The flat surface of the colon is covered by an epithelium composed of four differentiated cell types (enterocytes, enteroendocrine, goblet and Paneth cells) that invaginates at regular intervals to form crypts (fig 1). The bottom of the crypts is occupied by a few stem cells that give rise to actively dividing precursor cells that populate the bottom two‐thirds of the crypts.1 Proliferation occurs under the influence of growth factors from the Wnt family that may be produced by the underlying stromal cells underneath the stem cell compartment or by the epithelial cells themselves. The precursors migrate upward in an ordered fashion, which is also controlled by Wnt factors,2 and they stop proliferation when they reach the top third of the crypt, probably because they are too far from the Wnt source. Meanwhile, they continue their migration movement and colonise the surface of the colon. After about a week, epithelial cells undergo apoptosis and are shed in the lumen of the gut. The Paneth cells constitute an exception, as they migrate downward and occupy the very bottom of the crypt. Thus, the epithelium of the colon is under perpetual renewal. Wnt growth factors activate a cascade of intracellular events, which is known as the canonical Wnt pathway that ultimately leads to the expression of a genetic program controlling the co‐ordinated expansion, fate and sorting of the epithelial cell population. In colorectal cancer, epithelial cells initially proliferate inappropriately because they acquired mutations in components of the pathway, thereby mimicking the effect of a permanent Wnt stimulation. Thus, the mutated cell recapitulates a progenitor‐like phenotype, independent of its position in the epithelium.3 Canonical Wnt signalling has received considerable attention from cancer researchers over the years, because of its essential role in the homeostasis of the colonic epithelium and its deregulation in colorectal cancer and other tumours. This review attempts to summarise briefly our current understanding of the pathway in relation to its role in colorectal cancer development and to introduce how current knowledge may be used for the development of new treatments against this disease.
Figure 1 Schematic representation of the colonic epithelium. The epithelium has a flat surface that forms invaginations at regular intervals called crypts. The stem cells produce permanently proliferating precursors that migrate and differentiate as enterocytes (the absorptive cells of the colon), enteroendocrine cells (hormone‐secreting cells), goblet cells (mucus‐secreting cells) or Paneth cells (secreting antimicrobial toxins).
Molecular mechanisms of the canonical Wnt signal transduction pathway
The canonical Wnt signalling cascade controls cell behaviour by activating the transcriptional properties of DNA‐binding proteins of the T cell factor/lymphoid enhancer factor‐1 family (referred to as “TCF” below; fig 2). In the absence of Wnt signalling, TCFs block expression of Wnt target genes. Wnt induces stabilisation of cytosolic β‐catenin, which associates with TCFs in the nucleus, leading to the expression of specific target genes. Wnt factors can trigger the activation of at least two other, β‐catenin‐independent, pathways. They are referred to as non‐canonical signalling and their implication in colorectal cancer, if any, is not yet known.4
Figure 2 The Wnt pathway in the “off and on” states. Off: signalling is kept in an off state either in the absence of Wnt or when Wnt factors are prevented to bind to the membrane‐bound receptors Frizzled (Frz) and coreceptors low‐density lipoprotein receptor‐related protein (LRP). This is achieved either by the extracellular Wnt‐titrating inhibitors SFRPs (secreted Frz‐related peptides), WIF‐1 (Wnt inhibitory factor‐1) and Cerberus‐1(CER1), or by specific members of the Dickkopf (Dkk) family that stimulate the Kremen‐dependent endocytosis of LRP. In the cytoplasm, β‐catenin is tagged by phosphorylation (P) in the destruction complex containing the core components adenomatous polyposis coli (APC), axin/conductin and the kinases casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β). β‐Transducin repeat‐containing protein (βTrCP) targets phosphorylated β‐catenin to the proteasome where it is degraded. In the nucleus, the Wnt target genes are kept silent by the repressor Groucho interacting with DNA‐bound T cell factor (TCF). APC is thought to actively export β‐catenin. The inhibitors, inhibitor of β‐catenin (ICAT) and Chibby, can also repress β‐catenin activity in the nucleus. The occupancy of Frz and LRP by Wnt triggers the phosphorylation of the cytoplasmic tail of LRP by CK1 and GSK3β and the dishevelled (Dsh)‐dependent recruitment of axin on to phosphorylated LRP. In the nucleus, transcription of Wnt target genes is initiated by the displacement of Groucho, the interaction of β‐catenin with TCF and the recruitment of B cell lymphoma 9 (Bcl9), Pygopus, the mixed‐lineage leukaemia (MLL) methyltransferases, APC, C‐terminal‐binding protein (CtBP) and βTrCP.
In the absence of Wnt: the destruction complex
The levels of cytoplasmic β‐catenin are normally controlled by a multiprotein destruction complex that targets β‐catenin for degradation in proteasomes.5,6 This complex is assembled over the scaffold component axin or its homologue conductin, which contain binding domains for β‐catenin, the tumour suppressor adenomatous polyposis coli (APC), the kinases GSK3β and CK1α/ε (casein kinase 1α/ε). The main function of the destruction complex is to promote phosphorylation of β‐catenin, which is required to trigger ubiquitination of β‐catenin and its subsequent degradation in proteasomes. The phosphorylation sites are contained in the N‐terminal domain of β‐catenin and are hotspots for mutations in tumours (see below).
In the presence of Wnt
Wnts are glycoproteins7,8 where secretion is controlled specifically by the transmembrane protein Wntless/evenness interrupted.9,10 The production of active Wnt requires also a functional retromer, a multiprotein complex involved in intracellular protein trafficking.11 Only few Wnts activate the canonical Wnt/β‐catenin pathway. They bind to transmembrane receptors frizzleds and to coreceptors low‐density lipoprotein receptor‐related protein (LRP)‐5 and LRP‐6, which are essential for signal transmission. The interaction of Wnts with Frz receptors can be modulated by a number of secreted factors, which act as antagonists by binding to either Wnts or to the coreceptor LRP. The molecular events taking place immediately after activation of Frzs involve phosphorylation of LRP by GSK3β and CK1γ and the recruitment of axin to the plasma membrane in a manner dependent on the cytoplasmic component Dsh. This leads to the degradation of axin (reviewed by Cadigan and Liu4, and Tolwinski and Wieschaus12).
Stabilised β‐catenin enters the cell nucleus and associates with TCF/LEF transcription factors to activate the transcription of Wnt target genes. TCFs lack transactivation function and repress gene transcription in the absence of β‐catenin by interacting with Groucho corepressors. The transcriptional activation function is contributed by β‐catenin, which lacks any DNA‐binding activity.13,14 The transcriptional control exerted by TCF/β‐catenin complexes is under stringent positive and negative regulation mediated by various interacting factors, such as Bcl9, Pygopus, mixed‐lineage leukaemia histone methyltransferases, APC, Chibby, inhibitor of β‐catenin (ICAT), C‐terminal‐binding protein (CtBP) and components of chromatin‐remodelling complexes15 that are promising candidates to be tested for a putative role in tumorigenesis.
In vivo significance of the Wnt pathway
Several in vivo experiments in mice have shown that the Wnt pathway is essential for the maintenance of the proper architecture of the intestinal epithelium and that normal proliferation of the precursor cells depends on permanent activation of the pathway. Nuclear β‐catenin accumulates at the bottom of normal crypts, indicating a permanent stimulation of the Wnt pathway.2 Transgenic expression of the Wnt inhibitor Dickkopf1 (Dkk1) results in the loss of crypts in the intestine.16 Similarly, crypts are also lacking in the intestine of mice deficient for either Tcf417 or β‐catenin.18 In contrast, stimulation of the pathway through mutation of the negative regulator APC19,20,21 provokes the hyperproliferation of the epithelium. Similarly, mice transgenic for a constitutively stabilised form of β‐catenin develops intestinal adenomas.22 Thus, these studies highlight the importance of adequate Wnt signalling for the normal physiology of the intestine.
A large list of Wnt target genes has been compiled by investigations in model organisms and cellular systems (http://www.stanford.edu/∼rnusse/ for an updated list). Up to now, only few of these target genes have been shown to mediate the effects of Wnt signalling in vivo and to have an active role in colorectal cancer development when the pathway is aberrantly active. For example, the promoter of the proto‐oncogene c‐jun gene is controlled by TCF/β‐catenin complexes. In vivo, APC inactivation and simultaneous genetic ablation of c‐jun reduced the number and size of tumours.23 Cyclin D1 was initially reported as a β‐catenin target gene.24,25 However, conditional inactivation of APC in vivo showed that cyclin D1 is not important for the very early stages of intestinal tumorigenesis.26 Nevertheless, cyclin D1 becomes deregulated as a secondary event and it is an important factor for adenoma establishment and growth.27,28 Several proteases capable of degrading the extracellular matrix such as matrilysin/matrix metalloproteinase 7 were identified as Wnt targets.29,30 The matrix metalloproteinase matrilysin has an effect on cancer progression as deficiency of matrilysin in APC−/− mice resulted in a decrease of total tumour number and size.30 T lymphoma invasion and metastasis 1 (Tiam 1), a guanine nucleotide exchange factor for the Rac GTPase involved in cell adhesion and motility is also a Wnt‐responsive gene, expressed in the base of intestinal crypts and upregulated in mouse intestinal tumours and human colon adenomas. Tiam 1 deficiency was shown to considerably reduce the formation and growth of polyps in mice.31
TCFs control cell differentiation in the gut as shown by the finding that EphB2 (ephrin B2) and EphB3 receptors were downregulated by dominant‐negative TCF while their ligand EphB1 was upregulated. Further analysis in EphB knock‐out mice showed that this differential regulation served to prevent the intermingling of cells within the intestinal epithelium.2 This role in sorting out of cells could be of relevance for the development of intestinal adenomas as seen in APC−/− mice because mutant APC cells invaginate around the crypt‐villus junction and avoid migration into the region of high EphB expression at the top of the crypt. Surprisingly, EphB receptor expression was recently shown to be lost at the adenoma–carcinoma transition, suggesting a role in the suppression of cancer progression.32
More candidate Wnt target genes have been identified and their relevance for colon cancer tumorigenesis will be delineated in vivo using mouse models. Some of them will be of particular functional significance for tumour development and will represent good targets for future therapeutic investigations. We shall see now that aberrant activation of the pathway, as it occurs in colorectal cancer, results almost invariably through mutation of the two key regulators APC and β‐catenin.
APC mutations
Mutations of APC occur in a high proportion of sporadic colorectal carcinomas (up to 80%). Mutations of APC were first identified in the germline of patients with familial adenomatous polyposis (FAP, fig 3). These patients develop hundreds of polyps in the colon after inactivation of the remaining wild‐type allele revealing that APC behaves as a classical tumour suppressor.33 There are different phenotypes and thus different subclasses of FAP‐associated tumours which correlate with the position of the mutations in the APC gene.34 As APC mutations are detected very early in the adenoma–carcinoma sequence, it has been suggested that functional loss of APC is a prerequisite for progression towards malignancy.33 Indeed, several studies have shown that APC inactivation in vivo in mice is sufficient to initiate tumour development.19,20,21
Figure 3 Schematic structure of the adenomatous polyposis coli (APC) protein and distribution of APC mutations found in patients with familial adenomatous polyposis. (A) The 2843‐amino acid sequence displays an armadillo domain near the N terminus. Approximately the central third of the molecule is sufficient to target β‐catenin for degradation. This region contains different motifs that are repeated and dispersed along the sequence. The 15‐amino‐acid repeats (yellow) can bind β‐catenin, but their functional significance is still obscure. The 20‐amino‐acid repeats (green) can bind β‐catenin with a very high affinity when they are phosphorylated. The SAMP (serine–alanine–methionine–proline) repeats (red) represent docking sites for axin/conductin. Both the latter motifs are crucial for efficient degradation of β‐catenin, because they bring β‐catenin and axin/conductin in close proximity. The mutation cluster region (MCR) delineates the region where most mutations associated with either familial polyposis or sporadic colorectal cancer have been found. These mutations interrupt the open reading frame and result in the production of truncated APC fragments lacking roughly the C‐terminal half. As an example, the structure below shows truncated APC in the SW480 colon cancer cell line. (B) The frequency of germ line and somatic mutations from patients with FAP is shown as a function of the position of the mutation in the APC sequence. The scheme was taken from.6
The central region of the 2843 amino acid APC protein is most directly implicated in Wnt signalling6 (fig 3). It contains three 15‐amino‐acid repeats and seven 20‐amino‐acid repeats that all bind to β‐catenin. Unlike the 20‐amino‐acid repeats the 15‐amino‐acid repeats are not essential for down regulation of β‐catenin. Three so‐called SAMP repeats containing the core sequence serine–alanine–methionine–proline interact with the axin proteins.35
In colorectal tumours both alleles at the APC locus are affected, either by point mutations or through whole allele deletions. Point mutations of the APC gene occur mostly (about 60%) in a mutation cluster region (MCR) the 3′ end of which is located closely upstream to the sequence encoding the first SAMP repeat (fig 3). These mutations generate stop codons or frameshifts leading to the deletion of the C‐terminal half of the APC protein. The sequence of inactivating mutations in both APC alleles is not random (fig 4). A general rule, though not absolute, emerged from the detailed analysis of a large cohort of cases with FAP.34 If the first mutational hit occurs before the MCR or deletes the whole allele, then the second mutation will target the MCR. Inversely, if the first allele is mutated inside the MCR, the second mutational hit will either delete the second allele or will occur either inside or before the MCR. Thus, there is a strong selection for the removal of the C terminus of APC and more precisely for the elimination of the SAMP repeats located just downstream of the MCR. Experimental evidence indicates that mice expressing an APC molecule truncated just after the first SAMP repeat do not develop tumours.36 Thus, it is commonly accepted that the consequence of APC mutations is the removal of the interaction sites with axin/conductin,6 leading to the failure of assembling a functional destruction complex, which ultimately results in the constitutive stabilisation of β‐catenin.
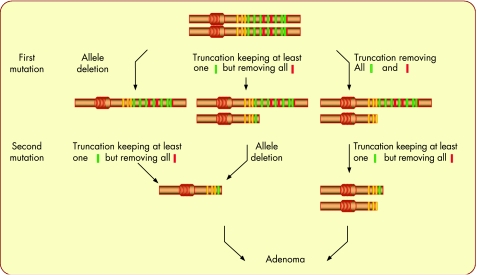
Figure 4 Schematic representation of the sequence of the mutational events affecting the adenomatous polyposis coli (APC) locus and leading to the development of colorectal adenomas. The colour code is the same as in fig 3. The scheme explains that in most cases, the combination of the two consecutive mutational events affecting the APC locus is not random and only permissive for the development of an adenoma when all SAMP repeats (red) have been removed, but at least one 20‐amino‐acid repeat (green) has been kept in the resulting structures. Any other combination is unlikely to provoke the emergence of an adenoma. Truncations result from mutations provoking premature termination of the open reading frame.
The truncated APC also lacks several nuclear export sequences (NESs), and the 3′ border of the MCR is located just upstream of a NES. Besides its function in regulating β‐catenin degradation APC appears to be involved in shuttling β‐catenin from the nucleus to the cytoplasm thereby avoiding its nuclear accumulation and interfering with its transcriptional activity.37 It has been proposed that this function of APC is important for its role as a tumour suppressor and that lack of β‐catenin shuttling through deletion of the NES contributes to the aberrant activation of the Wnt pathway in tumours.38 However, the APC‐dependent β‐catenin shuttling was challenged in a recent study showing that APC was influencing β‐catenin movements more by a passive retention mechanism in the cytoplasm than by an active nuclear export process.39
Hypermethylation of the wild‐type APC allele, and thus inhibition of expression are also found in some sporadic colorectal cancer and may constitute an alternative mechanism for APC gene inactivation.40 Indeed, mice carrying hypomorphic alleles of APC have been engineered and a reduction of APC to 15% of its normal level was sufficient to elicit the development of tumours.41 However, the analysis of APC mutations in FAP indicates that expression silencing through hypermethylation or deletion of both APC alleles is a very rare event.42 In contrast, colorectal cancer cells retain almost invariably a truncated APC product, probably because it fulfils a vital function crucial for the development of the tumour. Indeed, the reduction of the amount of APC to a very low level in a colorectal cancer cell line was shown to hamper proliferation and to inhibit DNA replication.43 Other functions associated with truncated APC are also documented. Thus, truncated APC fragments stimulate colorectal cancer cell migration44 and generate chromosomal instability.45
The 5′ border of the MCR is located just after the first 20‐amino‐acid repeat, reflecting a strong selective pressure for the retention of this repeat in the truncated APC fragment (fig 3 and fig 4). Thus, colorectal cancer cells from patients with FAP almost invariably express at least one truncated APC product containing the first 20‐amino‐acid‐repeat. The reason for this retention is not clear, but the strong selection for the presence of the repeat in truncated APC indicates that it must fulfil an important function.
β‐catenin and axin2/conductin mutations
β‐Catenin is mutated in a small percentage of colorectal carcinomas (around 10%). Most of the β‐catenin mutations preclude phosphorylation of the protein at the sites required for its degradation and constitutively stabilise the protein.6 In most cases, APC and β‐catenin mutations are linked to an increase of transcriptionally active β‐catenin and are mutually exclusive, reflecting their role in a common pathway.46 For instance, colon tumours with mutations in APC have a wild‐type β‐catenin gene, and vice versa, tumours with mutations in β‐catenin have wild‐type APC.
Mutations of β‐catenin have been mainly detected in microsatellite instabile colorectal tumours.47 These tumours are characterised by either sporadic or inherited mutations in DNA mismatch repair components. It appears that mutations in APC occur less frequently in these tumours than in mismatch repair proficient cases. Mismatch repair‐deficient colorectal cancer also show heterozygotic mutations in the axin2/conductin gene which resulted in truncated protein, possibly with a dominant‐negative action.48 A familial form of axin2 mutations was found to predispose to colorectal cancer and tooth agenesis.49
Cell–cell adhesion connection to Wnt signalling
It is well established from many studies that disturbances of cell junctions is a prerequisite for tumour invasion and metastasis.50,51 β‐catenin is also involved in the control of cell–cell adhesion by binding in a dynamic way to cadherin cell adhesion molecules and α‐catenin, thereby providing a link to the actin cytoskeleton (fig 5).52,53 The interactions of β‐catenin to TCF and E‐cadherin are mutually exclusive.54 It is not yet clear as to what extent the cell adhesion function of β‐catenin has a role in Wnt signalling,55 but a recent model describes how E‐cadherin‐bound β‐catenin can be released to contribute to transcription56 (fig 5). It was shown that the interactions of β‐catenin with α‐catenin and E‐cadherin are disturbed by tyrosine phosphorylation of β‐catenin on Tyr142 and Tyr654, respectively. In addition, phosphorylation on Tyr142 promotes binding of β‐catenin to the nuclear cofactor Bcl9‐2, thereby contributing to enhanced transcription of Wnt target genes. Deregulation of the tyrosine kinases phosphorylating β‐catenin or excessive expression of Bcl9‐2, as it has been shown in colorectal tumour samples, would promote transcription at the expense of cell–cell adhesion, both outcomes going toward malignancy. The same consequences may occur after loss of E‐cadherin expression. Interestingly, in primary colorectal tumours, cells located at the centre of the tumour display a polarised epithelial organisation with adherens junctions containing β‐catenin and E‐cadherin, whereas cells located at the periphery of the tumour are characterised by loss of junctional E‐cadherin and enrichment of nuclear β‐catenin.29
Figure 5 Connection of adherens junction to β‐catenin‐dependent transcription. In epithelial cells, the C terminus of β‐catenin is bound to E‐cadherin whereas the N terminus interacts with α‐catenin itself connected to the actin cytoskeleton. Phosphorylation by the tyrosine kinases c‐src, Fyn, Fer or Met at the indicated residues disrupts the interaction of β‐catenin with E‐cadherin and α‐catenin, respectively. Phosphorylation at Tyr142 creates a docking site for the nuclear cofactor B cell lymphoma 9‐2 (Bcl9‐2) that cooperates with β‐catenin for the transcription of Wnt target genes. TCF, T cell factor.
Other tumour‐related functions of APC
APC mutations are far more frequent than β‐catenin mutations in colorectal cancer. More cellular functions related to tumour progression have been assigned to APC in addition to its role in controlling cell proliferation, which may explain why APC is such a potent tumour suppressor. At the molecular level, the additional functions attributed to APC may mechanistically unify around its ability to bind to microtubules,57 stimulate their polymerisation,58 stabilise them59,60 and link them to the cell cortex.61,62,63 The functional interaction between APC and microtubules is thought to control cell migration, polarised cell division and chromosome segregation (fig 6).
Figure 6 Functions attributed to adenomatous polyposis coli (APC) and interacting partners (A). APC, represented by the red disc, controls polarised cell division (asymmetric stem cell division and symmetric epithelial cell division), migration, axonal growth and chromosomal segregation. These functions may reflect a common molecular mechanism, the control of microtubules dynamics. The bold D indicates a dominant function of truncated APC over full‐length APC. (B) APC interacts with several proteins involved in the regulation of cytoskeleton dynamics, which have been linked to either cell migration (blue) or polarised cell division (green). ASEF, APC‐stimulated guanine nucleotide exchange factor; hDLG, human Disc Large; IQGAP1, IQ domain‐containing, GTPase‐activating protein; mDIA, diaphanous; KAP3, kinesin super family‐associated protein 3; EB1, end binding 1.
APC and cell migration
Initially, it was shown that overexpression of full‐length APC in the mouse intestine causes disordered cell migration.64 Later, directed cell migration was shown to require the recruitment of APC to the leading edge of cells as they reorient in the direction of migration.65 The armadillo repeat domain of APC interacts with the APC‐stimulated guanine nucleotide exchange factor (ASEF) thereby activating Rac. The interaction of mutated but not wild‐type APC with ASEF was shown to promote migration of epithelial cells, suggesting a role of ASEF in tumour invasion and metastasis.44 A further evidence for a role of APC in cell migration was provided by the discovery of functional interactions with diaphanous that stabilise microtubules60 and with IQ domain‐containing, GTPase‐activating protein (IQGAP1) that links actin dynamics with microtubule stability. 66 In addition, APC has been implicated as an active player in the growth of axons.67 It seems clear that APC is required for proper cell migration, but the mechanisms involved are still in their infancy and it is not yet known how this function is related to colorectal cancer progression.
APC and polarised cell division
APC may be involved in polarised cell division (fig 6A). For epithelial cells, the plane of cell partitioning is perpendicular to the plane of the epithelium and it allows two daughter cells to align correctly in the epithelium without disturbing its flat surface. This type of cell division is called symmetrical epithelial division and its effects are reflected in the architecture of the epithelium, like the one covering the surface of the colon. Polarisation of cell division is also crucial for asymmetric cell division, the process that keeps one daughter cell in a stem cell pool after mitosis, while the second daughter cell joins the pool of differentiating cells. In the crypt of the colon, stem cell division has to be asymmetric, as one daughter cell remains in the stem cell pool. In both types of cell division, the plane of partitioning is dictated by the orientation of the microtubule spindle during mitosis and is represented by the metaphase equatorial plate. The spindle is thought to be anchored to the cell cortex at sites of cell–cell contact, the adherens junctions that emerged as essential components of the machinery.68
There are several lines of evidence indicating that APC contributes to the correct orientation of the mitotic spindle. In Drosophila, APC is recruited to the adherens junctions69 and spindle orientation and centrosome positions are perturbed on its deletion.61,62,70 As a result, epithelial cells and stem cells failed to carry out symmetric division along the planar axis and asymmetric division, respectively. APC can also bind to several components for which a role in spindle orientation has been shown. APC interacts with the microtubule‐binding protein EB1 that is required for symmetric epithelial division in Drosophila.70 APC interacts also with human Disc Large and human Scribble.71 In Drosophila, Dlg (Disc Large) and Scrib (Scribble) contribute to mitotic spindle orientation, regulate asymmetric cell division72 and their mutation results in a multilayered epithelium.73 Human Scribble depletion was shown to disrupt the adherens junctions74 and to relocalise APC.71 Most of the experiments linking APC to the control of mitotic spindle orientation were performed in Drosophila. However, a recent challenging study showed that in Drosophila embryos, null for both APC isoforms, neither spindle morphology or orientation, nor selection of the cell division plane were substantially affected.75 Despite these discrepancies, it remains that an implication of APC in polarised cell division in the colon would be an important contributing factor to tumorigenesis.
APC and chromosomal instability
APC has been localised not only to the mitotic spindle but also to kinetochores, the structures that link the microtubules of the spindle to the chromosomes. Mutation of APC in embryonic stem cells was associated with defects in chromosome segregation.76,77 Thus, loss of APC could lead to chromosomal instability and thereby promote cancer progression.
Interestingly, several other components of the Wnt pathway were also shown to be associated with microtubules and the mitotic spindle apparatus. Similar to APC, Dsh and axin were shown to cooperate in the control of microtubule stability.78 Also, both GSK3 and β‐catenin were found to be associated with the mitotic spindle and depletion of β‐catenin79 or inhibition of GSK activity80 led to disturbances of the mitotic spindle. Taken together it appears that the members of the β‐catenin destruction complex reunite at microtubules and, in particular, at the mitotic spindle. Whether this reflects a new functional property of the complex connected to the Wnt pathway remains to be determined. Obviously, localisation of these components at a delicate structure such as the mitotic spindle might have functional consequences for cancer development.
Therapeutic principles
The high prevalence of mutations activating the Wnt pathway in colorectal cancer makes it an attractive target for a pharmacological intervention aimed at inhibiting the activity of β‐catenin. The validity of this approach has been confirmed in several studies where interference with TCF/β‐catenin signalling led to inhibition of colorectal cancer cell proliferation. Thus, expression of either E‐cadherin81 or a dominant‐negative TCF mutant82 was sufficient to arrest cell growth, apparently by interfering with β‐catenin signalling. Similarly, knock‐down of β‐catenin by targeted inactivation83 or anti‐sense treatment84,85 inhibited the growth of colon carcinoma in mice. These examples provide proof of principle that interference with aberrant Wnt signalling could be of therapeutical value. Various strategies that are reviewed elsewhere86 have been envisaged to inhibit the activity of β‐catenin. Here, we will highlight only some of the most recent achievements that may be of therapeutic value.
There are several already established drugs previously known to be effective in the treatment of colorectal cancer that turned out later to target the Wnt pathway. Thus, treatment with non‐steroidal anti‐inflammatory drugs (NSAIDs) diminishes colorectal cancer incidence and mortality.87 The anticancer activity of NSAIDs is partly attributed to their ability to inhibit the activity of cyclooxygenases, key enzymes of prostaglandin synthesis. A recent study showed that even in the absence of a functional APC, the prostaglandin E2 (PGE2) can stimulate the proliferation of colon cancer cells by activating β‐catenin.88 Although molecular details remain unsolved, the signalling pathway initiated by PGE2 converges with the Wnt pathway at the level of axin, thus establishing a direct molecular link between the Wnt pathway and an inflammatory response initiated by PGE2. This study may provide a framework for the rational use of NSAIDs in the therapy of colon cancer.
The use of Wnt antagonists or other inhibitors that interfere with cell surface interactions of Wnts and their receptors could surprisingly provide a target for therapeutic intervention. Recently, a family of secreted Frz‐related proteins was identified that can compete with Frz for the Wnt ligands. The corresponding genes were preferentially hypermethylated, and therefore silenced in approximately 90% of human colon cancers tissues. It was also established that the Wnt pathway activated through mutation of β‐catenin or APC could be partially suppressed by these upstream ligand competitors.89 Thus, it may be possible to suppress Wnt signalling and therefore the tumour phenotype even in cancer cells containing APC or β‐catenin mutations, using components acting upstream in the pathway. Two other extracellular Wnt inhibitors, Dkk1 and Wnt inhibitory factor‐1, were also shown to be silenced by promoter hypermethylation in colon tumours and Dkk1 could inhibit the growth of tumours in mice despite the presence of a truncated APC.90
The identification of β‐catenin/TCF target genes expressed in colon cancer cells may offer novel opportunities for developing therapeutics against targets with critical role in colon cancer. A pertinent example lies in the discovery of histone deacetylase 2 (HDAC2) as a Wnt target gene. Increased HDAC2 expression is found in the majority of human colon cancer explants and its inhibition by valproic acid largely reduces adenoma formation in mice.67 These results therefore suggest that HDAC2 may be a particularly relevant target for therapy. Similarly, activation of the Wnt pathway upregulates the c‐myc proto‐oncogene91 and leads to alterations of c‐myc‐regulated genes, including ornithine decarboxylase (ODC), the first enzyme in polyamine synthesis. ODC has been associated with a risk of colorectal cancer. Selective inhibitors of ODC have been developed and one of them, difluoromethylornithine is under clinical evaluation as a preventive agent for colorectal cancer.92 As an additional example illustrating the importance of identifying the β‐catenin target genes for therapeutic purposes, the transcription factor SOX9 (sex‐determining region on the Y chromosome (Sry)‐related HMG box‐9) was shown to be expressed in the intestinal epithelium in a manner dependent on β‐catenin and TCF4 and in a pattern characteristic of Wnt targets.93 SOX9 transcriptionally represses another transcription factor, namely caudal‐related homeobox 2, which expression is reduced in most colorectal cancer.94 In parallel, it was shown that caudal‐related homeobox 2 deficiency results in chromosomal instability mediated by activation of the target of rapamycin (TOR) pathway.95 As chromosomal instability is a hallmark of colorectal cancer containing APC mutations, these results may make it possible to treat colorectal cancer with rapamycin derivatives or other inhibitors of the TOR pathway.
Since its discovery, RNA interference has become the method of choice to suppress gene expression in vitro. Small interfering RNAs (siRNAs), the mediators of RNA interference, are small double‐stranded RNAs that bind specifically to, and promote the degradation of a specific RNA, resulting in the silencing of the expression of a specific target gene. RNA interference is also emerging as a powerful tool for in vivo research and some reports have already shown the potential of siRNA as therapeutic agents in cancer.96 Moreover, gene silencing was achieved in response to systemic delivery of siRNA in monkeys.97 A proof of principle was obtained in the colon cancer field when it was shown that the survival of nude mice was greatly prolonged when they were implanted with adenocarcinoma cells pretreated with a siRNA against β‐catenin.98 In their experiments, the authors used an siRNA recognising wild‐type β‐catenin, but the specificity of RNA interference allows to discriminate a mutated variant from its wild‐type counterpart.99 Thus, it may be possible to target the stabilised isoforms of β‐catenin without altering the expression of wild‐type β‐catenin, known to fulfil important regulatory roles in many other tissues than the colon, in addition to its involvement in cell–cell adhesion. Similarly, the same logic may apply to mutant APC, as it was shown that down regulation of APC by RNA interference in a colon cancer cell line was inhibiting cell proliferation.43 Despite many problems linked to delivery in vivo, off‐target effects and toxicity100 technological enhancements may improve the efficacy of RNA interference as a reagent for clinical applications.101
Abbreviations
APC - adenomatous polyposis coli
ASEF - APC‐stimulated guanine nucleotide exchange factor
Bcl9‐2 - B cell lymphoma 9‐2
CK1 - casein kinase 1
Dkk1 - Dickkopf1
Dsh - dishevelled
Eph - ephrin
FAP - familial adenomatous polyposis
Frz - Frizzled
GSK3β - glycogen synthase kinase 3β
HDAC2 - histone deacetylase 2
-
LRP - low‐density lipoprotein receptor‐related protein
MCR - mutation cluster region
NES - nuclear export sequence
NSAIDs - non‐steroidal anti‐inflammatory drugs
ODC - ornithine decarboxylase
PGE2 - prostaglandin E2
SAMP - serine‐alanine‐methionine‐proline
siRNA - small interfering RNA
TCF - T cell factor
Footnotes
Competing interest: None.
References
- 1.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 2005434843–850. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H, Batlle E. EphB/EphrinB receptors and Wnt signaling in colorectal cancer. Cancer Res 2006662–5. [DOI] [PubMed] [Google Scholar]
- 3.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 2005306357–363. [DOI] [PubMed] [Google Scholar]
- 4.Cadigan K M, Liu Y I. Wnt signaling: complexity at the surface. J Cell Sci 2006119395–402. [DOI] [PubMed] [Google Scholar]
- 5.Seidensticker M J, Behrens J. Biochemical interactions in the Wnt pathway. Biochim Biophys Acta 20001495168–182. [DOI] [PubMed] [Google Scholar]
- 6.Polakis P. Wnt signaling and cancer. Genes Dev 2000141837–1851. [PubMed] [Google Scholar]
- 7.Cadigan K M, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev 1997113286–3305. [DOI] [PubMed] [Google Scholar]
- 8.Miller J R. The Wnts. Genome Biol 2002330001.1–3000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banziger C, Soldini D, Schutt C.et al Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 2006125509–522. [DOI] [PubMed] [Google Scholar]
- 10.Bartscherer K, Pelte N, Ingelfinger D.et al Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 2006125523–533. [DOI] [PubMed] [Google Scholar]
- 11.Coudreuse D Y, Roel G, Betist M C.et al Wnt gradient formation requires retromer function in Wnt‐producing cells. Science 2006312921–924. [DOI] [PubMed] [Google Scholar]
- 12.Tolwinski N S, Wieschaus E. Rethinking WNT signaling. Trends Genet 200420177–181. [DOI] [PubMed] [Google Scholar]
- 13.Behrens J, von Kries J P, Kuhl M.et al Functional interaction of beta‐catenin with the transcription factor LEF‐1. Nature 1996382638–642. [DOI] [PubMed] [Google Scholar]
- 14.Molenaar M, van de Wetering M, Oosterwegel M.et al XTcf‐3 transcription factor mediates beta‐catenin‐induced axis formation in Xenopus embryos. Cell 199686391–399. [DOI] [PubMed] [Google Scholar]
- 15.Sierra J, Yoshida T, Joazeiro C A.et al The APC tumor suppressor counteracts beta‐catenin activation and H3K4 methylation at Wnt target genes. Genes Dev 200620586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhnert F, Davis C R, Wang H T.et al Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf‐1. Proc Natl Acad Sci U S A 2004101266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korinek V, Barker N, Moerer P.et al Depletion of epithelial stem‐cell compartments in the small intestine of mice lacking Tcf‐4. Nat Genet 199819379–383. [DOI] [PubMed] [Google Scholar]
- 18.Ireland H, Kemp R, Houghton C.et al Inducible Cre‐mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta‐catenin. Gastroenterology 20041261236–1246. [DOI] [PubMed] [Google Scholar]
- 19.Oshima M, Oshima H, Kitagawa K.et al Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci U S A 1995924482–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata H, Toyama K, Shioya H.et al Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 1997278120–123. [DOI] [PubMed] [Google Scholar]
- 21.Sansom O J, Reed K R, Hayes A J.et al Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 2004181385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada N, Tamai Y, Ishikawa T.et al Intestinal polyposis in mice with a dominant stable mutation of the beta‐catenin gene. EMBO J 1999185931–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nateri A S, Spencer‐Dene B, Behrens A. Interaction of phosphorylated c‐Jun with TCF4 regulates intestinal cancer development. Nature 2005437281–285. [DOI] [PubMed] [Google Scholar]
- 24.Shtutman M, Zhurinsky J, Simcha I.et al The cyclin D1 gene is a target of the beta‐catenin/LEF‐1 pathway. Proc Natl Acad Sci U S A 1999965522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tetsu O, McCormick F. Beta‐catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999398422–426. [DOI] [PubMed] [Google Scholar]
- 26.Sansom O J, Reed K R, van de Wetering M.et al Cyclin D1 is not an immediate target of beta‐catenin following Apc loss in the intestine. J Biol Chem 200528028463–28467. [DOI] [PubMed] [Google Scholar]
- 27.Hulit J, Wang C, Li Z.et al Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice. Mol Cell Biol 2004247598–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilding J, Straub J, Bee J.et al Cyclin D1 is not an essential target of beta‐catenin signaling during intestinal tumorigenesis, but it may act as a modifier of disease severity in multiple intestinal neoplasia (Min) mice. Cancer Res 2002624562–4565. [PubMed] [Google Scholar]
- 29.Brabletz T, Jung A, Dag S.et al beta‐catenin regulates the expression of the matrix metalloproteinase‐7 in human colorectal cancer. Am J Pathol 19991551033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford H C, Fingleton B M, Rudolph‐Owen L A.et al The metalloproteinase matrilysin is a target of beta‐catenin transactivation in intestinal tumors. Oncogene 1999182883–2891. [DOI] [PubMed] [Google Scholar]
- 31.Malliri A, Rygiel T P, van der Kammen R A.et al The rac activator Tiam1 is a Wnt‐responsive gene that modifies intestinal tumor development. J Biol Chem 2006281543–548. [DOI] [PubMed] [Google Scholar]
- 32.Batlle E, Bacani J, Begthel H.et al EphB receptor activity suppresses colorectal cancer progression. Nature 20054351126–1130. [DOI] [PubMed] [Google Scholar]
- 33.Kinzler K W, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 199687159–170. [DOI] [PubMed] [Google Scholar]
- 34.Fearnhead N S, Britton M P, Bodmer W F. The ABC of APC. Hum Mol Genet 200110721–733. [DOI] [PubMed] [Google Scholar]
- 35.Behrens J, Jerchow B A, Wurtele M.et al Functional interaction of an axin homolog, conductin, with beta‐catenin, APC, and GSK3beta. Science 1998280596–599. [DOI] [PubMed] [Google Scholar]
- 36.Smits R, Kielman M F, Breukel C.et al Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev 1999131309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbro M, Henderson B R. Regulation of tumor suppressors by nuclear‐cytoplasmic shuttling. Exp Cell Res 200328259–69. [DOI] [PubMed] [Google Scholar]
- 38.Rosin‐Arbesfeld R, Cliffe A, Brabletz T.et al Nuclear export of the APC tumour suppressor controls beta‐catenin function in transcription. EMBO J 2003221101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieghoff E, Behrens J, Mayr B. Nucleo‐cytoplasmic distribution of {beta}‐catenin is regulated by retention. J Cell Sci 20061191453–1463. [DOI] [PubMed] [Google Scholar]
- 40.Esteller M, Sparks A, Toyota M.et al Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res 2000604366–4371. [PubMed] [Google Scholar]
- 41.Li Q, Ishikawa T O, Oshima M.et al The threshold level of adenomatous polyposis coli protein for mouse intestinal tumorigenesis. Cancer Res 2005658622–8627. [DOI] [PubMed] [Google Scholar]
- 42.Nathke I S. The adenomatous polyposis coli protein: the Achilles heel of the gut epithelium. Annu Rev Cell Dev Biol 200420337–366. [DOI] [PubMed] [Google Scholar]
- 43.Schneikert J, Behrens J. Truncated APC is required for cell proliferation and DNA replication. Int J Cancer 200611974–79. [DOI] [PubMed] [Google Scholar]
- 44.Kawasaki Y, Sato R, Akiyama T.et al Mutated APC and Asef are involved in the migration of colorectal tumour cells Asef, a link between the tumor suppressor APC and G‐protein signaling. Nat Cell Biol 20035211–215. [DOI] [PubMed] [Google Scholar]
- 45.Tighe A, Johnson V L, Taylor S S. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J Cell Sci 20041176339–6353. [DOI] [PubMed] [Google Scholar]
- 46.Morin P J, Sparks A B, Korinek V.et al Activation of beta‐catenin‐Tcf signaling in colon cancer by mutations in beta‐catenin or APC. Science 19972751787–1790. [DOI] [PubMed] [Google Scholar]
- 47.Kitaeva M N, Grogan L, Williams J P.et al Mutations in beta‐catenin are uncommon in colorectal cancer occurring in occasional replication error‐positive tumors. Cancer Res 1997574478–4481. [PubMed] [Google Scholar]
- 48.Liu W, Dong X, Mai M.et al Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta‐catenin/TCF signalling. Nat Genet 200026146–147. [DOI] [PubMed] [Google Scholar]
- 49.Lammi L, Arte S, Somer M.et al Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet 2004741043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev 19991815–30. [DOI] [PubMed] [Google Scholar]
- 51.Christofori G. Changing neighbours, changing behaviour: cell adhesion molecule‐mediated signalling during tumour progression. EMBO J 2003222318–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drees F, Pokutta S, Yamada S.et al Alpha‐catenin is a molecular switch that binds E‐cadherin‐beta‐catenin and regulates actin‐filament assembly. Cell 2005123903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada S, Pokutta S, Drees F.et al Deconstructing the cadherin‐catenin‐actin complex. Cell 2005123889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hulsken J, Birchmeier W, Behrens J. E‐cadherin and APC compete for the interaction with beta‐catenin and the cytoskeleton. J Cell Biol 19941272061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuphal F, Behrens J. E‐cadherin modulates Wnt‐dependent transcription in colorectal cancer cells but does not alter Wnt‐independent gene expression in fibroblasts. Exp Cell Res 2006312457–467. [DOI] [PubMed] [Google Scholar]
- 56.Brembeck F H, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta‐catenin. Curr Opin Genet Dev 20061651–59. [DOI] [PubMed] [Google Scholar]
- 57.Nathke I S, Adams C L, Polakis P.et al The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol 1996134165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munemitsu S, Souza B, Muller O.et al The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res 1994543676–3681. [PubMed] [Google Scholar]
- 59.Zumbrunn J, Kinoshita K, Hyman A A.et al Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol 20011144–49. [DOI] [PubMed] [Google Scholar]
- 60.Wen Y, Eng C H, Schmoranzer J.et al EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol 20046820–830. [DOI] [PubMed] [Google Scholar]
- 61.McCartney B M, McEwen D G, Grevengoed E.et al Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat Cell Biol 20013933–938. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita Y M, Jones D L, Fuller M T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 20033011547–1550. [DOI] [PubMed] [Google Scholar]
- 63.Reilein A, Nelson W J. APC is a component of an organizing template for cortical microtubule networks. Nat Cell Biol 20057463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong M H, Hermiston M L, Syder A J.et al Forced expression of the tumor suppressor adenomatosis polyposis coli protein induces disordered cell migration in the intestinal epithelium. Proc Natl Acad Sci U S A 1996939588–9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Etienne‐Manneville S, Hall A. Cdc42 regulates GSK‐3beta and adenomatous polyposis coli to control cell polarity. Nature 2003421753–756. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe T, Wang S, Noritake J.et al Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell 20047871–883. [DOI] [PubMed] [Google Scholar]
- 67.Zhou F Q, Zhou J, Dedhar S.et al NGF‐induced axon growth is mediated by localized inactivation of GSK‐3beta and functions of the microtubule plus end binding protein APC. Neuron 200442897–912. [DOI] [PubMed] [Google Scholar]
- 68.Perez‐Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell 2003112535–548. [DOI] [PubMed] [Google Scholar]
- 69.Bienz M, Hamada F. Adenomatous polyposis coli proteins and cell adhesion. Curr Opin Cell Biol 200416528–535. [DOI] [PubMed] [Google Scholar]
- 70.Lu B, Roegiers F, Jan L Y.et al Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature 2001409522–525. [DOI] [PubMed] [Google Scholar]
- 71.Takizawa S, Nagasaka K, Nakagawa S.et al Human scribble, a novel tumor suppressor identified as a target of high‐risk HPV E6 for ubiquitin‐mediated degradation, interacts with adenomatous polyposis coli. Genes Cells 200611453–464. [DOI] [PubMed] [Google Scholar]
- 72.Albertson R, Doe C Q. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol 20035166–170. [DOI] [PubMed] [Google Scholar]
- 73.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev 2004181909–1925. [DOI] [PubMed] [Google Scholar]
- 74.Qin Y, Capaldo C, Gumbiner B M.et al The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E‐cadherin. J Cell Biol 20051711061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCartney B M, Price M H, Webb R L.et al Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development 20061332407–2418. [DOI] [PubMed] [Google Scholar]
- 76.Fodde R, Kuipers J, Rosenberg C.et al Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol 20013433–438. [DOI] [PubMed] [Google Scholar]
- 77.Kaplan K B, Burds A A, Swedlow J R.et al A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol 20013429–432. [DOI] [PubMed] [Google Scholar]
- 78.Ciani L, Krylova O, Smalley M J.et al A divergent canonical WNT‐signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol 2004164243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaplan D D, Meigs T E, Kelly P.et al Identification of a role for beta‐catenin in the establishment of a bipolar mitotic spindle. J Biol Chem 2004 [DOI] [PubMed]
- 80.Wakefield J G, Stephens D J, Tavare J M. A role for glycogen synthase kinase‐3 in mitotic spindle dynamics and chromosome alignment. J Cell Sci 2003116637–646. [DOI] [PubMed] [Google Scholar]
- 81.Stockinger A, Eger A, Wolf J.et al E‐cadherin regulates cell growth by modulating proliferation‐dependent beta‐catenin transcriptional activity. J Cell Biol 20011541185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van de Wetering M, Oving I, Muncan V.et al Specific inhibition of gene expression using a stably integrated, inducible small‐interfering‐RNA vector. EMBO Rep 20034609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan T A, Wang Z, Dang L H.et al Targeted inactivation of CTNNB1 reveals unexpected effects of beta‐catenin mutation. Proc Natl Acad Sci U S A 2002998265–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roh H, Green D W, Boswell C B.et al Suppression of beta‐catenin inhibits the neoplastic growth of APC‐mutant colon cancer cells. Cancer Res 2001616563–6568. [PubMed] [Google Scholar]
- 85.Green D W, Roh H, Pippin J A.et al Beta‐catenin antisense treatment decreases beta‐catenin expression and tumor growth rate in colon carcinoma xenografts. J Surg Res 200110116–20. [DOI] [PubMed] [Google Scholar]
- 86.Dihlmann S, von Knebel D M. Wnt/beta‐catenin‐pathway as a molecular target for future anti‐cancer therapeutics. Int J Cancer 2005113515–524. [DOI] [PubMed] [Google Scholar]
- 87.Koehne C H, Dubois R N. COX‐2 inhibition and colorectal cancer. Semin Oncol 200431(Suppl 7)12–21. [DOI] [PubMed] [Google Scholar]
- 88.Castellone M D, Teramoto H, Williams B O.et al Prostaglandin E2 promotes colon cancer cell growth through a Gs‐axin‐beta‐catenin signaling axis. Science 20053101504–1510. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki H, Watkins D N, Jair K W.et al Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 200436417–422. [DOI] [PubMed] [Google Scholar]
- 90.Aguilera O, Fraga M F, Ballestar E.et al Epigenetic inactivation of the Wnt antagonist DICKKOPF‐1 (DKK‐1) gene in human colorectal cancer. Oncogene 2006 [DOI] [PubMed]
- 91.He T C, Sparks A B, Rago C.et al Identification of c‐MYC as a target of the APC pathway. Science 19982811509–1512. [DOI] [PubMed] [Google Scholar]
- 92.Gerner E W, Ignatenko N A, Lance P.et al A comprehensive strategy to combat colon cancer targeting the adenomatous polyposis coli tumor suppressor gene. Ann N Y Acad Sci 2005105997–105. [DOI] [PubMed] [Google Scholar]
- 93.Blache P, van de Wetering M, Duluc I.et al SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol 200416637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mallo G V, Rechreche H, Frigerio J M.et al Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down‐regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int J Cancer 19977435–44. [DOI] [PubMed] [Google Scholar]
- 95.Aoki K, Tamai Y, Horiike S.et al Colonic polyposis caused by mTOR‐mediated chromosomal instability in Apc+/Delta716 Cdx2+/− compound mutant mice. Nat Genet 200335323–330. [DOI] [PubMed] [Google Scholar]
- 96.Leung R K, Whittaker P A. RNA interference: from gene silencing to gene‐specific therapeutics. Pharmacol Ther 2005107222–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zimmermann T S, Lee A C, Akinc A.et al RNAi‐mediated gene silencing in non‐human primates. Nature 2006441111–114. [DOI] [PubMed] [Google Scholar]
- 98.Verma U N, Surabhi R M, Schmaltieg A.et al Small interfering RNAs directed against beta‐catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res 200391291–1300. [PubMed] [Google Scholar]
- 99.Martinez L A, Naguibneva I, Lehrmann H.et al Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci U S A 20029914849–14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grimm D, Streetz K L, Jopling C L.et al Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006441537–541. [DOI] [PubMed] [Google Scholar]
- 101.Behlke M A. Progress towards in vivo use of siRNAs. Mol Ther 200613644–670. [DOI] [PMC free article] [PubMed] [Google Scholar]