic inflammation. Peptides 199920267–273. [DOI] [PubMed] [Google Scholar]
- 3.Burton M B, Gebhart G F. Effects of intracolonic acetic acid on responses to colorectal distension in the rat. Brain Res 199567277–82. [DOI] [PubMed] [Google Scholar]
- 4.Burton M B, Gebhart G F. Effects of kappa‐opioid receptor agonists on responses to colorectal distension in rats with and without acute colonic inflammation. J Pharmacol Exp Ther 1998285707–715. [PubMed] [Google Scholar]
- 5.Kang Y M, Lamb K, Gebhart G F.et al Experimentally induced ulcers and gastric sensory‐motor function in rats. Am J Physiol Gastrointest Liver Physiol 2005288G284–G291. [DOI] [PubMed] [Google Scholar]
- 6.Al Chaer E D, Lawand N B, Westlund K N.et al Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol 1996762675–2690. [DOI] [PubMed] [Google Scholar]
- 7.Monconduit L, Bourgeais L, Bernard J F.et al Convergence of cutaneous, muscular and visceral noxious inputs onto ventromedial thalamic neurons in the rat. Pain 200310383–91. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein C N, Niazi N, Robert M.et al Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain 199666151–161. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Munakata J, Mayer E A.et al Perceptual responses in patients with inflammatory and functional bowel disease. Gut 200047497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farthing M J, Lennard‐jones J E. Sensibility of the rectum to distension and the anorectal distension reflex in ulcerative colitis. Gut 19781964–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer E A, Berman S, Suyenobu B.et al Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 2005115398–409. [DOI] [PubMed] [Google Scholar]
- 12.Verma‐Gandhu M, Bercik P, Motomura Y.et al CD4+ T cell modulation of visceral nociception in mice. Gastroenterology 20061301721–1728. [DOI] [PubMed] [Google Scholar]
- 13.Verdu E F, Bercik P, Verma‐Gandhu M.et al Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 200655182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper H S, Murthy S N, Shah R S.et al Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 199369238–249. [PubMed] [Google Scholar]
- 15.Barbara G, Vallance B A, Collins S M. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology 19971131224–1232. [DOI] [PubMed] [Google Scholar]
- 16.Laughlin T M, Bethea J R, Yezierski R P.et al Cytokine involvement in dynorphin‐induced allodynia. Pain 200084159–167. [DOI] [PubMed] [Google Scholar]
- 17.Reeve A J, Patel S, Fox A.et al Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 20004247–257. [DOI] [PubMed] [Google Scholar]
- 18.Larsson M H, Rapp L, Lindstrom E. Effect of DSS‐induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol Motil 200618144–152. [DOI] [PubMed] [Google Scholar]
- 19.Chadwick V S, Chen W, Shu D.et al Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 20021221778–1783. [DOI] [PubMed] [Google Scholar]
- 20.Ohman L, Isaksson S, Lundgren A.et al A controlled study of colonic immune activity and beta7+ blood T lymphocytes in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 20053980–986. [DOI] [PubMed] [Google Scholar]
- 21.Tornblom H, Lindberg G, Nyberg B.et al Full‐thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology 20021231972–1979. [DOI] [PubMed] [Google Scholar]
- 22.Gschossmann J M, Liebregts T, Adam B.et al Long‐term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig Dis Sci 20044996–101. [DOI] [PubMed] [Google Scholar]
- 23.Monnikes H, van dV I, Wollenberg B.et al Gastric perception thresholds are low and sensory neuropeptide levels high in helicobacter pylori‐positive functional dyspepsia. Digestion 200571111–123. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto H, Morise K, Kusugami K.et al Abnormal neuropeptide concentration in rectal mucosa of patients with inflammatory bowel disease. J Gastroenterol 199631525–532. [DOI] [PubMed] [Google Scholar]
- 25.Lu C L, Pasricha P J, Hsieh J C.et al Changes of the neuropeptides content and gene expression in spinal cord and dorsal root ganglion after noxious colorectal distension. Regul Pept 200513166–73. [DOI] [PubMed] [Google Scholar]
- 26.Philippe D, Dubuquoy L, Groux H.et al Anti‐inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest 20031111329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collin E, Mauborgne A, Bourgoin S.et al Kappa‐/mu‐receptor interactions in the opioid control of the in vivo release of substance P‐like material from the rat spinal cord. Neuroscience 199251347–355. [DOI] [PubMed] [Google Scholar]
- 28.Jessell T M, Iversen L L. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature 1977268549–551. [DOI] [PubMed] [Google Scholar]
- 29.Smith E M, Morrill A C, Meyer WJ I I I.et al Corticotropin releasing factor induction of leukocyte‐derived immunoreactive ACTH and endorphins. Nature 1986321881–882. [DOI] [PubMed] [Google Scholar]
- 30.Zurawski G, Benedik M, Kamb B J.et al Activation of mouse T‐helper cells induces abundant preproenkephalin mRNA synthesis. Science 1986232772–775. [DOI] [PubMed] [Google Scholar]
- 31.Antonijevic I, Mousa S A, Schafer M.et al Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci 199515(1 Pt 1)165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer M, Imai Y, Uhl G R.et al Inflammation enhances peripheral mu‐opioid receptor‐mediated analgesia, but not mu‐opioid receptor transcription in dorsal root ganglia. Eur J Pharmacol 1995279165–169. [DOI] [PubMed] [Google Scholar]