Abstract
Background
Activated hepatic stellate cells (HSCs) are an attractive target for antifibrotic therapy based on their key role in extracellular matrix accumulation during liver injury.
Aim
: To develop a system for regulable and cell‐specific gene expression in HSCs to enable targeted delivery of therapeutic genes.
Method
Two types of recombinant adenoviral vectors were constructed, one expressing the Cre gene under the surveillance of specific promoters and the other containing a potent expression unit that was activated by Cre recombinase‐mediated recombination to remove an upstream lox‐flanked “stuffer” sequence, thereby amplifying the expression of downstream transgene of interest while maintaining specificity.
Results
When the promoter of the collagen 1A2 gene drove Cre recombinase expression in primary quiescent rat HSC, modest green fluorescence protein (GFP) expression was observed. However, in activated HSC, the collagen promoter effectively drove Cre recombinase activity, as assessed by the increased expression of GFP. In contrast, GFP expression was barely observed when the collagen promoter was expressed in hepatocytes. HSC‐specific expression of Smad7 considerably reduced the expression of type I collagen in culture and decreased fibrosis in two liver fibrosis models. Finally, to achieve targeted clearance of activated HSC in culture and in vivo, thymidine kinase was selectively expressed under the control of the collagen promoter, which conferred cell‐specific killing by ganciclovir leading to reduced fibrosis.
Conclusion
Our results show the potential utility of transcriptionally controlled gene therapy using a Cre/loxP system to ameliorate hepatic fibrosis in vivo.
Liver fibrosis is characterised by a reversible accumulation of extracellular matrix in hepatic parenchyma following chronic injury.1 Persistent fibrosis leads to cirrhosis, an end‐stage and irreversible illness, with life‐threatening complications. Thus, establishment of novel therapies against liver fibrosis is urgently needed.
In normal liver, hepatic stellate cells (HSCs) encircle the sinusoids as liver‐specific pericytes that store vitamin A‐associated lipid droplets.2 Following persistent liver injury, HSCs become activated, detach from the sinusoids, and subsequently undergo myofibroblastic transformation.3 This cellular process is characterised by a loss of lipid droplets, enhanced production of extracellular matrix including type I collagen, expression of smooth muscle‐α actin (αSMA) and platelet‐derived growth factor (PDGF) receptor, and proliferation in response to PDGF‐BB.4 Because HSCs have a crucial role in hepatic fibrogenesis, they are attractive targets for antifibrotic therapy. However, targeting of therapeutic molecules solely to this cell type in vivo remains a potential obstacle.
Adenoviral vectors can be used to effectively deliver genes to various cells and tissues.5 However, a major disadvantage is their lack of cell‐specific targeting. To overcome this problem, unique promoters can be utilised to regulate gene expression in a cell‐type‐specific manner. Although the magnitude of gene expression using specific promoters is generally low and may not be sufficient for effective gene therapy, expression can be augmented by using coinfection of recombinant adenoviruses equipped with a Cre/loxP system.6
HSCs are phenotypically related to fibroblasts, smooth muscle cells and also neural cells, based on their expression of type I collagen,7 desmin,8 vimentin,9 αSMA,10 glial fibrillar acidic protein (GFAP),11 neural cell adhesion molecule,12,13 nestin14 and synaptophysin.15 Based on these features, we have constructed recombinant adenoviruses that express Cre recombinase under the control of promoters of either type I collagen, desmin or GFAP to induce specific gene expression in HSC. We found that among these three promoters, type I collagen promoter is the most suitable for the regulation of exogeneous gene expression in activated HSC. Furthermore, we show here that adenovirus‐mediated overexpression of Smad7 or thymidine kinase (TK) under the control of type I collagen promoter attenuated fibrosis induced by bile duct ligation (BDL) or thioacetamide (TAA) treatment in rats.
Materials and methods
Animals
Pathogen‐free male Wistar rats were obtained from SLC (Shizuoka, Japan). Animals were housed at a constant temperature and had free access to laboratory chow and water ad libitum. Procedures were performed under the control of the animal care committee of Osaka City University in accordance with the Guideline on Animal Experiments in Osaka City University.
Construction of recombinant adenovirus vector and virus purification
We constructed two kinds of recombinant adenovirus vectors using the adenovirus Cre/loxP kit (TAKARA, Tokyo, Japan). The promoter adenovirus vectors of AxAw collagen (17COL)‐Cre, desmin‐Cre and GFAP‐Cre were constructed by the insertion of each promoter cDNA into the Swa I cloning site of cosmid pAxAwNCre. Collagen promoter (17COL) consists of an upstream enhancer region of a 1.5‐kb length between −17 kb and −15.5 kb from the transcription initiation site and the proximal promoter of pro type IA2 collagen.16,17 The mouse desmin promoter consists of a 4‐kb upstream region from −4005 to the transcription initiation site.18 The mouse GFAP promoter contains the promoter region between −2567 and +12.19 Cosmid pAxCALNL‐GFP, pAxCALNL‐LacZ and pAxCALNL‐Smad7 were constructed by the insertion of the green fluorescent protein (GFP) cDNA, LacZ cDNA and mouse Smad7 (mSmad7), respectively, into the Swa I cloning site of cosmid pAxCALNLw. Although cosmid pAxCALGL‐TK was constructed similarly by the insertion of thymidine kinase cDNA, cosmid pAxCALGL‐TK was additionally inserted GFP into cDNA between two loxP sites. GFP and LacZ were used to check the actual running of this Cre/loxP system in in vitro and in vivo experimental conditions. Using the COS‐TPC method,20 recombinant adenoviruses of COL‐Cre, desmin‐Cre, GFAP‐Cre, CAG‐Cre, LNL‐GFP, LNL‐LacZ, LNL‐mSmad7 and LGL‐TK were generated by transfecting the cosmids of individual adenoviruses into 293 cells as described in the manufacture's protocol. The titre of the recombinant adenoviruses was measured by the 50% tissue culture infectious dose (TCID50) method.21
Preparation of primary‐cultured HSC and hepatocytes and cell lines
HSC were isolated from male Wistar rats as previously described.22 Isolated HSC were plated at 5×105 cells/cm3. Hepatocytes were isolated from male Wistar rats (200 g) as previously described.23 The purity of isolated hepatocytes was 99% and their viability was approximately 95% as estimated by a trypan blue dye exclusion test. Hepatocytes were suspended at a density of 1.5×104 cells/cm3. LX‐2 was used as the human HSC line, and Huh7 and HepG2 were used as human hepatoma cell lines.
Adenoviral infection of cultured liver cells or cell lines
HSC and hepatocytes were infected with 50 multiplicity of infection (MOI = plaque‐forming unit (PFU)/cell number) of LNL‐GFP together with 50 MOI of 17COL‐Cre, CAG‐Cre, desmin‐Cre or GFAP‐Cre at day 1 of primary culture. GFP expression was observed at day 3. Activated HSC were treated similarly at day 7 of primary culture and observed at day 9. GFP expression was documented using an LSM 510 Laser Scanning Microscope (Zeiss, Oerkochen, Germany). Subconfluent LX‐2 cells were infected with 50 MOI of 17COL‐Cre or CAG‐Cre and 50 MOI of LNL‐mSmad7, LGL‐TK, LNL‐GFP or LNL‐LacZ.
Animal models
Two mechanistically distinct models of experimental cirrhosis were used in rats, either by TAA administration or by bile duct occlusion. A scheme of the experimental design is shown in fig 1B.
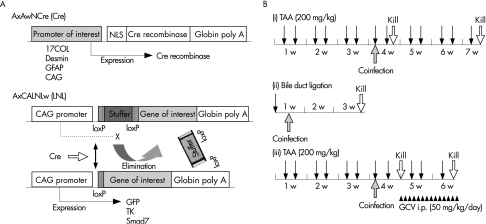
Figure 1 Scheme of recombinant adenoviruses and in vivo experimental protocol. (A) Constructs of recombinant adenoviral vectors. Recombinant adenoviral vector expresses the Cre gene under the control of specific promoters (17COL, Desmin, GFAP or cytomegalovirus enhancer, chicken β‐actin promoter and a part of 3′ untranslated region of rabbit β‐globin (CAG)). Another adenoviral vector contains a potent expression unit that is activated by Cre recombinase‐mediated removal of an upstream loxP‐flanked stuffer sequence. As a consequence, coinfection of these two types of adenoviral vectors to the cells induces transcription of the down stream transgene of interest (GFP, Smad7 and thymidine kinase). (B) Models of liver fibrosis. (i) Liver fibrosis was induced by intraperitoneal injection of TAA (200 mg/kg body weight) twice a week up to 7 weeks. Then, rats received totally 2×109 PFU of adenovirus vectors per rat via tail vein and were continuously injected with TAA for further few days, 1 week or 3 weeks. (ii) Bile duct ligation‐induced liver fibrosis was generated by ligating common bile duct for 3 weeks. Rats were received a single administration of total 2×109 PFU of adenovirus vectors per rat via tail vein at 4 days after the ligation of common bile duct. (iii) Rats were treated with TAA as in (i). Then, rats were intraperitoneally injected with ganciclovir (GCV) at a dose of 50 mg/kg/day for 4 days or 2 weeks. CAG, cytomegalovirus enhancer, chicken β‐actin promoter and a part of 3′ untranslated region of rabbit β‐globin; COL, collagen; GCV, ganciclovir; GFAP, glial fibrillar acidic protein; GFP, green fluorescent protein; LNL, loxP‐neo‐loxP.
Analyses of liver fibrosis
Fixed liver tissues were dehydrated and then embedded in Polybed. Sections were cut at a thickness of 4 μm. After deparaffinisation and hydration, the sections were stained for 1 h in 0.1% (w/v) Sirius red (Direct Red 80, Aldrich, Milwaukee, Wisconsin, USA) in a saturated aqueous solution (about 1.2% w/v) of picric acid (Wako, Osaka, Japan). After staining, the slides were rinsed for 30 min in 0.01 N HCl to remove unbound dye. After dehydration by an alcohol series, the slides were mounted and observed under a light microscope. For semiquantitative analysis of liver fibrosis, 10 fields from each slide were randomly selected, recorded and the red‐stained area per total area (mm2/mm2) was measured using Macscope Analyzer (Mitani Corporation, Fukui, Japan).
Immunofluorescence staining of apoptotic cells and αSMA
Immunofluorescence staining of apoptotic cells was performed by terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick‐end labelling (TUNEL) using the commercial ApopTag Peroxidase In Situ Oligo Ligation Apoptosis Detection Kit (CHEMICON, Temecula, California, USA). Staining of αSMA (Sigma Chemical, Saint Louis, Missouri, USA), a specific marker for activated stellate cells and myofibroblasts, was performed according to the previously described method.24 The sections were observed under a conventional fluorescent microscope and photographed using a cooled CCD camera (DP‐70, Olympus, Tokyo, Japan).
Assay of hydroxyproline content
To determine the amount of hydroxyproline, 100 mg of wet liver sample was subjected to acid hydrolysis as previously described.25 The data were expressed as hydroxyproline (mg)/wet liver weight (g).
Western blot analysis
Samples were subjected to sodium dodecyl sulphate‐polyacrylamide gel electrophoresis and then transferred on to a nitrocellulose membrane (Bio‐Rad, Hercules, California, USA). After blocking with 5% skim milk, the membranes were treated with primary antibodies and then successively with peroxidase‐conjugated secondary antibodies. After washing, immunoreactive bands were visualised by using ECL detection reagent (Amersham, Buckinghamshire, UK) and documented by LAS 1000 (Fuji Photo Film, Tokyo, Japan).
Quantitative real‐time polymerase chain reaction analysis of mRNA expression
Expression of rat collagen IA2 (COL IA2) mRNA and mSmad7 was measured by reverse transcription polymerase chain reaction (RT‐PCR) method by using Taq‐Man One‐Step RT‐PCR Master Mix Reagents and the Applied Biosystems Prism7700 (PE Appliedsystems, Foster City, California, USA) according to the previously reported procedure.26 Total RNA was isolated from HSC or whole liver tissues using ISOGEN (Nippon Gene, Tokyo, Japan). Table 1 shows the primers and an oligonucleotide probe used. Individual gene expression was normalised by glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA expression. As a standard reaction, cDNA corresponding to 200, 40, 8 and 1.6 ng of total RNA from one sample was examined and used as reference value. We analysed each sample by using 100 ng of total RNA. The conditions for RT‐PCR were as follows: 20 min at 50°C, 10 min at 95°C, and then 40 cycles of amplification for 15 s at 94°C and 1 min at 60°C.
Table 1 Sequences of primers and probes used for real‐time reverse transcription polymerase chain reaction.
| Probe | Sequence |
|---|---|
| Rat collagen 1A2 | |
| Forward | 5′‐AAGGGTCCTTCTGGAGAACC‐3′ |
| Reverse | 5′‐TGGAGAGCCAGG GAGACCCA‐3′ |
| Probe | 5′‐CAGGGTCTTCTTGGTGCTCCCGGTAT‐3′ |
| Mouse smad7 | |
| Forward | 5′‐GACTCCAGGACGCTGTTGGT‐3′ |
| Reverse | 5′‐CCATGGTTGCTGGATGAACT‐3′ |
| Probe | 5′‐AGTGTTCCCTGGTTTCTCCATCAAGGCT‐3′ |
| Rodent GAPDH | |
| Forward | 5′‐TGCACCACCAACTGCTTAG‐3′ |
| Reverse | 5′‐GGATGCAGGGATGATGTTC‐3′ |
| Probe | 5′‐CAGAAGACTGTGGATGGCCCCTC‐3′ |
GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase.
Cell survival assay
Cell survival was measured using Alamar blue assay (BIOSOURCE, Nivelles, Belgium) according to the manufacturer's instructions.27 In brief, 20 μl of Alamar blue were added to 6‐well plates containing Dulbecco's modified Eagle's medium and 10% fetal calf serum. Absorbance at 530 nm was measured by using a microplate reader (Wallac 1420 ARVOsx, P‐E Applied Biosystems, Foster City, California, USA).
Laser capture microdissection
After staining and fixation, a laser capture microdissection (LCM) system LM200 (ARCTURUS Bioscience, Mountain View, California, USA) was used to isolate areas of fibrous septae from tissue sections. These areas were collected on a CapSure HS LCM Cap provided with the system. After LCM, total RNA was extracted from the captured tissue using ISOGEN (Nippon Gene, Tokyo, Japan).
Statistical analysis
Values shown in the figures represent means (SD) of five or more independent samples. The results were analysed by the unpaired Student's t test. Statistical significance was set at p <0.05.
Results
GFP expression by the two recombinant adenoviral vectors equipped with Cre/loxP system in HSC and hepatocytes
We first investigated whether adenoviral gene expression regulated by the Cre/loxP system was effective in primary‐cultured HSC. Both Ad‐LNL‐GFP and Ad‐Cre controlled under either a specific promoter or CAG, were infected in quiescent and activated primary rat HSC. GFP was observed in almost all HSCs when the CAG promoter was used to express Cre recombinase. GFP was detectable in a small number of HSC when either desmin or GFAP promoter was used (data not shown). However, by using 17COL promoter, GFP expression was induced moderately in quiescent HSC and was greatly enhanced in activated HSC (fig 2A). The specific activity of 17COL promoter in HSC was confirmed by using primary‐cultured hepatocytes, Huh7 and HepG2. When the CAG promoter was used to express Cre recombinase, GFP expression was observed in almost all of these cells. However, the 17COL promoter failed to do so (fig 2B). Based on these data confirming its cell specificity in cultured HSC, we used the 17COL promoter in subsequent experiments.
Figure 2 Adenovirus‐mediated green fluorescence protein (GFP) expression in hepatic stellate cells and hepatocytes. (A) Expression of GFP in primary‐cultured HSC. Each 50 MOI of LNL‐GFP and Cre‐expressing adenovirus (CAG‐Cre or 17COL‐Cre) was coinfected in quiescent and activated rat primary‐cultured HSC. The HSC were observed at 2 days after infection under a LSM 510 Laser Scanning Microscope. (B) Expression of GFP in rat primary hepatocytes, Huh7 or HepG2. Each 50 MOI of LNL‐GFP and Cre‐expressing adenovirus (CAG‐Cre or 17COL‐Cre) was coinfected in primary‐cultured hepatocytes, Huh7 and HepG2. The cells were observed at 2 days after infection under a LSM 510 Laser Scanning Microscope. CAG, cytomegalovirus enhancer, chicken β‐actin promoter and a part of 3′ untranslated region of rabbit β‐globin; HSC, hepatic stellate cell.
Expression of mSmad7 and thymidine kinase in LX‐2 using 17COL‐Cre
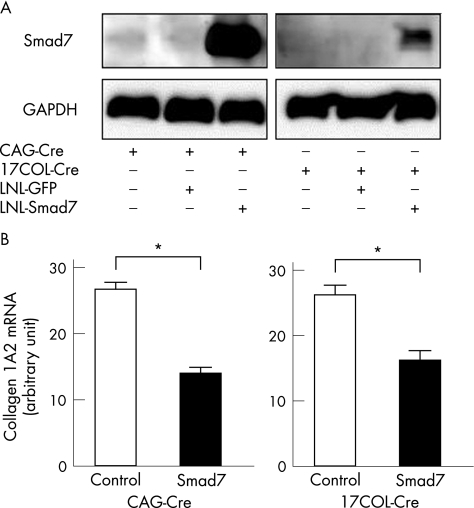
We assessed the potential of this system to downregulate stellate cell activation by expressing mSmad7 in LX‐2. Western blot confirmed the appearance of mSmad7 in LX‐2 infected with LNL‐mSmad7 and 17COL‐Cre although the 17COL promoter appeared to be less active than CAG promoter (fig 3A). Expression of mSmad7 decreased collagen 1A2 mRNA expression in LX‐2 (fig 3B). A similar result was also observed in activated rat HSC in primary culture (data not shown).
Figure 3 Effect of mSmad7 induced by cytomegalovirus enhancer, chicken β‐actin promoter and a part of 3′ untranslated region of rabbit β‐globin (CAG) or 17COL promoter on the expression of collagen mRNA. (A) mSmad7 expression in LX‐2. Each 50 multiplicity of infection of loxP‐neo‐loxP (LNL)‐mSmad7 and Cre‐expressing adenovirus (CAG‐Cre or 17COL‐Cre) was coinfected in LX‐2 cells. The cells were harvested at 2 days after infection. The cell lysates were prepared for immunoblotting to determine the expression of mSmad7 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH). (B) Collagen 1A2 mRNA expression. The steady‐state levels of endogenous collagen 1A2 mRNA were determined by real‐time reverse transcription polymerase chain reaction. Relative expression levels of collagen 1A2 mRNA were normalised against those of GAPDH mRNA. Data are mean (SD) obtained from five independent tests. *p<0.05. COL, collagen.
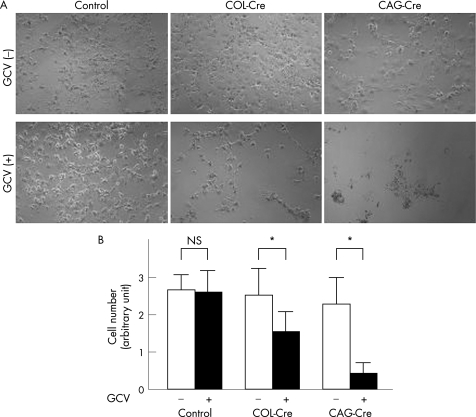
Next, we assessed the feasibility of expressing thymidine kinase in HSC to confer susceptibility to cell killing by GCV. Sensitivity of LX‐2 to GCV was analysed after infection of LGL‐TK and either CAG‐Cre or 17COL‐Cre. As shown in fig 4A, GCV alone was not cytotoxic, LX‐2 underwent cell death when coinfected with CAG‐Cre and LGL‐TK. The combinations 17COL‐Cre and LGL‐TK were also effective, but less so than CAG‐Cre and LGL‐TK. Alamar blue assays quantitatively confirmed these observations (fig 4B). These observations using the Cre/lox‐P system with 17COL promoter confirmed the relatively strong and specific expression of genes of interest in HSC.
Figure 4 Effect of thymidine kinase induced by cytomegalovirus enhancer, chicken β‐actin promoter and a part of 3′ untranslated region of rabbit β‐globin (CAG) or 17COL promoter on the survival of LX‐2. (A) Morphology of LX‐2. Each 50 multiplicity of infection of LGL‐TK and Cre‐expressing adenovirus (CAG‐Cre or 17COL‐Cre) were coinfected in LX‐2 cells. LX‐2 infected only LGL‐TK were used as control. Cells were cultured in the presence or absence of ganciclovir (GCV; 5 μg/ml) for 2 days after infection. Then, the cells were observed under a phase‐contrast microscope. (B) Alamar blue assay. The cells were treated as in (A) and used for Alamar blue assay as described in Materials and Methods. Data are mean (SD) obtained from five independent tests. NS, not significant. *p<0.05.
Suppression of liver fibrosis by mSmad7 expression in HSC in vivo
It has been reported that 46k Da coxsackie/adenovirus receptor is induced in activated HSC and augments the adenoviral transfection into this phenotype of HSC compare with quiescent HSC.28 Additionally, Nakamura et al29 showed that adenovirus‐mediated gene expression was preferentially shown in septal cells rather than hepatocytes in cirrhotic liver. In this context, we investigated the transduction efficacy of CAG‐Cre and LNL‐GFP into hepatocytes and HSC in intact or BDL‐treated rat livers after systemic administration of the viruses. GFP‐positive cells were dominantly hepatocytes in intact liver while they were the sinusoid lining cells in BDL‐treated liver (supplementary fig D1; see supplementary figures available online at http://gut.bmjjournals.com/supplemental). HSC isolated from intact liver rarely showed GFP while almost 50% of HSC isolated from BDL‐treated liver expressed GFP (supplementary fig D2).
Repetitive injection of TAA for 4–7 weeks induced prominent hepatic fibrosis with central–central bridging (fig 5A and supplementary figs A, B). A single administration of 17COL‐Cre and LNL‐mSmad7 via tail vein at 4 weeks considerably reduced the extent of fibrosis as confirmed by the morphometric analysis of Sirius red‐stained liver sections and the quantitative estimation of hydroxyproline content in the liver (fig 5A–C). The specific expression site of mSmad7 mRNA in the rat liver infected with 17COL‐Cre and LNL‐mSmad7 was determined by comparing its expression in fibrous region with that in non‐fibrous regions that were separately obtained by LCM (fig 5D). As shown in fig 5E, mSmad7 mRNA was abundantly expressed in fibrous regions as compared with that in non‐fibrous regions. Furthermore, the western blot also showed that expression of Smad7 protein was significantly higher in fibrous regions than in non‐fibrous regions (fig 5F). The expression of αSMA was hardly detectable in non‐fibrous region compared with fibrous regions.
Figure 5 Preventive effect of 17COL promoter‐induced mSmad7 expression on the progression of thioacetamide (TAA)‐induced hepatic fibrosis. Rats were treated as shown in fig 1B‐i. (A) Sirius red staining. Fibrotic septa caused by TAA treatment (control) were dramatically suppressed by mSmad7 expression using 17COL‐Cre and loxP‐neo‐loxP (LNL)‐Smad7. LNL‐green fluorescent protein was coinfected as control. (B, C) Estimation of liver fibrosis. The degree of hepatic fibrosis was quantified by measuring the area positive for Sirius red staining (B) and hydroxyproline content (C). Data obtained from eight rats in each group are the mean (SD). *p<0.05. (D–F) Fibrous (F) and non‐fibrous (N) regions were separately cut using LCM as described in Materials and Methods section (D). (E) Expression of mSmad7 mRNA in the individual area was measured by real‐time reverse transcription polymerase chain reaction. Relative expression of mSmad7 mRNA was normalised against that of glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA. Data obtained from six rats in each group are the mean (SD). *p<0.05. (F) Western blot. Expression of Smad7, smooth muscle‐α actin (αSMA) and GAPDH was determined by immunoblotting.
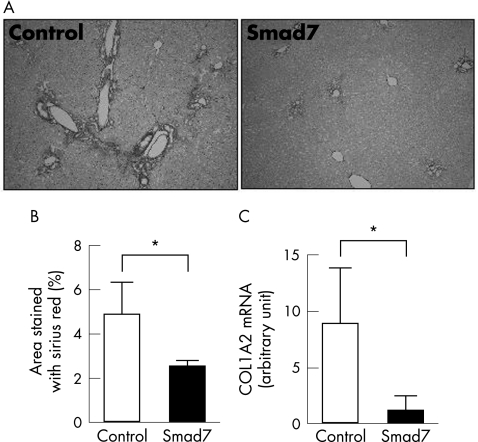
In BDL rat liver, fibrotic areas developed around the periportal zones in 3 weeks. As shown in fig 6A and supplementary fig B, collagen promoter‐specific expression of mSmad7 dramatically decreased this fibrotic change. Image analysis of Sirius red‐stained liver sections showed a significant decrease in the percentage of fibrotic area after ectopic mSmad7 expression (fig 6B). Expression of collagen 1A2 mRNA was also dramatically decreased (fig 6C).
Figure 6 Preventive effect of 17COL promoter‐induced mSmad7 expression on the progression of BDL‐induced hepatic fibrosis. Rats were treated as shown in fig 1B‐ii. (A) Sirius red staining. Fibrotic changes caused by BDL treatment (control) were dramatically suppressed by mSmad7 expression using 17COL‐Cre and LNL‐Smad7. LNL‐green fluorescent protein was coinfected as control. (B) Estimation of liver fibrosis. The degree of hepatic fibrosis was quantified by measuring the area positive for Sirius red staining. Data are obtained from six rats in each group and represent the mean (SD). *p<0.05. (C) The steady‐state levels of endogenous collagen 1A2 mRNA were determined by real‐time RT‐PCR. Relative expression levels of collagen 1A2 mRNA were normalised against these of glyceraldehyde‐3‐phosphate dehydrogenase mRNA. Data obtained from six rats in each group are the mean (SD). *p<0.05.
A liver‐specific therapeutic effect of this system was confirmed by using bleomycin‐induced lung fibrosis model, which indicated that lung fibrosis was not attenuated by systemic injection of 17COL‐Cre and LNL‐mSmad7 (supplementary fig C).
Suppression of liver fibrosis by thymidine kinase expression in HSC in vivo
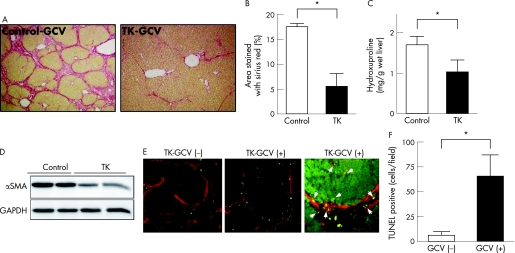
Next, we examined whether HSC death induced by GCV after the expression of thymidine kinase could suppress liver fibrosis induced by TAA administration. TAA was administered to rats for 6 weeks; after 4 weeks of TAA, rats were injected with 17COL‐Cre and LNL‐thymidine kinase, following with intraperitoneal administration of GCV (50 mg/kg/day) every day for 2 weeks. As depicted in fig 7A and supplementary fig B, liver fibrosis was dramatically decreased by these treatments. Morphometric analysis of Sirius red‐stained liver sections and estimation of hydroxyproline content in the liver quantitatively confirmed these observations (fig 7B,C). αSMA expression was also suppressed (fig 7D). Actual death of activated HSC by the expression of thymidine kinase after GCV injection was estimated by TUNEL staining. As shown in fig 7E, cell death was observed mainly along fibrotic septae in GCV‐injected rat liver, whereas it was hardly detectable in rat liver without GCV. TUNEL positivity was also seen in activated HSCs that were αSMA positive within the hepatic parenchyma. Semiquantitative analysis showed that the number of TUNEL‐positive cells in GCV‐treated liver were 10 times more than that in GCV non‐injected one (fig 7F).
Figure 7 Effect of 17COL promoter‐induced thymidine kinase on the progression of thioacetamide (TAA)‐induced hepatic fibrosis. Rats were treated as shown in fig 1B, iii. (A) Sirius red staining. Fibrotic septum caused by TAA treatment (control) was dramatically suppressed by ganciclovir (GCV) treatment together with the expression of thymidine kinase under the control of 17COL promoter (thymidine kinase‐GCV). LNL‐GFP was coinfected as control. (B, C) Estimation of liver fibrosis. The degree of hepatic fibrosis was quantified by measuring the area positive for Sirius red‐staining (B) and hydroxyproline content (C). Data obtained from six rats in each group are the mean (SD). *p<0.05. (D) Western blot. Lysates of the livers were prepared from two groups (control‐GCV and thymidine kinase‐GCV). Expression of smooth muscle‐α actin (αSMA) and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was determined by immunoblotting. (E) Double immunofluorescence staining for αSMA (red) and TUNEL (green). TUNEL‐positive cells were few in number in the liver infected with 17COL‐thymidine kinase (left). After GCV injection, TUNEL‐positive cells became apparent along αSMA‐positive fibrotic septae (middle). Higher magnification indicates that TUNEL positivity was observed in αSMA‐positive cells in fibrotic septae (arrowheads) and was also seen in αSMA‐positive parenchymal sinusoidal HSCs (arrows; right). (F) Estimation of TUNEL‐positive cells. For semiquantitative analysis of cell death, the number of TUNEL‐positive cell per field was counted in 10 fields from each slide randomly selected. TUNEL‐positive cell numbers increased 10‐fold in GCV‐injected rat livers compared to levels in non‐treated rats. GCV. *P<0.05
Discussion
Adenoviral vectors are a highly efficient gene expression system both in cultured cells and in tissues.5 In the present study, we show that a Cre/loxP system can be used to augment gene expression levels in vivo sufficiently to achieve meaningful effects on gene expression and liver fibrosis. To do so, we constructed an adenovirus expressing Cre recombinase under the control of the 17COL promoter and were able to amplify gene expression specifically in HSC and myofibroblasts under the control of a potent CAG promoter present in the second adenovirus vector. Coinfection of these two adenoviral vectors successfully augmented the expression of therapeutic genes specifically in HSC both in culture and in the liver in vivo. Furthermore, we showed that coinfection of 17COL‐Cre and LNL‐mSmad7 or LGL‐TK dramatically suppressed the progression of hepatic fibrosis.
Several transfection methods and vector systems have been studied to deliver foreign genes into the liver. However, all have some limitations; retroviral vectors, which are used most often in clinical practice, are not suitable for gene therapy for liver fibrosis because their transfection efficiency is relatively low even in dividing cells, including partially hepatectomised liver. Although adenoviral vectors have been used less in clinical trials, they appear to become a more efficient delivery system of foreign genes into the liver but are not typically targeted to single cell types. For example, inhibition of growth factors such as soluble form of PDGF receptor,30 cytokines such as interferon α,31 MMP‐1,32 MMP‐8,33 SOD,34 and telomerase35 have been introduced as adenoviral transgenes. Among them, blockage of TGFβ signalling pathways via adenoviral vectors expressing either truncated type II TGFβ receptor,36 the ecto‐domain of TGFβRII fused to the Fc, soluble TGFβ receptor37 or Smad738 have successfully suppressed experimental hepatic fibrosis. However, it should be noted that blocking of signalling might favour neoplastic transformation of hepatocytes, as TGFβ is a tumour suppressor gene. However, in principle, this problem could be overcome by using cell‐specific targeting systems that avoid hepatocytes.
Up to 5 kb length of promoter sequence is typically utilised to generate the adenoviral Cre/loxP system. We used the promoters of three genes, collagen I, desmin or GFAP. Cosmid pAx17COL‐NCre was generated by the insertion of a strong tissue‐specific COL1A2 enhancer (−17.0 to −15.5 kb) that is linked to the −350 minimal promoter sequence having TGFβ response element and interferon γ response element.16,17 The cosmid pAxDesmin‐NCre was generated by the insertion of 4‐kb upstream of desmin transcription initiation point that contains a CArG/octamer overlapping element that can bind the serum response factor.18 The cosmid pAxGFAP‐NCre was generated by the insertion of 2.5‐kb upstream of GFAP transcription start point containing AP‐2, nuclear factor I and cyclic AMP‐responsive element.19 Desmin and GFAP are intermediate filament proteins that are fully expressed in HSC. In the present in vitro experiments, however, activity of these two promoters induced by our Cre/loxP system was unexpectedly weak in activated HSC (data not shown), possibly because these vectors did not contain sufficient regulatory sequences to drive high‐level expression. In contrast, 17COL promoter effectively expressed transgenes in activated HSC.
As indicated above, the 17COL promoter contains the −350 minimal promoter sequence containing TGFβ‐ response elements and interferonγ‐response elements in addition to a strong tissue‐specific enhancer. However, the level of gene expression induced by a simple 17COL promoter was unexpectedly low. In contrast, our gene reporter assay showed that 17COL promoter‐dependent Cre/loxP system yielded a 10‐fold higher GFP expression compared with a simple 17COL promoter in plasmid transfection experiments performed in LX‐2 cells. These results strengthen the usefulness of Cre/loxP system for regulable and augmented gene expression in a target cells. The αSMA promoter was another potential candidate promoter for achieving specific gene expression in HSC using Cre/loxP system. However, Magness et al39 recently showed the heterogeneity of hepatic fibrogenetic cell populations; by using a double transgenic mouse model in which GFP and RFP are driven by collagen I and αSMA promoters, respectively, they clearly showed that αSMA and collagen I are not always coexpressed in HSC and myofibroblasts in culture and in fibrotic liver. On the other hand, Takeji and Miwa40 showed that renal fibrosis induced by unilateral ureteric obstruction is augmented in SMA‐null mice; proliferation, activity of migration and expression of collagen type I are all increased in αSMA‐null fibroblasts. These data suggest that αSMA may have an antifibrotic function. Furthermore, the length of αSMA promoter (5.3 kb) required in these studies is beyond the cloning capacity of the present cosmid. Thus, the αSMA promoter was not an ideal candidate for use in our studies.
In addition to Smad7 and thymidine kinase, the system we have used in these studies has been used successfully with other target genes. Overexpression driven by the COL1A2 promoter of RasN17, a dominant negative Ras, whose 17th Ser is mutated to an Asp, dramatically decreased the expression of cyclin D1, resulting in the suppression of HSC proliferation (data not shown). Adenovirus‐mediated expression of YB‐1, a TGFβ/Smad signal repressor, under the control of the identical promoter inhibited HSC activation and considerably suppressed hepatic fibrosis in carbon tetrachloride‐treated mouse liver.41
A disadvantage of the adenovirus vector system is the inability to perform repetitive administrations due to the acquisition of immune response against the virus. To avoid this problem, Haegel‐Kronenberger et al42 recently reported an approach to transiently suppress the immune system using dimeric human monoclonal antibodies against CD40 and CD80. Thummala et al43 reported another method in which CTLA4Ig, an immune modulatory gene, is incorporated into the vector containing the transgene of interest. Adenoviral vectors that do not express any viral proteins have been developed, and several reports have reinforced their utility.44,45,46 Tominaga et al47 showed that retrograde administration of recombinant adenoviruses into the common bile duct was effective in hepatic transgene expression, using LacZ as a reporter gene without immunosuppressive treatment. A combination of these variations combined with our approach may lead to decreased toxicity and prolongation of transgene expression. Ultimately, these advances may collectively lead to the development of clinically meaningful gene therapy approaches in patients with fibrotic liver disease.
Supplementary Material
Acknowledgements
We thank Dr Izumu Saito for his generous gift of cosmid pAxAwNCre. We also thank Dr Kohei Miyazono for his generous gift of mouse Smad7 cDNA.
Abbreviations
BDL - bile duct ligation
CAG - cytomegalovirus enhancer, chicken β‐actin promoter and a part of 3′ untranslated region of rabbit β‐globin
COL - collagen
GAPDH - glyceraldehyde‐3‐phosphate dehydrogenase
GCV - ganciclovir
GFAP - glial fibrillar acidic protein
GFP - green fluorescent protein
HSC - hepatic stellate cell
LCM - Laser capture microdissection
LNL - loxP‐neo‐loxP
MOI - multiplicity of infection
PDGF - platelet‐derived growth factor
PFU - plaque‐forming unit
RT‐PCR - reverse transcription polymerase chain reaction
αSMA - smooth muscle‐α actin
TAA - thioacetamide
TUNEL - terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick‐end labelling
Footnotes
Funding: This work was supported in part by a grant from the Ministry of Education, Science, Sports and Culture of Japan (C16590150 to KI), and the NIH (DK56601 to SLF), as well as the Feld Fibrosis Program (to SLF).
Competing interests: None.
References
- 1.Friedman S L. Liver fibrosis—from bench to bedside. J Hepatol 200338(Suppl 1)S38–S53. [DOI] [PubMed] [Google Scholar]
- 2.Wake K. Perisinusoidal stellate cells (fat‐storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A‐storing cells in extrahepatic organs. Int Rev Cytol 198066303–353. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K, Wakahara T, Wang Y Q.et al In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology 1999291760–1767. [DOI] [PubMed] [Google Scholar]
- 4.Pinzani M. Platelet‐derived growth factor receptor expression in hepatic stellate cells: how too much of a good thing can be bad. Hepatology 199522997–999. [DOI] [PubMed] [Google Scholar]
- 5.St George J A. Gene therapy progress and prospects: adenoviral vectors. Gene Ther 2003101135–1141. [DOI] [PubMed] [Google Scholar]
- 6.Sato Y, Tanaka K, Lee G.et al Enhanced and specific gene expression via tissue‐specific production of Cre recombinase using adenovirus vector. Biochem Biophys Res Commun 1998244455–462. [DOI] [PubMed] [Google Scholar]
- 7.Maher J J, Bissell D M, Friedman S L.et al Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest 198882450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoi Y, Namihisa T, Kuroda H.et al Immunocytochemical detection of desmin in fat‐storing cells (Ito cells). Hepatology 19844709–714. [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Hui A Y, Albanis E.et al Human hepatic stellate cell lines, LX‐1 and LX‐2: new tools for analysis of hepatic fibrosis. Gut 200554142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramadori G, Veit T, Schwogler S.et al Expression of the gene of the alpha‐smooth muscle‐actin isoform in rat liver and in rat fat‐storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol 199059349–357. [DOI] [PubMed] [Google Scholar]
- 11.Niki T, De Bleser P J, Xu G.et al Comparison of glial fibrillary acidic protein and desmin staining in normal and CCl4‐induced fibrotic rat livers. Hepatology 1996231538–1545. [DOI] [PubMed] [Google Scholar]
- 12.Knittel T, Aurisch S, Neubauer K.et al Cell‐type‐specific expression of neural cell adhesion molecule (N‐CAM) in Ito cells of rat liver. Up‐regulation during in vitro activation and in hepatic tissue repair. Am J Pathol 1996149449–462. [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatani K, Seki S, Kawada N.et al Expression of neural cell adhesion molecule (N‐CAM) in perisinusoidal stellate cells of the human liver. Cell Tissue Res 1996283159–165. [DOI] [PubMed] [Google Scholar]
- 14.Niki T, Pekny M, Hellemans K.et al Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology 199929520–527. [DOI] [PubMed] [Google Scholar]
- 15.Cassiman D, van Pelt J, De Vos R.et al Synaptophysin: a novel marker for human and rat hepatic stellate cells. Am J Pathol 19991551831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inagaki Y, Truter S, Ramirez F. Transforming growth factor‐beta stimulates alpha 2(I) collagen gene expression through a cis‐acting element that contains an Sp1‐binding site. J Biol Chem 199426914828–14834. [PubMed] [Google Scholar]
- 17.Bou‐Gharios G, Garrett L A, Rossert J.et al A potent far‐upstream enhancer in the mouse pro alpha 2(I) collagen gene regulates expression of reporter genes in transgenic mice. J Cell Biol 19961341333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mericskay M, Parlakian A, Porteu A.et al An overlapping CArG/octamer element is required for regulation of desmin gene transcription in arterial smooth muscle cells. Dev Biol 2000226192–208. [DOI] [PubMed] [Google Scholar]
- 19.Miura M, Tamura T, Mikoshiba K. Cell‐specific expression of the mouse glial fibrillary acidic protein gene: identification of the cis‐ and trans‐acting promoter elements for astrocyte‐specific expression. J Neurochem 1990551180–1188. [DOI] [PubMed] [Google Scholar]
- 20.Miyake S, Makimura M, Kanegae Y.et al Efficient generation of recombinant adenoviruses using adenovirus DNA‐terminal protein complex and a cosmid bearing the full‐length virus genome. Proc Natl Acad Sci USA 1996931320–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanegae Y, Makimura M, Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn J Med Sci Biol 199447157–166. [DOI] [PubMed] [Google Scholar]
- 22.Kawada N, Seki S, Inoue M.et al Effect of antioxidants, resveratrol, quercetin, and N‐acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology 1998271265–1274. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda K, Kubo S, Hirohashi K.et al Mechanism that regulates nitric oxide production by lipopolysaccharide‐stimulated rat Kupffer cells. Physiol Chem Phys Med NMR 199628239–253. [PubMed] [Google Scholar]
- 24.Ikeda K, Kawada N, Wang Y Q.et al Expression of cellular prion protein in activated hepatic stellate cells. Am J Pathol 19981531695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuno M, Akita K, Moriwaki H.et al Prevention of rat hepatic fibrosis by the protease inhibitor, camostat mesilate, via reduced generation of active TGF‐beta. Gastroenterology 20011201784–1800. [DOI] [PubMed] [Google Scholar]
- 26.Uyama N, Shimahara Y, Okuyama H.et al Carbenoxolone inhibits DNA synthesis and collagen gene expression in rat hepatic stellate cells in culture. J Hepatol 200339749–755. [DOI] [PubMed] [Google Scholar]
- 27.Olaso E, Ikeda K, Eng F J.et al DDR2 receptor promotes MMP‐2‐mediated proliferation and invasion by hepatic stellate cells. J Clin Invest 20011081369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q, Que L G, Rockey D C. Adenovirus‐mediated gene transfer to nonparenchymal cells in normal and injured liver. Am J Physiol Gastrointest Liver Physiol 2002282G565–G572. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Akiyoshi H, Saito I.et al Adenovirus‐mediated gene expression in the septal cells of cirrhotic rat livers. J Hepatol 199930101–106. [DOI] [PubMed] [Google Scholar]
- 30.Borkham‐Kamphorst E, Herrmann J, Stoll D.et al Dominant‐negative soluble PDGF‐beta receptor inhibits hepatic stellate cell activation and attenuates liver fibrosis. Lab Invest 200484766–777. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K, Aoki K, Ohnami S.et al Adenovirus‐mediated gene transfer of interferon alpha improves dimethylnitrosamine‐induced liver cirrhosis in rat model. Gene Ther 200310765–773. [DOI] [PubMed] [Google Scholar]
- 32.Iimuro Y, Nishio T, Morimoto T.et al Delivery of matrix metalloproteinase‐1 attenuates established liver fibrosis in the rat. Gastroenterology 2003124445–458. [DOI] [PubMed] [Google Scholar]
- 33.Siller‐Lopez F, Sandoval A, Salgado S.et al Treatment with human metalloproteinase‐8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology 20041261122–33 (discussion 949, [DOI] [PubMed] [Google Scholar]
- 34.Zhong Z, Froh M, Wheeler M D.et al Viral gene delivery of superoxide dismutase attenuates experimental cholestasis‐induced liver fibrosis in the rat. Gene Ther 20029183–191. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph K L, Chang S, Millard M.et al Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 20002871253–1258. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Sakata R, Ueno T.et al Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine‐treated rats. Hepatology 200032247–255. [DOI] [PubMed] [Google Scholar]
- 37.Ueno H, Sakamoto T, Nakamura T.et al A soluble transforming growth factor beta receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum Gene Ther 20001133–42. [DOI] [PubMed] [Google Scholar]
- 38.Dooley S, Hamzavi J, Breitkopf K.et al Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 2003125178–191. [DOI] [PubMed] [Google Scholar]
- 39.Magness S T, Bataller R, Yang L.et al A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology 2004401151–1159. [DOI] [PubMed] [Google Scholar]
- 40.Takeji M, Miwa T. Gene expression system of aortic smooth muscle actin and its role in acute inflammation. The 27th Annual Meeting of the Molecular Biology Society of Japan; 8–10 December 2004, Yokohama, Japan 20042PA–468.
- 41.Inagaki Y, Kushida M, Higashi K.et al Cell Type‐Specific Intervention of Transforming Growth Factor beta/Smad Signaling Suppresses Collagen Gene Expression and Hepatic Fibrosis in Mice. Gastroenterology 2005129259–268. [DOI] [PubMed] [Google Scholar]
- 42.Haegel‐Kronenberger H, Haanstra K, Ziller‐Remy C.et al Inhibition of costimulation allows for repeated systemic administration of adenoviral vector in rhesus monkeys. Gene Ther 200411241–252. [DOI] [PubMed] [Google Scholar]
- 43.Thummala N R, Ghosh S S, Lee S W.et al A non‐immunogenic adenoviral vector, coexpressing CTLA4Ig and bilirubin‐uridine‐diphosphoglucuronateglucuronosyltransferase permits long‐term, repeatable transgene expression in the Gunn rat model of Crigler‐Najjar syndrome. Gene Ther 20029981–990. [DOI] [PubMed] [Google Scholar]
- 44.Kim I H, Jozkowicz A, Piedra P A.et al Lifetime correction of genetic deficiency in mice with a single injection of helper‐dependent adenoviral vector. Proc Natl Acad Sci U S A 20019813282–13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morsy M A, Gu M, Motzel S.et al An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci U S A 1998957866–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitani K, Graham F L, Caskey C T.et al Rescue, propagation, and partial purification of a helper virus‐dependent adenovirus vector. Proc Natl Acad Sci USA 1995923854–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tominaga K, Kuriyama S, Yoshiji H.et al Repeated adenoviral administration into the biliary tract can induce repeated expression of the original gene construct in rat livers without immunosuppressive strategies. Gut 2004531167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.