Abstract
Aim
To study the role of mucus in the spatial separation of intestinal bacteria from mucosa.
Patients and methods
Mucus barrier characteristics were evaluated using histological material obtained by biopsy from purged colon, colon prepared with enema and material from untreated appendices fixed with non‐aqueous Carnoy solution. Bacteria were evaluated using fluorescence in situ hybridization, with bacterial 16S RNA probes and related to the periodic acid Schiff alcian blue stain. Biopsies from controls (n = 20), patients with self‐limiting colitis (SLC; n = 20), ulcerative colitis (n = 20) and 60 randomly selected appendices were investigated.
Results
The mucosal surface beneath the mucus layer was free of bacteria in ⩾80% of the normal appendices and biopsies from controls. The thickness of the mucus layer and its spread decreased with increasing severity of the inflammation; the epithelial surface showed bacterial adherence, epithelial tissue defects and deep mucosal infiltration with bacteria and leucocytes. Bacteria and leucocytes were found within mucus in all biopsy specimens from patients with ulcerative colitis, SLC, and acute appendicitis. The concentration of bacteria within mucus was inversely correlated to the numbers of leucocytes.
Conclusions
The large bowel mucus layer effectively prevents contact between the highly concentrated luminal bacteria and the epithelial cells in all parts of the normal colon. Colonic inflammation is always accompanied by breaks in the mucus barrier. Although the inflammatory response gradually reduces the number of bacteria in mucus and faeces, the inflammation itself is not capable of preventing bacterial migration, adherence to and invasion of the mucosa.
Accepted hypotheses for the aetiology of inflammatory bowel disease (IBD) suggest that contact between intestinal bacteria and mucosal surfaces triggers and perpetuates the colonic inflammation.1 The host is first exposed to intestinal bacteria at the mucus layer, which covers the mucosal surface. The mucus is a hydrated polymeric gel with a thickness of 50–800 μm, which is composed of two layers: a loosely adherent layer removable by suction and a layer firmly attached to the mucosa.2,3 The mucus is secreted by goblet cells and is composed of proteins, carbohydrates and lipids. Its main constituent is a glycoprotein (mucin). Various changes of mucus properties are well documented in IBD,4,5,6,7 but the pathogenetic relevance of these changes is uncertain. The role of mucus in the transit, mediation or prevention of bacterial contact with the epithelial cells is largely unknown.8 Recent advances in the application of the 16S RNA‐based fluorescence in situ hybridization (FISH) for studies of Carnoy fixed paraffin wax‐embedded intestinal tissues allow monitoring of bacterial communities within the well preserved mucus.9,10 However, difficulties still remain in obtaining material that is representative for the in vivo situation. It is especially important that studies on interactions between mucus and intestinal bacteria exclude biases associated with purging or preoperative antibiotic treatment. This can be done for the left colon by comparing data from purged intestines and intestines prepared only by enemas. Unfortunately, the cleansing effect of enemas cannot be satisfactorily extended further than the midtransverse colon. Autopsy material is also inappropriate as early/immediate postmortem changes occur under the influence of an aggressive faecal flora. Furthermore, for acute abdominal surgery broad‐spectrum antibiotics and purging are routinely used for elective abdominal surgery in Germany. However, preoperative use of antibiotics is not universal and some hospitals perform emergency appendectomies in uncomplicated cases without use of preoperative antibiotics with excellent results. Appendices are also removed routinely during laparoscopy in Germany, both when acute appendicitis is suspected, and also prophylactically when the appendix is normal. The appendix is part of the cecum, from which it originates and resembles histologically. Appendictomy provides presently the only opportunity to monitor the mucus barrier representative of the right colon in an unmanipulated gut, and to study and compare normal tissues with those with acute inflammation of different severity.
This study aimed at investigating the characteristics of the barrier for intestinal bacteria within the mucus layer, under normal as well as pathological conditions. Biopsies from purged colon are compared with biopsies from colon pretreated by enemas (left colon) and with material of whole appendices (right colon) removed by appendictomy without pretreatment.
Patients and methods
All patients were investigated and gave informed consent according to the protocol approved by the ethics commission of the Charité Hospital, Humboldt University, Berlin, Germany. The study included three groups: normal controls, patients with self‐limiting colitis (SLC) and ulcerative colitis. Ten people in each group were purged and underwent full colonoscopy and another 10 underwent sigmoidoscopy after an enema. The diagnosis of ulcerative colitis was made according to established criteria.11 Patients with subtotal colitis or pancolitis of low–moderate activity treated with 3 g mesalazine orally were included. Patients occasionally treated with prednisolone or azathioprine, were also included. Patients receiving other treatment were excluded. SLC was defined as first time colitis that macroscopically and histologically resembled infectious colitis and had an appearance and histology atypical for IBD. Bowel preparation for colonoscopy was performed using senna compounds (75 ml Clean‐Prep, Mundipharma, Limburg, FRG) and 2–3 l polyethylene glycol with electrolytes solution (Golytely, Braintree Laboratories, Braintree, Massachusetts, USA). The sigmoidoscopy was performed after glycerol enemas. None of the patients in all groups had been treated with antibiotics in the 2 months before the study. Biopsy specimens were taken, when possible from macroscopically non‐inflamed or less inflamed areas both in the cecum (right) and sigmoid (left) colon.
In addition, material from 60 appendices removed by laparoscopic emergent appendictomy was investigated. Patients operated within 24 h after onset of symptoms and without history of previous episodes indicating possible chronicity were included. In 11 patients the appendix showed no histological signs of acute appendicitis. The clinical diagnosis of acute appendicitis was confirmed clinically and histologically in 49 patients; 21 patients had catarrhal appendicitis and 25 had suppurative appendicitis. Three appendices with carcinoid, tubulovillous adenoma and appendicitis oxyurica were excluded. Table 1 presents the age and sex of patients.
Table 1 Baseline data for patients and control subjects.
| Appendicitis | Controls | SLC | UC | ||||
|---|---|---|---|---|---|---|---|
| No | Catarrhal | Suppurative | |||||
| Appendictomy | A | 11 | 21 | 25 | |||
| Colonoscopy | C | 10 | 10 | 10 | |||
| Sigmoidoscopy | S | 10 | 10 | 10 | |||
| Mean age (years) | A | 31.7 | 26.8 | 26 | |||
| C | 50.1 | 39.5 | 48.8 | ||||
| S | 49.2 | 37.4 | 47.1 | ||||
| Male/female ratio | A | 7/4 | 6/15 | 7/18 | |||
| C | 5/5 | 7/3 | 4/6 | ||||
| S | 4/6 | 6/4 | 3/7 | ||||
A, appendectomy; C, Colonoscopy; S, Sigmoidoscopy; SLC, self‐limiting colitis; UC, ulcerative colitis.
Tissue preparation
Tissues were fixed in non‐aqueous Carnoy solution12, (2 h for biopsies and 6 h for appendices), processed and then embedded into paraffin wax blocks using standard techniques. Sections of 4 μm thickness were placed on SuperFrost slides (R Langenbrinck, Emmendingen, Germany). One of two successive sections was hybridised with the universal Eub 338 probe to enumerate bacteria, the other was stained with alcian blue/periodic acid Schiff (PAS) to asses mucus and leucocytes. The background fluorescence of human tissue allowed tissue structures to be seen by FISH. The photomicrographs of alcian blue/PAS stain and FISH were overlaid using Adobe Photoshop imaging software to confirm the correct interpretation of the data.
Microscopic evaluation of the mucus barrier and FISH
Microscopy was performed with the Nikon e600 fluorescence microscope (Nikon, Tokyo, Japan) and photo documented with a Nikon DXM1200 camera, and software (Nikon). Probes were synthesised with Cy3, FITC or Cy5 fluorescent dye at the 5′end (MWG Biotech, Ebersberg, Germany). The hybridisation with Eub 33813 Cy3 probe universal for Eubacteria was performed at 46°C to visualise all bacteria. Photographs were made consecutively from the entire surface of the section (×400 magnification) with overlap of joining microscopic fields. The single shots were composed to figures representing the complete surface visually using PowerPoint imaging software. Light microscopic figures of successive sections stained with alcian blue/PAS were used for evaluation of mucus and leucocytes. All photos were made in real colours and not manipulated except for brightness and contrast. The characteristics of mucus were evaluated separately for faeces, mucus, adjacent mucosa and mucosal compartments by two independent investigators. Separate high power (×1000 magnification) photo series were made for regions with bacteria adherent to the epithelial surface, bacteria within crypts, for fissures filled with bacteria, abscesses and regions of tissues infiltrated by bacteria. Bac 303,14 EREC,15 Fprau,16 Ebac17 probes representing Bacteroides, Eubacterium rectale, Clostridium coccoides, Fusobacterium prausnitzii and Enterobacteriaceae cluster were applied in different combinations including Eub 338 probe to evaluate the bacterial diversity in multi‐colour analysis. The hybridisations were performed according to standard protocols and always counterstained with DAPI. Additional hybridisations with lysozyme and with the non‐sense probe Non 33813 were performed to test for gram‐positive bacteria and non‐specific binding. The quantification of bacteria was based on the assumption that a 10‐μl sample with a cell concentration of 107 cells per ml contains 40 cells per average microscopic field at magnification of 1000.9
The mucus barrier for intestinal bacteria within single groups was evaluated by the:
Number of patients without mucus on the epithelial surface of the probes (the crypts could be filled, but the mucus within crypts was not evaluated if the epithelial surface was not covered with mucus).
Mucus thickness (a representative region covering at least 20% of the epithelial surface was chosen for this measurement). Three side‐by‐side measurements were performed with the middle measurement placed at the site of the maximal mucus thickness, and both other reading lines located left and right from the first at the distance of 100 μm. A mean value was used for each probe.
Percent of the epithelium covered with mucus. The length of the mucus layer was divided by the length of visible epithelial layer in the whole section ×100.
Number of patients with bacteria and leucocytes within mucus. Even when cautiously handled, the mucus layer could not be preserved all over the epithelial surface. Especially in large sections of appendices, the shrinkage of faeces led regularly to partial defects of the mucus. Bacteria and leucocytes were enumerated within a 30 μm broad region of the mucus adjacent to the mucosa to reduce errors in interpretation.
Percentage of the mucus containing bacteria. The microphotographs of complete biopsies or tissue sections were overlaid with a 5 μm micrometer net. In a 30 μm band of mucus adjacent to the mucosa the number of quadrates containing bacteria were divided by number of quadrants of mucus free from bacteria ×100.
Percentage of the mucus containing leucocytes was evaluated with the same technique as above (5).
Concentrations of faecal bacteria (for appendices only): numbers of faecal bacteria were calculated within a square of 10×10 μm, which was placed over a representative region in the centre of the intestinal lumen. A mean value of 5 measurements was used for each patient.
Concentrations of bacteria within mucus: numbers of mucus‐penetrating bacteria were calculated within a square of 10×10 μm, which was placed over mucus next to the epithelial surface. A mean value of 5 measurements was calculated.
Number of patients with bacteria adherent to the epithelial surface.
Percentage of the epithelium covered with adherent bacteria.
-
Number of patients with focal defects of the epithelial layer including:
-
-
A. Defects of single epithelial cells within intact epithelial layer. The epithelial cells are filled with bacteria and surrounded by normal epithelial cells. The underlying submucosa is not denuded.
-
-
B. Aphthoid defects of the epithelial layer (1–5 depleted epithelial cells). The underlying submucosa is denuded and covered with bacteria or leucocytes.
-
-
C. Widespread damage of epithelial surface with loss of mucosal integrity, leucocytes confluent between submucosal and faecal compartment, bacteria mixed with leucocytes and diffusely infiltrating tissue.
-
-
Percentage of the surface of the intestinal lumen (faeces) filled with leucocytes evaluated as above (5).
Number of patients with leucocytes attached to the mucosa.
Percentage of the epithelium covered with attached leucocytes.
-
Number of patients with bacteria invading submucosa including:
-
-
A. Fissure.
-
-
B. Diffuse infiltration of the mucosa.
-
-
C. Microabscess.
-
-
Percentage of the tissue surface showing bacterial invasion.
Statistics
Mean values and standard deviations (SDs) were calculated and expressed as mean (SD). Using analysis of variance a p value of <0.05 was considered significant.
Results
Mucus barrier in non‐inflamed appendices and normal controls
Table 2 summarises the numerical data of the characteristics of the mucus barrier. The architecture of the mucosa was intact. Leucocytes filled 3 to 15% of the surface (compartment above the mucus layer) within the lumen of normal appendices. The faecal bacterial concentrations were high. No adherent bacteria or bacteria invading mucosa were observed. The cross‐sections of 6 appendices without inflammation were nearly completely covered with mucus (fig 1) and disturbed only by scratches and rips caused by mechanical manipulation. The sections were not circular in 5 appendices. The intestinal contents were partially lost, the mucus layer was well preserved. Mucus defects caused by shrinkage of faeces were irregularly observed at the border between mucus and faeces. The mucus layer in the appendix was 131 (51) μm thick (mean (SD)) when measured within the epithelial areas with intact mucus. The mucosa adjacent areas of the mucus were free of bacteria in 9/11, and free of leucocytes in 10/11 patients with normal appendices. The percentage of mucus containing bacteria was <7%. The percentage of mucus containing leucocytes was <0.5%.
Table 2 Mucus barrier characteristics.
| Appendix | Cecum | Sigmoid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Catarrhal | Suppurative | Controls | SLC | UC | Controls | SLC | UC | ||
| Appendectomy | A | 11 | 21 | 25 | ||||||
| Colonoscopy | C | 10 | 10 | 10 | 10 | 10 | 10 | |||
| Sigmoidoscopy | S | 10 | 10 | 10 | ||||||
| Number of patients with mucus depletion | A | 0 | 0 | 12 | ||||||
| C | 0 | 0 | 4 | 0 | 1 | 3 | ||||
| S | 0 | 4 | ||||||||
| Mucus thickness | A | 131 (51) | 26 (29) | 7 (10)* | ||||||
| C | 83 (49) | 54 (71) | 22 (21)‡ | 70 (46) | 42 (35) | 31 (39)NS | ||||
| S | 56 (21) | 36 (17) | 27 (33)NS | |||||||
| Percent of the epithelium covered with mucus | A | 87 (14) | 53 (23) | 6 (9)* | ||||||
| C | 58 (24) | 30 (26) | 16 (22)§ | 57 (26) | 31 (30) | 22 (25)¶ | ||||
| S | 49 (20) | 34 (24) | 17 (22)¶ | |||||||
| Number (%) of patients with bacteria and leucocytes within mucus | A | 2 (18) | 21 (100) | 13 (100) | ||||||
| C | 2 (20) | 10 (100) | 6 (100) | 1 (10) | 9 (100) | 7 (100) | ||||
| S | 3 (30) | 10 (100) | 6 (100) | |||||||
| Percentage of the mucus containing bacteria | A | 5–7 | 53 (37) | 94 (14)* | ||||||
| C | 5.3 (7) | 58 (24) | 53 (27) | 5 (3.3) | 41 (26) | 40 (30)* | ||||
| S | 1.2 (1) | 63 (36) | 51 (26) | |||||||
| Percentage of the mucus containing leucocytes | A | <0.5 | 10 (15) | 78 (25) | ||||||
| C | 0 | 7 (6) | 13 (6) | 0 | 3 (3) | 5 (3) | ||||
| S | 0 | 7 (5) | 9 (7) | |||||||
| Mean concentrations of faecal bacteria ×1010/ml (SD) | A | 45 (17) | 8.1 (7.3) | 0.7 (1.5)* | ||||||
| Mean concentrations of bacteria within mucus ×1010/ml (SD) | A | <0.005 | 4.8 (5) | 0.6 (1.2)* | ||||||
| C | 0.03 (0.1) | 0.8 (1.1) | 0.1 (0.3) | 0.01 (0.03) | 1.1 (0.7) | 0.3 (0.5)* | ||||
| S | 0.01 (0.02) | 1.2 (0.9) | 0.2 (0.5) | |||||||
| Number of patients with bacteria adherent to the epithelial surface | A | 0 | 14 | 18† | ||||||
| C | 1 | 4 | 6† | 1 | 6 | 8† | ||||
| S | 0 | 6 | 8 | |||||||
| Percentage of the epithelium covered with adherent bacteria | A | 0 | 15 (21) | 47 (41*) | ||||||
| C | 8 (3) | 21 (16) | 52 (31) | 4 (4) | 26 (17) | 45 (34)* | ||||
| S | 1 (0.6) | 40 (30) | 44 (38) | |||||||
| Number of patients with focal defects of the epithelial layer including | A | 0 | 21 | 25† | ||||||
| C | 0 | 6 | 6† | 0 | 4 | 7† | ||||
| S | 0 | 5 | 6 | |||||||
| Single epithelial cells invaded by bacteria | A | 0 | 13 | 5 | ||||||
| C | 0 | 3 | 1 | 0 | 3 | 0 | ||||
| S | 0 | 2 | 0 | |||||||
| Patchy/aphthoid defects denuding submuosa | A | 0 | 10 | 15 | ||||||
| C | 0 | 2 | 4 | 0 | 1 | 3 | ||||
| S | 0 | 2 | 4 | |||||||
| Spread epithelial surface damage/epithelial loss | A | 0 | 0 | 15 | ||||||
| C | 0 | 1 | 1 | 0 | 0 | 0** | ||||
| S | 0 | 0 | 0** | |||||||
| Percentage of the intestinal lumen filled with leucocytes | A | 7 (3) | 64 (32) | 75 (32) | ||||||
| C | – | – | – | – | – | – | ||||
| S | ||||||||||
| Number of patients with leucocytes attached to the mucosa | A | 0 | 6 | 25* | ||||||
| C | 0 | 5 | 5† | 0 | 2 | 6† | ||||
| S | 4 | 4 | ||||||||
| Percentage of the epithelial surface covered with attached leucocytes | A | <1 | 30 (24) | 72 (40)* | ||||||
| C | 0 | 2 (2) | 14 (9)† | 0 | 5–7 | 11 (3)† | ||||
| S | 5 (3) | 2 (1) | ||||||||
| Number of patients with bacteria invading submucosa including: | A | 0 | 7 | 25* | ||||||
| C | 0 | 1 | 5 | 0 | 2 | 4† | ||||
| S | 1 | 3 | ||||||||
| Fissure | A | 0 | 4 | 11 | ||||||
| C | 0 | 1 | 4 | 0 | 2 | 3 | ||||
| S | 0 | 1 | 3 | |||||||
| Diffuse infiltration of the mucosa | A | 6 | 19 | |||||||
| C | 0 | ** | ** | 0 | ** | ** | ||||
| S | 0 | ** | ** | |||||||
| Microabscess | A | 1 | 9 | |||||||
| C | 0 | 0 | 1 | 0 | 0 | 2 | ||||
| S | 0 | 1 | 2 | |||||||
| Percentage of tissue surface with bacterial invasion | A | 0 | 5 (6) | 31 (37)* | ||||||
| C | 0 | ** | ** | 0 | ** | ** | ||||
| S | 0 | ** | ** | |||||||
*Marks rows with differences that are significant (p<0.001), including a comparison of single groups.
†The significant differences were not calculated for 0 value of the controls. The differences between groups with positive values within the row were not significant.
‡p<0.042.
§p<0.003.
¶p<0.02.
**The bacterial invasion of mucosal tissues in the biopsies was difficult to distinguish from biases caused by mechanical handling of the biopsy. High concentrations of bacteria within mucus of patients with ulcerative colitis and SLC could have been pressed into the tissues. Therefore, no evaluation was attempted in regions showing epithelial defects. No infiltration was observed in areas adjacent to basal membrane below the intact epithelial layer.
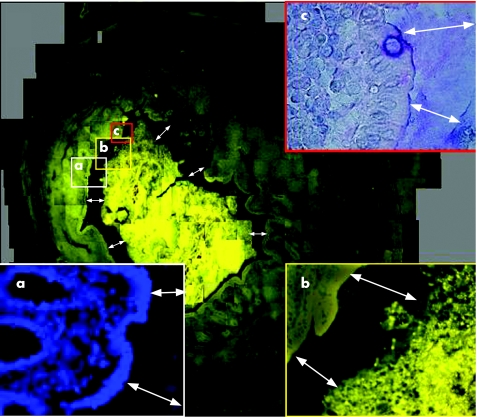
Figure 1 Hybridisation of a cross‐section of a normal appendix with the Eub 338 probe universal for all bacteria. The weakness of the fluorescence signals does not allow us to make FISH photomicrographs at this low resolution. The cross‐section of the appendiceal surface is therefore composed of about 400 overlapping FISH photomicrographs at a magnification of ×400. The mucosal tissues can be well distinguished due to background fluorescence of human tissues. The mucus layer can be perceived as a continuous gap (double headed arrows) between mucosal surface and bacterial masses located in the lumen of the appendix. The mucus layer positively hybridised with the Eub 338 probe universal for all bacteria. No nuclear structures that could be referred to bacterial DNA or leucocytes nuclei can be detected within this gap using 4,6‐diamidino‐2‐phenylindole (DAPI) stain (a, ×400). Bacteria are separated from the epithelial surface along the whole circumference. The epithelial layer is intact. Small scratches and rips are obviously artefacts. Bacteria within mucus, mucosa adherent, intracellular, or invading bacteria are absent at larger magnification of FISH (b). (c) Alcian/periodic acid Schiff (PAS) stain of corresponding section ×400. No leucocytes can be seen within intact mucus.
The mucus layer in biopsy specimens taken during colonoscopy in control patients was only partially preserved. The epithelial surfaces flanking the torn‐out portions of the biopsy were regularly worn‐out. Nevertheless, in all biopsy specimens from the control group, the epithelial surface was covered with mucus an average 60%, with the smallest proportion being 30%. The mean (SD) mucus thickness was 83 (49) μm in the cecum and 70 (46)/56 (21) μm in the sigmoid colon of purged versus enema prepared control patients. No epithelial defects or submucosal bacteria were detected. The mucus was free of leucocytes in all control patients. Bacteria were found within mucus in about 20% of the biopsy specimens, and covered 1–10% of the mucus surface. In one control patient, a string of single adherent bacteria (mainly Escherichia coli) was observed attached to the epithelial surface.
Mucus barrier in inflamed appendices and biopsy specimens from patients with SLC and ulcerative colitis
The characteristics of the mucus barrier such as the surface of the epithelium covered by mucus and the mucus thickness were reduced >10‐fold in suppurative appendicitis compared with normal appendices. The mucus layer was completely absent in 12/25 patients and only rudimentary, found as small island, in the remaining 13. The mucus layer could be partially seen in all patients with catarrhal appendicitis. However, the mucus thickness and the surface covered by the mucus were significantly reduced compared with the mucus layer of the non‐inflamed appendices. All differences were highly significant (p<0.001).
The thickness of the mucus layer and the surface covered by mucus were also reduced in biopsy specimens from patients with SLC, and even more so in the biopsy specimens from patients with ulcerative colitis when compared with normal controls; however, the differences in the thickness of the mucus layer in the sigmoid colon did not always reach statistical significance.
The mucus close to the mucosa contained bacteria, leucocytes or both in all samples of inflamed intestine (figs 2 and 3). The concentrations of mucus‐penetrating and adherent bacteria were highest in catarrhal appendicitis and in patients with SLC. The percentage of epithelium covered by adherent bacteria or adherent leucocytes was the highest in suppurative appendicitis and in ulcerative colitis. A strong negative association was observed between bacterial concentration in faeces (appendices) or in the mucus layer (biopsies and appendices) and the number of leucocytes within the mucus layer. The bacterial concentrations decreased with increased migration of leucocytes into the intestinal lumen, especially in suppurative appendicitis, where leucocytes could completely fill the intestinal lumen. The occurrence and severity of focal epithelial defects increased progressively from catarrhal to suppurative appendicitis and from SLC to ulcerative colitis, despite increasing numbers of leucocytes and decreasing concentrations of bacteria within the mucus layer.
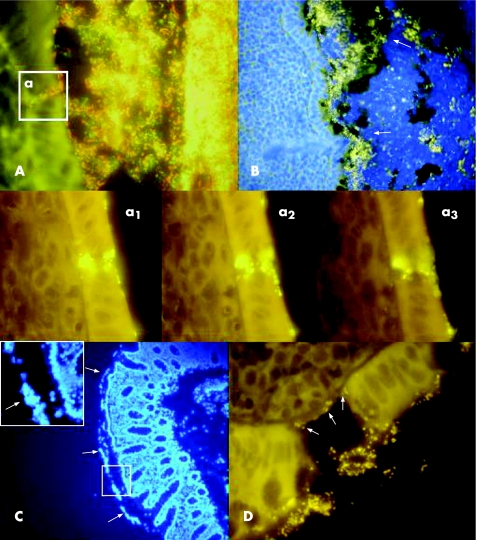
Figure 2 Mucus barrier in catarrhal appendicitis (A,B) and ulcerative colitis (C,D). (A) The mucus is infiltrated by bacteria in catarrhal appendicitis. The Bac 303 Cy3 probe (Bacteroides) is orange, the EREC Cy5 probe (Eubacterium rectale‐Clostridium coccoides) is red, all other bacteria (Eub 338 FITC probe) are green. The bacteria within mucus, the adherent bacteria and all invading bacteria are composed of diverse faecal flora. The insertion A shows a typical focal defect in the mucosa, which was often observed in catarrhal appendicitis. The fields a1–a3 show a similar defect using the universal Eub 338 Cy3 probe for hybridisation to achieve a better resolution. Photomicrographs made at different focus levels clearly show that bacteria are not overlaid, but located within single epithelial cells. The surrounding epithelial cells appear intact. The underlying submucosa is not denuded. (B) The simultaneous photomicrographs of DAPI fluorescence (blue colour represents mainly nuclei of human cells) and orange fluorescence of the universal bacterial Eub 338 Cy3 probe. The nuclei of leucocytes fill the lumen of the appendix, especially the portions on top of the mucus, but are also seen within the mucus layer (the outer border of the mucus is marked by white arrows) and attached to the mucosa. The bacterial concentrations are noticeably reduced in regions with high leucocyte counts. (C) DAPI stain of the biopsy specimen from a patient with active ulcerative colitis. The nuclei of leucocytes within mucus are well seen (white arrows). Migration of leucocytes into the mucus in inflammation is a common phenomenon, generally seen in both SLC and ulcerative colitis. (D) Eub 338 Cy3 hybridisation (all bacteria, orange fluorescence) of tissue from a patient with active ulcerative colitis. The background fluorescence of the tissues allows visualisation of the aphthoid lesions of the epithelial layer and denuded submucosa. Bacteria are directly attached to the denuded submucosa (arrows).
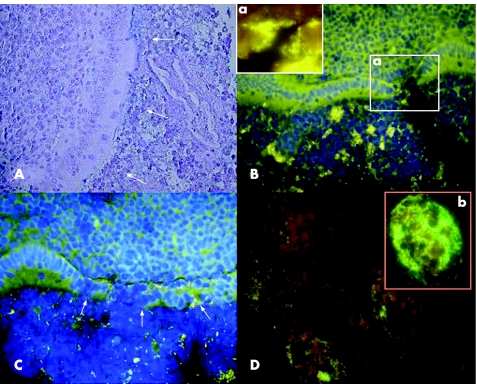
Figure 3 Mucosal barrier in suppurative appendicitis. (A) Alcian blue/periodic acid Schiff stain. The appendiceal lumen is nearly completely filled with leucocytes in suppurative appendicitis. The mucus is absent even in cases where the mucosal integrity is preserved. Leucocytes are observed in direct contact with the epithelial surface without any visible mucus gap in between (white arrows). (B,C) Simultaneous photomicrograph of DAPI fluorescence (blue colour represents mainly nuclei of human cells) and orange fluorescence of the universal bacterial Eub 338 Cy3 probe are presented. The lumen of the intestine is filled with leucocytes, and scattered bacteria. Single bacteria and bacterial islands orange fluorescence are seen between leucocytes filling the lumen of the appendix. The inset (a) in B shows an aphthoid epithelial defect with bacteria attached to the submucosa (Eub 338 Cy3 orange fluorescence). The arrows in the C point towards a more extended epithelial defect. (D) Simultaneous multi‐colour hybridisation. The bacteria hybridised with the Bac 303 Cy3 probe (Bacteroides) are orange and the bacteria hybridised with the Fprau Cy5 probe (Fusobacterium prausnitzii) are red, all other bacteria are stained with FITC and appear green. The insertion (b) shows a photomicrograph of a microabscess within the same section. The infiltrating bacteria and the bacteria within abscess are of various microbial species and the numbers of single bacterial species vary significantly.
The epithelial defects in catarrhal appendicitis were typically represented by invasion of single epithelial cells within the border layer of intact epithelium, surrounded by apparently normal epithelial cells and filled with bacteria (fig 2A,a1–a3). Similar lesions were observed in biopsy specimens from patients with SLC; however, the concentrations of bacteria within single epithelial cells were considerably lower. Epithelial defects with denuded submucosa were typical for suppurative appendicitis and for patients with ulcerative colitis (figs 2B,3B,D). They often appeared as aphthoid ulcer with 3–10 adjacent cells absent within the epithelial layer (typical for ulcerative colitis), but were sometimes widespread and damaged large surface areas. The aphtoid defects were never >10 epithelial cells (maximum length of 50 μm) in ulcerative colitis. The aphthoid defects were confluent in suppurative appendicitis often leaving only small islands of intact epithelium, and the widely denuded submucosa was covered by both adherent bacteria and attached leucocytes. The mucosal integrity was completely destroyed in one‐third of patients with suppurative appendicitis. Leucocytes both within the submucosal tissue and within the intestinal lumen were often confluent. Bacteria in low concentrations were diffusely mixed with lymphocytes and they deeply infiltrated mucosa and contributed to both fistulas and microabscesses (fig 3D).
Influence of enema versus purging on the mucus barrier
The mucus barrier characteristics were generally lower in enema‐treated than in purged colons, however, the differences were not always consistent and never statistically significant (table 2).
Composition of the bacteria
The bacteria within the mucus, the adherent bacteria and the bacteria invading single epithelial cells or diffusely spread into the submucosa always consisted of multiple species (figs 2A, 3D). At least four different bacterial groups were detected in each location. The composition of bacteria within the mucus, the adherent bacteria and the invading bacteria were similar within the biopsy material from the same patient, but varied between individual patients. Clusters of Bacteroides and Eubacteriumrectale‐Clostridiumcoccoides were always present, contributing each at least 10% of the Eub 338 visible population (universal probe). Fprau (Fusobacterium prausnitzii) and Ebac (Enterobacteriaceae) were often less numerous and sometimes absent.
Discussion
The central role of immunity in maintaining mucosal integrity is well founded, but the precise mechanisms by which the host recognises and handles the intestinal flora are still poorly understood. The intestinal epithelial layer is generally regarded to be exposed to a rich intestinal flora with a multitude of microbial components, both benign and potentially pathogenic. However, the healthy mucosa remains immunologically quiescent, despite the potentially hostile environment, and will only react when closely confronted with obligate pathogens.18,19 Traditionally the lack of immune response to the “normal” faecal flora is explained as consequence of so‐called immune tolerance, implicating the existence of sophisticated immune mechanisms, capable of discriminating between “benign” and “pathogenic” microorganisms, and responding in accordance with its potential threat.20,21 The problem with the last explanation is the high diversity of the intestinal flora. The bacteria in the colon will often reach concentrations of 1012/g faeces and include >500 different species with their effect unknown. None of the presently available laboratory methods are capable of monitoring the exact composition of the intestinal flora and its changes, but we suggest that the host does it in a real time manner. Further, difficulties in understanding how tolerance works arises from the fact that nearly all the intestinal bacteria are pathogenic under certain conditions. Bacteroides fragilis, Clostridiumperfingens, Clostridium difficile, E coli, Streptococcus and Enterococcus are pathogens, if translocated to submucosal intraperitoneal or intravasal compartments. They are usually regarded as harmless commensals as they are present in large quantities in every healthy intestine.
Convincingly, our data show that the postulated contact between intestinal bacteria and mucosa is a fiction.22 A mucus gel covers the mucosa and separates the luminal bacteria from the epithelial surface throughout the colon in healthy individuals. Although the thickness of the mucus layer may vary, the 30 μm zone in the mucus close to mucosa is free of bacteria—for example, the mucosa is, in reality, germ free. It is impossible to correctly enumerate diffusely spread bacteria in concentrations <104/ml using FISH, this is one bacterium per four microscopic fields.9 However, negative bacterial culture and data from quantitative polymerase chain reaction with universal bacterial primer performed on washed biopsy specimens from controls support our present results.23,24
Histological investigations of the mucus of the large colon of humans were, until today, performed mainly on surgically removed material after the use of a variety of fixation and staining techniques.4,5,6 The role of mucus in separating bacteria from the mucosa was mentioned in some of these studies, but never systematically investigated and quantified in regard to the location, spread and thickness of the mucus layer. Mucus is extremely sensitive to manipulations and biases. The vulnerability of the mucus and ambiguity of previous results led to opposing interpretations. The immune inclusion hypothesis postulates that the host specifically maintains (via IgA, nutrients and other factors) a sessile bacterial biofilm adherent to the mucosa. The bacterial biofilm will grow in the mucus matrix (immune inclusion) and will thereby prevent contact of pathogenic bacteria with the intestinal mucosal wall (immune exclusion). Evidence supporting this hypothesis was summarised in two recently published reviews.22,25 A bacterial biofilm attached to the mammal intestinal mucosa (baboon) was shown by electron microscopy and immunohistochemistry.26 In vitro experiments showed that immunoglobin A and mucins facilitated biofilm formation by normal human gut flora and by E coli on cultured, fixed human epithelial cell surfaces—for example, conditions under which non‐adherent bacteria are repeatedly washed away.22 The immune inclusion hypothesis is seemingly not in accordance with the findings from human studies using in situ hybridisation with 16S RNA based probes. Four groups of investigators using different fixation and hybridisation protocols reported independently during the past 6 years a lack of bacteria in normal mucus, but raised bacterial numbers in mucus from patients with colonic inflammation and IBD.23,27,28,29
However, all these studies were based on material obtained during colonoscopy or elective surgery. The human colon was purged before intervention in all patients, which makes it possible that the reported absence of bacteria within mucus could be the result of high concentrations of the electrolyte solutions used for purging. In addition, preoperative use of antibiotics can even confuse the findings further. The advantage of the present study is that it investigates the mucus barrier on material obtained by biopsy from purged patients, patients prepared with enemas and on material of untreated appendices. The observed mean mucus thickness was somewhat lower than previously reported from investigations on surgically removed tissues.12 However, the characteristics of the mucus barrier for intestinal bacteria did not differ between patients purged or prepared with enemas, and were also in accordance with results obtained from untreated appendices. A complete separation of bacteria from mucosa was found in practically all normal controls, regardless of the method used for preparation of the patients for endoscopy. By contrast, none of the samples from patients with colonic inflammation showed an intact mucus barrier. The mucus was either penetrated by bacteria, a bacteria‐leucocyte mix, or bacteria and inflammatory cells were directly attached to the epithelial surface when the mucus was completely depleted.
Our data do not support the immune inclusion hypothesis in humans. We found no spatial structures or accumulations of bacteria, which could be interpreted as a protective bacterial biofilm. Bacteria observed within mucus were scattered irregularly, unorganised, and clearly associated with colonic inflammation and signs of a progressive breakdown of the mucus barrier. The mucus thickness and spread was lower in suppurative than in catarrhal appendicitis, and in patients with ulcerative colitis than in SLC. The proportion of mucus containing bacteria, the percentage of the epithelial surface covered by adherent bacteria, the number of epithelial defects and ulcerations, the occurrence of submucosal infiltration, fissures and microabscesses was all increased accordingly.
Nothing seemed to indicate that inflammation was primarily responsible for the observed leakage. Contrarily, the concentrations of bacteria within faeces, mucus or adherent bacteria were inversely related to the number of leucocytes in all inflammatory groups and the inflammatory response was clearly antibacterial. The inverse relationship was even more obvious when the number of bacteria and leucocytes in single probes were plotted (data not shown). The highest bacterial concentrations within mucus were observed in catarrhal appendicitis and SLC, where the leucocyte response was moderate compared with ulcerative colitis and suppurative appendicitis. The bacterial boundary towards the host moved with the growing severity of inflammation from the periphery of the outer mucus regions towards the mucosal surface, progressively involving epithelial layer, submucosa and deep tissues, despite pronounced antibacterial activity and generally falling bacterial concentrations. This microbial invasion was unselective. In each sample, bacteria within mucus, adherent bacteria, bacteria within single epithelial cells, bacteria attached to the denuded submucosa and invasive bacteria were composed of a mix including at least four different bacterial groups. This indicates that the antibacterial properties of the mucosal immune response are not as specific as generally assumed. Practically all types of faecal bacterial groups participate in the invasion of the mucosa when the barrier integrity is lost.9,23 It appears that the restitution of the intact mucus barrier is the only possibility to stop an inflammation in progress, to restore the immunological equilibrium and to maintain intestinal health.
Abbreviations
DAPI - 4,6‐diamidino‐2‐phenylindole, a usual DNA stain
FISH - fluorescence in situ hybridization
FITC - fluorescein isothiocyanate
IBD - inflammatory bowel disease
PAS - periodic acid Schiff
SLC - self‐limiting colitis
Footnotes
Competing interests: None.
All authors are responsible for the research and have participated in the concept and design; analysis and interpretation of data; drafting or revision of the manuscript, and they have approved the manuscript as submitted.
References
- 1.Podolsky D. Inflammatory bowel disease. N Engl J Med 2002347417–429. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenberger L M. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol 199557565–583. [DOI] [PubMed] [Google Scholar]
- 3.Atuma C, Strugala V, Allen A.et al The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 2001280922–929. [DOI] [PubMed] [Google Scholar]
- 4.Pullan R D, Thomas G A, Rhodes M.et al Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. GUT 199435353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strugala V, Allen A, Dettmar P W.et al Thickness and continuity of the colonic mucus layer in active and quiescent ulcerative colitis. GUT 200047(Suppl III)A219. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes J M. Mucins and inflammatory bowel disease. Q J Med 19979079–82. [DOI] [PubMed] [Google Scholar]
- 7.Deplancke B, Gaskins H R. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 200173(Suppl)1131–1141. [DOI] [PubMed] [Google Scholar]
- 8.Corfield A P, Myerscough N, Longman R.et al Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. GUT 200047589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swidsinski A, Weber J, Loening‐Baucke V.et al Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. JCM 2005433380–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swidsinski A, Loening‐Baucke V, Lochs H.et al Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol 200581131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman R W, Selby W S, Jewell D P. Controlled trial of intravenous metronidazole as an adjunct to corticosteroids in severe ulcerative colitis. GUT 1986271210–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo K, Ota H, Akamatsu T.et al Histochemistry of the surface mucous gel layer of the human colon. GUT 199740782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amann R, Krumholz L, Stahl D A. Fluorescent‐oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 1990172762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manz W, Amann R, Ludwig W.et al Application of a suite of 16S rRNA‐specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga‐flavobacter‐bacteroides in the natural environment. Microbiology 19961421097–1106. [DOI] [PubMed] [Google Scholar]
- 15.Franks A H, Harmsen H J, Raangs G C.et al Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group‐specific 16S rRNA‐targeted oligonucleotide probes. Appl Environ Microbiol 1998643336–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suau A, Rochet V, Sghir A.et al Fusobacterium prausnitzii and related species represent a dominant group within the human fecal flora. Syst Appl Microbiol 200124139–145. [DOI] [PubMed] [Google Scholar]
- 17.Bohnert J, Hübner B, Botzenhart K. Rapid identification of Enterobacteriaceae using a novel 23S rRNA‐targeted oligonucleotide probe. Int J Hyg Environ Health 200220377–82. [DOI] [PubMed] [Google Scholar]
- 18.Perdue M H. The mucosal antigen barrier: cross talk with mucosal cytokines. AJP GI 19992771–5. [DOI] [PubMed] [Google Scholar]
- 19.Sansonetti P J. War and peace at mucosal surfaces. Nat Rev Immunol 20044953–964. [DOI] [PubMed] [Google Scholar]
- 20.Cobrin G M, Abreu M T. Defects in mucosal immunity leading to Crohn's disease. Immunological Reviews 2005206277–295. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Q, Walker W A. Innate immunity of the gut: mucosal defense in health and disease. J Pediatr Gastroenterol Nutr 200438463–473. [DOI] [PubMed] [Google Scholar]
- 22.Everett M L, Palestrant D, Miller S E.et al Immune exclusion and immune inclusion: a new model of host‐bacterial interactions in the gut. Clin Appl Immunol Rev 20044321–332. [Google Scholar]
- 23.Swidsinski A, Ladhoff A, Pernthaler A.et al Mucosal flora in inflammatory bowel disease. Gastroenterology 200212244–54. [DOI] [PubMed] [Google Scholar]
- 24.Martin H M, Campbell B J, Hart C A.et al Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterolgy 200412780–93. [DOI] [PubMed] [Google Scholar]
- 25.Bollinger R R, Everett M L, Palestrant D.et al Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology 2003109580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palestrant D, Holzknecht Z E, Collins B H.et al Secretory IgA associated biofilms are a normal feature of bacterial growth in the mammalian gut. Ultrastruct Pathol 20042823–27. [PubMed] [Google Scholar]
- 27.Schultsz C, Van Den Berg F M, Ten Kate F W.et al The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology 19991171089–1097. [DOI] [PubMed] [Google Scholar]
- 28.Kleessen B, Kroesen A J, Buhr H J.et al Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol 2002371034–1036. [DOI] [PubMed] [Google Scholar]
- 29.van der Waaij L A, Harmsen H J, Madjipour M.et al Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA‐based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm Bowel Dis 200511865–871. [DOI] [PubMed] [Google Scholar]