Polyphenols are phytochemicals that are abundant in food and beverages derived from plants. Although no deficiency state has been described for them, increased intake of polyphenols appears to protect against disease in virtue of their anti‐inflammatory and vasculoprotective properties. This article focuses on four polyphenols with established anti‐inflammatory properties: resveratrol, epigallocatechin gallate, curcumin and quercetin. In rodents, ingestion or systemic administration of these agents inhibits nuclear factor κ B‐dependent gene expression and induces phase II antioxidant and detoxifying proteins. Conditions prevented and/or ameliorated by these polyphenols include inflammatory colitis and acute pancreatitis. Polyphenols also attenuate ischaemia‐reperfusion injury and endotoxemic sepsis, which has a role in the development of multiple organ dysfunction in severe acute pancreatitis. Enteral nutrition has an important role in the management of inflammatory bowel disease (IBD)—mainly of Crohn's disease, and of acute pancreatitis. Parenteral nutrition is reserved for refractory cases and disease‐associated complications. Artificial nutrition attempts to safely administer the essential and otherwise beneficial constituents of food to patients with an impaired ability to ingest or digest food; yet, polyphenols are not included in the formulas. We suggest that the addition of polyphenols to artificial nutritional formulas would improve the outcome of patients with IBD and acute pancreatitis in need of enteral or parenteral nutrition.
Polyphenols, a group of secondary plant metabolites, are non‐essential nutrients that probably contribute to human health
Plants, like other unicellular and multicellular organisms, contain ubiquitous organic molecules (eg, amino acids, carbohydrates and fatty acids) termed primary metabolites that are essential to cell structure and basic metabolism. These compounds also serve as substrates for the synthesis of an array of chemicals called secondary plant metabolites, which are accumulated at lower concentrations and are more variably distributed among different species. Once thought to be waste products, these agents are now considered to have a role in ecological interactions with friendly and hostile microorganisms and macroorganisms, and protection from environmental stressors. After their ingestion, certain secondary metabolites from edible plants interact beneficially with the regulatory domains of functional proteins that are shared by plants and herbivores, due to evolutionary conservation. Vitamins, for instance, act as enzyme coactivators and their insufficient intake results in some disease.1,2,3 Polyphenolic substances (PPLs), the subject of this review, belong to an ill‐defined group of secondary plant metabolites called phytochemicals or bioactive compounds in food, for which a deficiency state has not been described (denying them the title of essential micronutrients) but they have attracted attention as mediators of the disease‐preventive effect of a healthful diet.4,5,6,7,8,9,10,11,12,13,14,15 PPLs' status as a non‐essential, but an established health‐promoting dietary constituent is similar to that of the marine‐life‐derived, long chain ω‐3 polyunsaturated fatty acids (LC PUFAs), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These can be synthesised to some extent from the shorter‐chain, plant‐derived, essential ω‐3 PUFA, α‐linolenic acid. Thus, EPA and DHA are not considered essential nutrients for adults.9 Yet, since the initial interest in their vasculoprotective properties during the 1970s, a formidable body of knowledge from mechanistic, epidemiological and large clinical studies has evolved, defining a role for the LC ω‐3 PUFAs in the secondary prevention of cardiovascular disease.16,17 Several studies have also shown the importance of supplementing artificial nutrition formulas with these fatty acids, by showing that they reduce morbidity, and possibly mortality, in several clinical scenarios.18,19 Thus, modern nutritional science has expanded its focus from overt nutrient‐deficiency states to the relationship of diet and nutritional status to the industrialised world's common diseases, including the benefits derived from consuming certain non‐essential bioactive components of food.9,20 Although interest in PPLs' effect on health is growing, it is more recent21 than the investigations dealing with the ramifications of LC ω‐3 PUFA consumption. In addition, the growing list of identified PPLs, their variable distribution in different foods, their low bioavailability and their extensive and differential intestinal and hepatic metabolism have hampered defining their significance in the prevention of disease.12,13,14,15,21,22 The current level of evidence precludes recommending that patients take PPL‐containing supplements to treat or prevent disease, rather than eat a variety of healthful foods with emphasis on plant sources.9,13,21 Nevertheless, PPLs are increasingly recognised as health‐promoting phytochemicals as: (1) they attenuate animal models of many human illnesses that have an inflammatory component;13,21 (2) their consumption by humans on a healthful plant‐based diet is approximately 1 g a day—considerably higher than that of vitamins C and E and β‐carotene;13 (3) they have anti‐inflammatory, antioxidative and insulin‐sensitising effects and have enhanced endothelial function in clinical trials;23,24,25,26,27,28 and (4) they have reduced cardiovascular disease in epidemiological studies.29
Polyphenols inhibit pro‐inflammatory transcription factors and enhance cytoprotective ones in vivo
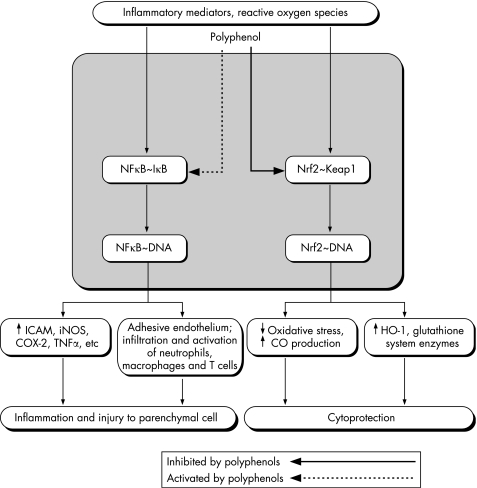
PPLs are powerful free radical scavengers in vitro,13 but present opinion holds that their in vivo anti‐inflammatory effect stems from their interaction with proteins involved in signal transduction and gene expression.12,13,21,22,30 One important target of PPLs' action in vivo is the pivotal inflammatory transcription factor, nuclear factor κ B (NFκB; fig 1). Usually bound to and inactivated by the cytoplasmic inhibitor of κ B (IκB), NFκB is released from its association with the latter by activated IκB kinases (IκKs). Pathogen‐associated molecular patterns (eg, endotoxin), inflammatory cytokines (eg, tumour necrosis factor‐α (TNFα)), T‐cell‐activating signals and reactive oxygen species trigger signal transduction cascades that converge on, phosphorylate and rapidly activate IκKs. Released NFκB is then translocated into the nucleus where it promotes the expression of inflammatory gene products such as TNFα, inducible nitric oxide synthase (iNOS), cyclo‐oxygenase (COX)‐2, intercellular adhesion molecule and induces inflammatory activation of lymphocytes, monocyte/macrophages and endothelial cells.31 Although it may protect parenchymal cells from apoptosis, NFκB is considered to be a target for the treatment of diverse pathological conditions with an inflammatory component.31,32 By inhibiting IκK phosphorylation and/or preventing proteasomal degradation of IκB, numerous PPLs attenuate the in vivo NFκB activation induced in inflammatory disease states.12,13 These include resveratrol, which is found in grapes and wine and probably contributes to their cardioprotective effects;33,34 epigallocatechin gallate (EGCG), an anti‐inflammatory component of green tea;35 curcumin, the major anti‐inflammatory PPL in turmeric;36,37 and quercetin, which is found in apples, onions, leafy green vegetables and tea.38 Interestingly, the anti‐inflammatory properties of NSAIDs and salicylates are also partially attributable to inhibition of IκK.39 As PPLs do not appear to prevent COX‐1‐mediated synthesis of constitutive/protective prostaglandins, they may be safer, if less powerful, anti‐inflammatory agents.
Figure 1 Polyphenols attenuate injury to parenchymal cells by down‐regulating inflammatory genes and up‐regulating cytoprotective ones. COX‐2, cyclo‐oxygenase‐2; HO‐1, haem‐oxygenase‐1; IκB, inhibitor of κ B; ICAM, intercellular adhesion molecule; iNOS, inducible nitric oxide synthase; NFκB, nuclear factor κ B; Nrf2, nuclear factor E2‐related factor; TNFα, tumour necrosis factor‐α.
In addition to down‐regulating the expression of inflammatory mediators, preneoplastic lesions also induce in vivo the transcription of cytoprotective phase II antioxidant and detoxifying molecules, such as haem‐oxygenase‐1 (HO‐1) and glutathione‐related enzymes40,41,42,43 (fig 1). These are part of the endogenous defence system against xenobiotic and chemical toxicity and protect from carcinogenesis and inflammatory and autoimmune disease.44 Electrophilic PPLs release nuclear factor E2‐related factor (Nrf2) from its complex with the cytoskeleton‐associated protein Keap1, through an interaction with thiols in Keap1. Nrf2 can then bind to the antioxidant responsive element, a regulatory element of phase II genes.43 Interestingly, the Nrf2 pathway is physiologically activated by the very mediators of inflammatory pathways, such as increased oxidative stress, certain protein kinase(s) C and mitogen‐activated protein kinases, perhaps as a counter‐regulatory mechanism to reduce collateral tissue injury during inflammation.44 Thus, through differential modulation of gene expression, PPLs inhibit the injurious consequences of cell injury, oxidative stress and inflammation while enhancing cytoprotective responses. Other genomic and non‐genomic actions of different PPLs are reviewed elsewhere.12,13,29,33,34,35,36,42
Let artificial nutrition be thy medicine
Artificial nutrition formulas are an attempt to partially reconstruct food from its essential and healthful components, in a form that can be safely administered by the enteral or parenteral route.45 Over the years, formulas have been modified in an attempt to replicate healthful food intake, correct malnutrition, attenuate catabolism, inflammation and immunosuppression and to enhance disease resolution and healing.45,46 To this end, formulas may include pharmacological doses of nutrients (eg, glutamine and ω‐3 PUFAs), non‐essential constituents of food with healthful and disease‐modifying properties (eg, dietary fibre, prebiotics and probiotics), and even molecules not related to food (eg, transforming growth factor‐β).46,47,48,49 Yet, a patient who temporarily or permanently becomes dependent on enteral or parenteral nutrition is deprived of PPLs that she/he may have previously obtained from fruit, vegetables, tea, wine, chocolate or spices.
Although consumption of phytochemicals as a supplement does not necessarily confer the same benefit as ingesting foods rich in these compounds, addition of PPLs to otherwise complete nutritional formulas would bring them a step closer to what has been termed the “entire biological package” of food.51
By inhibiting NFκB, inclusion of the PPLs discussed here in artificial nutritional formulas may boost their therapeutic effect in acute and chronic diseases that necessitate enteral nutrition or parenteral nutrition and in which NFκB activation is implicated, such as sepsis,52 acute respiratory distress syndrome,53 postoperative organ dysfunction,54 cachexia,55 IBD56 and acute pancreatitis.57
It has previously been proposed that phytochemicals other than vitamins may be important for patients receiving artificial nutrition.45 The rest of this article reviews the literature suggesting that administration of the four above‐mentioned PPLs is beneficial in IBD and acute pancreatitis, thus offering a rationale for their inclusion in artificial nutrition formulas for patients with these conditions.
Polyphenols for IBD
Crohn's disease and ulcerative colitis, the two forms of IBD, are multifactorial disorders resulting from a dysfunctional epithelial, innate and adaptive immune response to intestinal microorganisms. Pharmacological treatment typically targets the ensuing robust autoimmune and inflammatory response that damages the gastrointestinal mucosa, impairing its absorptive and protective barrier function.58 Most patients with IBD will suffer at some stage a degree of nutritional deficiency, owing to any combination of anorexia, malabsorption, enteropathic protein and blood loss, and a systemic inflammatory‐catabolic response. These have deleterious intestinal and extraintestinal consequences.58,59,60
Paediatric and adult patients with Crohn's disease and malnutrition may need enteral nutrition and rarely parenteral nutrition to replenish macronutrients and micronutrients and enhance anabolism and growth. In patients with active Crohn's disease, enteral nutrition is effective as a remission‐inducing and a glucocorticoid‐sparing treatment to maintain remission. Parenteral nutrition may benefit malnourished patients before major surgery, those with spontaneous or postsurgical enterocutaneous fistulas and possibly also glucocorticoid‐resistant patients. Patients with Crohn's disease having short bowel syndrome after extensive resection of the intestines often depend on artificial nutrition as a source of nutrients. The benefit of nutritional support in ulcerative colitis has received less interest and is presently less supported.58,59,60
Studies in rodent models of IBD61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77 (table 1) indicate that administration of PPLs is effective in preventing and treating intestinal inflammation and injury. Acute or chronic colitis was induced in these studies by intrarectal administration of dinitrobenzene sulphate,63,71 or trinitrobenzene sulphate,61,62,66,67,68,69,71,72,73 addition of dextran sulphate sodium (DSS) to the drinking water,64,75,76,77 or by knock‐out of the interleukin‐2 gene65 (IL‐2‐/‐ mice). Rodents were treated with PPLs before, during and/or after induction of colitis, by oral (added to food or drinking water or through an orogastric tube),61,62,64,65,66,67,68,70,71,72,73,74,75,76,77 rectal72 or intraperitoneal63,69,70 administration. They were killed, and indices of disease were assessed between 48 h and 6 weeks after induction of colitis. Resveratrol,61,62 EGCG/green tea PPL extract,63,64,65 curcumin66,67,68,69,70,71 and quercetin and its naturally occurring glycones72,73,74,75,76,77 (quercetrin and rutin) reduced mortality rates, attenuated colonic (eg, diarrhoea, bloody stools) and extracolonic (eg, weight loss) signs of disease, colon macropathology and micropathology (eg, hyperaemia, ulcerations, inflammatory infiltrate, serosal adhesions) and/or indices of inflammation and autoimmunity (eg, colonic myeloperoxidase and NFκB activity, increased TNFα, IL‐1β, IL‐12, iNOS and reduced IL‐10, Crohn's disease 4+ T cell and neutrophil infiltration). In studies that assessed several dosages, the ratio between the highest and lowest effective dose ranged between 2 and 6, suggesting a relatively wide therapeutic window.
Table 1 Prophylactic and therapeutic effects of polyphenol administration in rodent models of inflammatory colitis.
| Polyphenol | Model, reference | Route, dose, duration and timing of administration | Outcome | Comments |
|---|---|---|---|---|
| Resveratrol | TNBS enema in Wistar rats61 | i.g. 5 or 10 mg/kg resveratrol 48, 24 and 1 h before and 24 h after TNBS | Reversal of weight loss; ↑ stool consistency, ↓colonic macropathology (presence and degree/extent of hyperaemia, ulceration, inflammation, adhesions), ↓ colonic histopathology (presence and degree/extent of necrosis, inflammatory infiltrate and mucus depletion); ↓ mucosal IL‐1β, COX‐2, PGD2 levels; ↓ colonic MPO activity | PGE2 levels were not significantly reduced; 5 mg/kg resveratrol improved fewer parameters than 10 mg/kg |
| TNBS enema in Wistar rats62 | i.g. 10 mg/kg/day resveratrol for 14 days starting 24 h after TNBS | ↑ Stool consistency; colonic macropathology (weight, presence and degree/extent of hyperaemia, ulceration, inflammation, adhesions), ↓ colonic histopathology (presence and degree/extent of necrosis, inflammatory infiltrate, fibrosis and mucus depletion); ↓ mucosal TNFα and p65 NFκB; ↓ colonic MPO activity; ↑ mucosal PGE2; ↑ epithelial apoptosis | PGE2 levels were reduced during the chronic stage of colitis in untreated animals; resveratrol normalised PGE2 levels | |
| EGCG/green tea polyphenol extract | DNBS enema, in Sprague–Dawley rats63 | i.p. 50 mg/kg/day green tea polyphenol extract 1 day before and 4 days after DNBS | Reversal of weight loss; ↓ diarrhoea; ↓ colon weight and macropathology; ↓ histopathology (presence and degree/extent of oedema, necrosis, neutrophil infiltrate, haemorrhage); ↓ colonic TNFα, ICAM‐1 and nitrotyrosine levels; ↓ colonic MPO activity; | |
| ↑ Colonic HO‐1 | ||||
| DSS in drinking water, in BALB/C mice64 | p.o. green tea polyphenol extract (in food) for 3 days before and 7 days after DSS | ↓ Weight loss and diarrhoea; ↑ colon length; ↓histopathology (presence and degree/extent of inflammatory infiltrate, mucosal expansion, crypt epithelium disruption, ulceration); ↓ epithelial cytoskeleton distortion/fragmentation (as per laser scanning confocal microscopy); ↓ serum rGSH, SAA, TNFα, ↑ serum Hct and GSSG | Colonic glutathione status not significantly improved | |
| IL‐2‐/‐ C57BL/6J mice65 | p.o. green tea polyphenol extract (5 g/l in drinking water) for 6 weeks after disease establishment | ↑ Weight gain, ↓ colon weight; ↓ histopathology (presence and degree of inflammatory infiltrate, epithelial hyperplasia, goblet cell depletion, erosions, ulcerations and crypt abscesses); ↓ ex vivo colonic TNFα and IFN‐γ, ↓plasma SAA levels, ↑ Hct | IL‐2‐/‐ mice spontaneously develop autoimmune disease characterised by colitis, haemolytic anaemia and cachexia; all components responded to green tea intake | |
| Curcumin | TNBS enema in Wistar rats66 | p.o. curcumin (2% in food) for (a) 3 days before or (b) 14 days after TNBS | ↑ Survival; ↓ weight loss; ↓ colon histopathology (presence and degree/extent of inflammatory infiltrate, thickening of colon wall, goblet cell depletion); ↑ colonic IκB; ↓ colonic NFκB activation and IL‐1β mRNA (both groups) | Prophylactic (group a) and therapeutic (group b) curcumin showed similar efficacy to prophylactic sulphasalisine |
| TNBS enema in BALB/c mice67 | i.g. curcumin at 25, 50, 100 or 300 mg/kg/day for 10 days before and 8 days after TNBS administration | ↓ Weight loss; ↓ diarrhoea; ↓ colon weight and macropathology (presence and degree/extent of inflammation and ulceration); ↓ histopathology (presence and degree/extent of inflammatory infiltrate, thickening of colon wall, goblet cell depletion); ↓ colon MPO activity; ↓ colon NO, O2− (groups b–d). ↓ colon serine protease and NFκB activity; ↓ colon IFN‐γ, IL‐12 mRNA, ↑ IL‐4 mRNA (assessed in group b only). | Doses of 50–300 mg/kg/day were similarly effective, but 25 mg/kg/day did not significantly improve colitis. Curcumin attenuated the expression of T‐helper 1 profile of cytokines | |
| TNBS enema in C57BL/6 and BALB/c mice68 | p.o. curcumin in food at (a) 2% for 3 days before; (b) 0.5% for 7 days after; (c) 2% for 7 days after; (d) 5% for 7 days after TNBS administration or (e) 2% for 5 days starting 2 days after TNBS | ↑ Survival (group a); ↓ weight loss (groups a–d); ↓ histopathology (presence and degree of inflammatory infiltrate, vascularity, thickening of colon wall) (groups a,c,d); ↓ mucosal CD4+ T cell infiltration, NFκB activation and levels of IL‐6, IL‐12,TNFα and IFN‐γ mRNA (assessed in group a only) | ||
| TNBS enema in Sprague–Dawley rats69 | i.p. 30 mg/kg/day curcumin for 14 days after TNBS | ↑Survival; ↓weight loss; macropathology (hyperaemia, ulceration, inflammation, adhesions); ↓ micropathology (epithelial necrosis, destruction of glands, inflammatory infiltrate, ↑granulation tissue); ↓colonic IL‐1, TNFα, IFN‐γ mRNA, ↑colonic IL‐4 mRNA; | Curcumin tended to be more effective than both dexamethasone 2 mg/kg/day and combined dexamethasone and curcumin. Only curcumin increased formation of PGJ2 and granulation tissue | |
| ↑ Colonic PPAR‐γ and 15d‐PGJ2 | ||||
| TNBS enema in Sprague–Dawley rats70 | i.p. 30 or 60 mg/kg/day curcumin for 14 days after TNBS | ↑Survival; ↓macropathology (hyperaemia, ulceration, inflammation, adhesions); ↓ micropathology (epithelial necrosis, destruction of glands, inflammatory infiltrate); ↓ colon MPO activity; ↓colonic iNOS, COX‐2, TNFα, IFN‐γ mRNA, ↑PGE2 (30 and 60 mg/kg/day) | ||
| DNBS enema in C3H mice71 | p.o. curcumin (0.25% in food) for 5 days before and 5 days after DNBS | ↓ Colon macropathology (presence and degree of hyperaemia, bowel wall thickening, ulceration, inflammation; ↓ colon histopathology (presence and degree of inflammatory infiltrate, ulceration, necrosis); ↓colonic IL‐1β; ↓colonic MPO, p38 MAPK and NFκB activity | ||
| Quercetin and its naturally occurring glycones, quercetrin and rutin | TNBS enema in Sprague–Dawley rats72 | (a) i.g. rutin 10 mg/kg/day from day 1 to 6 after TNBS; | ↓ Colon macropathology (presence and degree/extent of hyperaemia, inflammation ulceration, scabbing, stricture, serosal adhesion); ↓colon MPO activity (rutin and quercetin 25‐100 μM/day) | p.r. quercetin 10 μM/day was ineffective. PO rutin and PR quercetin were as effective as PO sulphasalazine and PR 5‐ASA; rutin was metabolised to quercetin by colonic microbial glycosidases |
| (b) p.r. quercetin (enema) 10, 25, 50 or 100 μM/day from day 1 to 6 after TNBS | ||||
| TNBS enema in Wistar rats73 | i.g. quercetrin 1 or 5 mg/kg (single dose) 2 h before TNBS | ↓ Colonic alkaline phosphatase and NOS activity; ↑ colonic water and electrolyte absorption; ↓ colonic MDA (1 and 5 mk/kg) | No reduction in colonic MPO activity, perhaps due to the single dose and/or insufficient time for pharmacological effect | |
| TNBS enema in Wistar rats74 | Acute colitis: i.g. quercitrin 0.125, 0.25, 0.5, 1, 5, 10, 25 or 50 mg/kg 2 h before to and 24 h after TNBS; | Acute colitis: ↓ diahroea, ↓ colon macropathology, ↓ colon MPO and AP activity, ↑ colon glutathione content, ↑ colon fluid absorption. (1 and 5 mg/kg only) | 0.125–0.5 mg/kg and 10–50 mg/kg were not effective in acute colitis; quercetrin 1 and 5 mg/kg did not reduce markers of inflammation and oxidative stress in chronic colitis despite reduced macropathology | |
| Chronic colitis: i.g. quercitrin 1 or 5 mg/kg, 2 h before to and daily for 2–4 weeks after TNBS | Chronic colitis: ↓ diahroea, ↓ colon macropathology, ↑ colon fluid absorption (1 and 5 mg/kg/day) | |||
| DSS in drinking water, in Wistar rats75 | i.g. quercetin or quercetrin, 1 mg/kg/day for 10 days with DSS. | ↓ Disease activity index (weight loss, diarrhoea, blood in faeces), ↓ colonic MPO and NFκB activity, ↓ colonic IL‐1β, TNFα, iNOS (quercetrin) | i.g. quercetin was ineffective. Quercetrin is metabolised by colonic microbial rhamnosidases to the active quercetin | |
| DSS in drinking water, in ICR mice76 | p.o quercetin or rutin (0.1% in food) for 7 days before and 7 days concomitantly with DSS | ↓ Weight loss; ↓ colonic shortening; ↓ histopathology (presence and degree/extent of oedema, inflammation, regenerative changes, fibrosis); ↓ colon and pΦ IL‐1β, ↓ colon IL‐1β, IL‐6, GM‐CSF and COX‐2 mRNA (rutin) | i.g. quercetin was ineffective | |
| DSS in drinking water, in Wistar rats77 | p.o quercitrin in drinking water: (a) 1 mg/kg/day together with DSS for 8 days; (b) 5 mg/kg/day together with DSS for 8 days; (c) 1 mg/kg/day after 10 days of DSS, for 5 days | ↓ Disease activity index (weight loss, stool consistency, rectal bleeding) (all groups); ↓histopathology (presence and degree/extent of inflammatory infiltrate, ulceration, mucus depletion, mitotic activity in crypts, oedema, vascularity, fibrin deposition) (a,c); ↓ colon MPO and iNOS activity (a,c); ↑ colon glutathione content (all groups); ↓ colon NFκB activation | Early administration of quercitrin at 1 mg/kg/day improved more parameters than did 5 mg/kg/day; colon LTB4 levels were not reduced by quercitrin | |
| (assessed in group c only) |
AP, alkaline phosphatase; 5‐ASA, 5‐aminosalicylic acid; CCK‐8, cholecystokinin octapeptide; COX, cyclo‐oxygenase; CYP, cytochrome P450; 15d‐PGJ2, 15‐deoxy‐D 12, 14‐prostaglandin J2; DNBS, dinitrobenzene sulphate; DSS, dextran sulphate sodium; EGCG, epigallocatechin gallate; ICAM, intercellular adhesion molecule; IFN, interferon; i.g., intragastric; i.p., intraperitoneal; p.o., oral; i.v., intravenous; s.c., subcutaneous; iNOS, inducible nitric oxide synthase; MAPK, mitogen‐activated protein kinase; MDA, malondialdehyde; MPO, myeloperoxidase; NFκB, nuclear factor κ B; PPAR, peroxisome proliferator‐activated receptor; SAA, serum amyloid A; TNBS, trinitrobenzene sulphate; TNF, tumour necrosis factor.
Quercetrin (3‐rhamnosylquercetin) and rutin (3‐O‐rhamnosyl‐glucosyl‐quercetin), the two commonly occurring glycones of quercetin, lack anti‐inflammatory properties in vitro but act as pro‐drugs when ingested for the treatment of colitis. They are not well absorbed in the small intestine and are metabolised in the colon to the locally active aglycone form, quercetin, by microbial rhamnosidases.72,75 Quercetin was found to be effective when administered intrarectally,72 but its ingestion failed to ameliorate colitis despite the efficacy of its glycosides,72,73,74,75,76,77 probably due to its avid absorption in the small bowel. Protection against small intestinal disease was not discussed in these studies, but their findings suggest that ingestion of PPL glycones may not be beneficial for inflammation proximal to the colon.
Inhibition of NFκB and of leucocyte and T‐cell infiltration and activation probably contributes to PPLs' therapeutic effect in colitis.56 PPL induction of HO‐1,41,42 which reduces oxidative stress and increases carbon monoxide formation, may also blunt injury in IBD.77,78,79 A 2‐week treatment with curcumin, itself a peroxisome proliferator‐activated receptor‐γ agonist,80,81,82 increased the intestinal level of this transcription factor and its endogenous agonist 15‐deoxy‐D 12, 14‐prostaglandin J2.69 Thus, intestinal peroxisome proliferator‐activated receptor‐γ activation, which inhibits NFκB and attenuates colitis,83 may also underlie curcumin's therapeutic action. PGE2 levels are reduced in some models of chronic colitis, and may be increased by PPLs, despite reduced transcription of COX‐2.62,69,70 In one study, curcumin, but not dexamethasone, increased the formation of granulation tissue 2 weeks after induction of trinitrobenzene sulphate colitis.69 Thus, PPLs may somehow modulate the eicosanoid response such as to promote the resolution of inflammation and enhance wound healing.
Animal studies suggest that PPLs improve graft function and survival after organ transplantation, acting alone or synergistically with cyclosporine or mycophenolate mofetil, suggesting that they have immunosuppressive properties as well.84,85,86 Oral curcumin and quercetin reduced acute rejection and immunosuppressive‐drug side effects in a double‐blind study on cadaveric renal transplant recipients.87 Also of pertinence to the nutritional support of patients with IBD in a catabolic state is the finding that intraperitoneal administration of resveratrol for 10 days attenuated skeletal muscle cachexia in tumour‐bearing mice.88 A small pilot study showed that oral curcumin therapy improved clinical symptoms, histopathology and laboratory indices in all five patients with ulcerative colitis and four out of five patients with Crohn's disease who had an insufficient response to conventional treatments.89 The patients with ulcerative colitis received 550 mg curcumin twice a day for 1 month followed by the same dose once a day for another month. Patients with Crohn's disease were treated with 360 mg curcumin three times a day for 1 month followed by 360 mg four times a day for two additional months.89 Finally, the preliminary results from a 6‐month placebo‐controlled trial of curcumin therapy in 89 patients with ulcerative colitis in remission were recently presented.90 All patients were on 5‐aminosalicylic acid therapy. Relapse was seen in 5% of curcumin‐treated and 21% of placebo‐treated patients, and no serious adverse effects were reported.90
Polyphenols for acute pancreatitis
Acute pancreatitis is an inflammatory process triggered by a number of instigating factors, with variable involvement of peri‐pancreatic tissues and remote organs. After initial acinar cell injury by a specific pathogenic agent or process, the release and activation of proteases and leucocyte infiltration exacerbate a destructive autodigestive and inflammatory cascade. Most cases are mild and resolve spontaneously within 48 h with supportive therapy. But in patients with severe acute pancreatitis (SAP), spillover of inflammatory molecules can initiate early or late multiple organ dysfunction syndrome.90 Early enteral nutrition, rather than nothing per os or parenteral nutrition, is the recommended approach for patients with severe, and especially necrotising acute pancreatitis, despite the rationale behind avoiding stimulation of the pancreas in such a scenario. Parenteral nutrition is reserved for patients with SAP that develop critical illness and in whom attempts at administering enteral nutrition have failed.91,92
PPLs are protective in experimental acute pancreatitis93,94,95,96,97,98,99,100,101,102,103,104 induced either by injection of tert‐butyl hydroperoxide79 or sodium taurocholate into the pancreatic duct,81,82,85 intraperitoneal83 or intravenous86 injection of cerulein, intraperitoneal injection of DL‐ethionine84 or intravenous cholecystokinin octapeptide (CCK‐8) together with oral ethanol (table 2).86 Resveratrol,79,80,81,82 EGCG/green tea PPL extract83,84 and curcumin85,86 attenuate acute pancreatitis in rodents when administered before, concomitantly to or after the instigating agent. Pancreatic damage (pancreatic macropathology and micropathology, neutrophil infiltrate, trypsin activity, lipoperoxides and inflammatory cytokines) was reduced in all studies except for two that were performed by the same group,102,103 and in which curcumin did reduce serum amylase, TNFα and IL‐6 as well as bacterial translocation.
Table 2 Prophylactic and therapeutic effects of polyphenol administration in rodent models of acute pancreatitis.
| Polyphenol | Model and reference | Route, dose, duration and timing of administration | Outcomes | Comments |
|---|---|---|---|---|
| Resveratrol | ButOOH injection into pancreatic duct of Wistar rats94 | i.p. resveratrol 2 mg/day for 8 days before ButOOH injection (rats weighed ∼300 g) | ↓Pancreatic weight, ↓pancreatic histopathology (acinar vacuolisation, focal oedema, necrosis, haemorrhage), ↓ pancreatic carbonyl and SH groups, ↓acinar RES cistern dilation and mitochondrial swelling (per electron microscopy), ↓serum amylase activity | Administration of diethylstilbesterol, a synthetic analogue of resveratrol, was equally effective |
| Taurocholate injection into pancreatic duct of Sprague–Dawley rats95,96 | i.p. resveratrol 30 mg/kg (single dose) after taurocholate injection | ↓ Pancreatic histopathology (haemorrhage, microthrombi, exudates, inflammatory infiltrate), ↓ pancreatic NFκB activity, ↓pancreatic TNFα and IL‐8, ↓lung histopathology (alveolar septum thickening, interstitial oedema, inflammatory infiltrate), ↓lung water content and capillary permeability, ↓ lung MPO activity, ↓ lung ICAM‐1, ↓ blood viscosity | ||
| Taurocholate injection into pancreatic duct of Sprague–Dawley rats97 | i.v. 10 mg/kg resveratrol 5 min after sodium taurocholate | ↓Micropathology (intralobular and interlobular oedema, inflammatory infiltrate, haemorrhage, necrosis); ↓PΦ NFκB activation and iNOS activity; ↓serum TNFα, IL‐1β and NO | ||
| Taurocholate injection into pancreatic duct of Sprague–Dawley rats98 | i.v. 20 mg/kg resveratrol after sodium taurocholate | ↓Macropathology (oedema, necrosis, haemorrhage, saponification); ↓amount and turbidity of ascitic fluid; ↓micropathology (intralobular and interlobular oedema, inflammatory infiltrate, haemorrhage, thrombosis, necrosis); ↓pancreatic TBARS, ↓pancreatic MPO activity, ↑pancreatic SOD | ||
| s.c. CCK‐8 in Wistar rats99 | i.p. 10 mg/kg resveratrol 30 min before CCK‐8 | ↓ Pancreatic wet weight, ↓pancreatic histopathology (acinar cell vacuolisation, intralobule and interlobule oedema, inflammatory infiltrate) | ||
| EGCG/green tea polyphenol extract | i.p. cerulein in Wistar rats100 | p.o. green tea polyphenol extract (in drinking water) for 10 days before i.p. cerulein | ↓ Pancreatic wet weight, ↓pancreatic histopathology (acinar cell vacuolisation, intralobule and interlobule oedema), ↓ pancreatic MDA, ↓ serum amylase | |
| i.p. DL‐ethionine in Wistar rats101 | p.o. green tea polyphenol extract (in drinking water) for 10 days before i.p. DL‐ethionine) | ↓ Pancreatic wet weight, ↓ pancreatic histopathology (acinar cell necrosis, intralobule and interslobule oedema), ↓ pancreatic MDA, ↓ serum amylase | ||
| Curcumin | Taurocholate injection into pancreatic duct of Wistar rats102 | (a) i.g. curcumin 100 mg/kg/day for 20 days before and 6 days after taurocholate administration; (b) as in group (a) and i.p. ciprofloxacillin and metronidazole for 6 days after taurocholate administration | ↓ Bacterial translocation; ↓ serum amylase, MDA and NO | Combination of curcumin and antibiotics produced better results than curcumin alone, but neither groups reduced pancreatic histopathologic scores |
| Taurocholate injection into pancreatic duct of Wistar rats103 | i.g. curcumin 100 mg/kg/day for 20 days before and 6 days after taurocholate administration | ↓ Serum TNFα and Il‐6 | Curcumin did not reduce tissue injury | |
| (1) i.v. cerulein or (2) p.o. ethanol + i.v. CCK‐8 in Sprague–Dawley rats104 | i.v. curcumin 200 mg/kg (single dose) concomitantly to cerulein/CCK‐8 | ↓ Pancreatic histopathology, ↓pancreatic trypsin activity, ↓neutrophil infiltration, ↓pancreatic NFκB and AP‐1 activation, ↑pancreatic IκB, ↓pancreatic IL‐6, TNFα, iNOS mRNA, ↓serum amylase and lipase | Curcumin did not reduce CCK‐mediated amylase secretion, suggesting that the reduced pancreatic trypsin activity is due to its antineutrophil effect |
CCK‐8, cholecystokinin octapeptide; EGCG, epigallocatechin gallate; ICAM, intercellular adhesion molecule; iNOS, inducible nitric oxide synthase; i.g., intragastric; i.p., intraperitoneal; p.o., oral; i.v., intravenous; s.c., subcutaneous; MDA, malondialdehyde; MPO, myeloperoxidase; NFκB, nuclear factor κ B; SOD, sphincter of Oddi dysfunction; TNF, tumour necrosis factor.
Inhibition of NFκB57 and enhanced expression of HO‐1105 may mediate PPLs' protective effects in acute pancreatitis.
Some evidence suggests that administration of PPLs early in the course of acute pancreatitis may prevent the development of multiple organ dysfunction and septic shock: resveratrol attenuated SAP‐associated acute respiratory distress syndrome.95,96 Prophylactic administration of PPLs attenuates ischaemia‐reperfusion injury to the bowel,106,107 which has been implicated in bacterial translocation, pancreatic infection and development of sepsis in SAP.108,109 PPLs also protect against ischaemia‐reperfusion injury to the kidneys, liver and heart, 110,111,112,113,114,115,116 and may thus reduce dysfunction of these organs in the context of the severe inflammatory response syndrome. Finally, pre‐emptive administration of PPLs significantly improves survival rates in rodent models of endotoxinemia,117,118,119 whereas intravenous curcumin treatment attenuates the liver injury, systemic inflammation and mortality associated with caecal ligation and puncture, even when initiated 5 h after the puncture.80
Some considerations for future research and development
Beyond confirming their efficacy, the animal studies reviewed here contribute little to the formulation of a PPL extract (ie, which PPL(s), what dosage(s) would be effective when administered with enteral nutrition/parenteral nutrition). Effective dosage(s) varied among different models, rodent species and studies. Controlled clinical studies of PPL supplementation90 should provide a closer approximation, but the slow coadministration of PPLs together with nutrient‐rich enteral nutrition/parenteral nutrition formulas may influence PPLs' pharmacodynamics. For instance, quercetrin supplementation potentiates ω‐3 PUFAs' anti‐inflammatory effect in DSS‐induced colitis120 but quercetin inhibits the induction of heat shock protein 70 (hsp70),121 which partially mediates the beneficial response to glutamine.122,123 What then would be the sum effect of combining quercetin (or its glycones) with an “immunonutritional” package containing glutamine and ω‐3 PUFAs? Other PPLs actually induce hsp70 or at least do not interfere with its cytoprotective properties.124,125
Uncertainties notwithstanding, the animal studies do suggest that PPLs have a wide therapeutic window, and that potentially, numerous combinations/dosages would be beneficial. PPLs generally remain non‐toxic, even at relatively high doses126,127,128,129,130 and safety studies in healthy volunteers, followed by dose‐finding/safety studies in patients receiving enteral nutrition/parenteral nutrition should help identify therapeutic regimens.
Food–drug interactions are another issue which needs to be discussed. Quercetin inhibits cytochrome P450 (CYP) 3A4131 and increases blood levels of cyclosporine (which is occasionally used to treat IBD)132 in healthy volunteers.133 Consumption of a green tea extract did not interfere with CYP3A4‐mediated or 2D6‐mediated drug metabolism.134 To the best of our knowledge, the effect of curcumin and resveratrol on clinically relevant pharmacokinetics in humans has not been assessed. Despite their potential to increase cyclosporine levels, the PPLs reviewed here actually protect against cyclosporine‐induced nephrotoxicity.135,136,137,138,139,140 while enhancing its therapeutic immunosuppressive effect (in organ transplantation).85,86
In conclusion, PPLs are anti‐inflammatory and cytoprotective constituents of plant‐derived food that reduce the severity of experimental IBD and acute pancreatitis and may safely enhance the therapeutic effect of enteral and parenteral nutrition in patients with these conditions. Further preclinical and clinical studies are indicated.
The body responds to cell injury by initiating an inflammatory response aimed at immobilising, destroying and removing the injurious agent, and by cytoprotective adaptations that reduce direct and co‐lateral damage to parenchymal cells. Numerous injurious agents initiate intracellular cascades that converge on transcription factors, such as NFκB and Nrf2, allowing their translocation to the nucleus where they enhance, respectively, the transcription of pro‐inflammatory and cytoprotective genes. Through their divergent effect on NFκB and Nrf2, PPLs inhibit the synthesis of potentially injurious mediators while enhancing that of antioxidative and anti‐inflammatory ones.
Acknowledgements
The authors thank Dr Alexandra Mahler for her excellent editorial assistance.
Footnotes
iAs olives are rich in lipophilic PPLs, olive oil‐based formulas may contain remnants that have not been removed during processing. Olive PPLs are antioxidative, anti‐inflammatory and vasculoprotective in humans and it has been suggested that they, rather than the mono‐unsaturated fatty acids, are the anti‐inflammatory component found in dietary olive oil and in nutritional formulas based on it. If this is the case, it would further support a beneficial role for combining PPLs in artificial nutrition.
Competing interests: None.
References
- 1.Balandrin M F, Klocke J A, Wurtele E S.et al Natural plant chemicals: sources of industrial and medicinal materials. Science 1985281154–1160. [DOI] [PubMed] [Google Scholar]
- 2.Verpoorte R. Plant secondary metabolism. In: Verpoorte R, Alfermann AW, eds. Metabolic engineering of plant secondary metabolism. Dordrecht: Kluwer Academic Publishers, 20001–29.
- 3.Eastwood M A. A molecular biological basis for the nutritional and pharmacological benefits of dietary plants. QJM 20019445–48. [DOI] [PubMed] [Google Scholar]
- 4.Kris‐Etherton P M, Hecker K D.et al Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med 2002113(Suppl 9B)71S–88S. [DOI] [PubMed] [Google Scholar]
- 5.Hu F B. Plant‐based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr 200378(Suppl 3)544S–551S. [DOI] [PubMed] [Google Scholar]
- 6.Most M M. Estimated phytochemical content of the dietary approaches to stop hypertension (DASH) diet is higher than in the Control Study Diet. J Am Diet Assoc 20041041725–1727. [DOI] [PubMed] [Google Scholar]
- 7.Dryden G W, Song M, McClain C. Polyphenols and gastrointestinal diseases. Curr Opin Gastroenterol 200622165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rock C L. Nutrition in the prevention and treatment of disease. In: Goldman L, Ausellio DA, eds. Cecil textbook of medicine. 22nd edn. Philadelphia, PA: Saunders, 2004. 108–1311
- 9.Bidlack W R, Wang W. Designing functional foods. In: Shils ME, et al eds. Modern nutrition in health and disease. 10th edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2005
- 10.Simopoulos A P. The traditional diet of Greece and cancer. Eur J Cancer Prev 200413219–230. [DOI] [PubMed] [Google Scholar]
- 11.Schaffer S, Muller W E, Eckert G P.et al Proceedings of the 1st International Conference on Polyphenols and Health. Vichy, France, November 18–21, 2004. Am J Clin Nutr 200581(Suppl 1)215S–335S. [PubMed] [Google Scholar]
- 12.Scalbert A, Manach C, Morand C.et al Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 200545287–306. [DOI] [PubMed] [Google Scholar]
- 13.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 199856317–333. [DOI] [PubMed] [Google Scholar]
- 14.Kroon P A, Clifford M N, Crozier A.et al How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr 20048015–21. [DOI] [PubMed] [Google Scholar]
- 15.Harper C R, Jacobson T A. Usefulness of omega‐3 fatty acids and the prevention of coronary heart disease. Am J Cardiol 2005961521–1529. [DOI] [PubMed] [Google Scholar]
- 16.Kris‐Etherton P M, Harris W S, AHA Nutrition Committee. American Heart Association et al Omega‐3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol 200323151–152. [DOI] [PubMed] [Google Scholar]
- 17.Heller A R, Rossler S, Litz R J.et al Omega‐3 fatty acids improve the diagnosis‐related clinical outcome. Crit Care Med 200634972–979. [DOI] [PubMed] [Google Scholar]
- 18.Kreymann K G, Berger M M, Deutz N E.et al ESPEN Guidelines on Enteral Nutrition: intensive care. Clin Nutr 200625210–223. [DOI] [PubMed] [Google Scholar]
- 19.Heimburger D C. Nutrition's interface with health and disease. In: Goldman L, Ausellio DA, eds. Cecil textbook of medicine, 22nd edn. Philadelphia, PA: Saunders, 200449–54.
- 20.Scalbert A, Johnson I T, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr 200581(Suppl 1)215S–17S. [DOI] [PubMed] [Google Scholar]
- 21.Kris‐Etherton P M, Lefevre M, Beecher G R.et al Bioactive compounds in nutrition and health‐research methodologies for establishing biological function: the antioxidant and anti‐inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr 200424511–538. [DOI] [PubMed] [Google Scholar]
- 22.Heiss C, Kleinbongard P, Dejam A.et al Acute consumption of flavanol‐rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 2005461276–1283. [DOI] [PubMed] [Google Scholar]
- 23.Marrugat J, Covas M I, Fito M.et al SOLOS Investigators. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation—a randomized controlled trial. Eur J Nutr 200443140–147. [DOI] [PubMed] [Google Scholar]
- 24.Schroeter H, Heiss C, Balzer J.et al (‐)‐Epicatechin mediates beneficial effects of flavanol‐rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A 20061031024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lekakis J, Rallidis L S, Andreadou I.et al Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil 200512596–600. [DOI] [PubMed] [Google Scholar]
- 26.Ruano J, Lopez‐Miranda J, Fuentes F.et al Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J Am Coll Cardiol 2005461864–1868. [DOI] [PubMed] [Google Scholar]
- 27.Grassi D, Lippi C, Necozione S.et al Short‐term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr 200581611–614. [DOI] [PubMed] [Google Scholar]
- 28.Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol 20051677–84. [DOI] [PubMed] [Google Scholar]
- 29.Hughes D A. Plant polyphenols: modifiers of immune function and risk of cardiovascular disease. Nutrition 200521422–423. [DOI] [PubMed] [Google Scholar]
- 30.Barnes P J, Karin M. Nuclear factor‐kappa B: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 19973361066–1071. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Yamamoto Y, Wang Q M. The IKK NF‐kappa B system: a treasure trove for drug development. Nat Rev Drug Discov 2004317–26. [DOI] [PubMed] [Google Scholar]
- 32.de la Lastra C A, Villegas I. Resveratrol as an anti‐inflammatory and anti‐aging agent: mechanisms and clinical implications. Mol Nutr Food Res 200549405–430. [DOI] [PubMed] [Google Scholar]
- 33.Baur J A, Sinclair D A. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 20065493–506. [DOI] [PubMed] [Google Scholar]
- 34.Frei B, Higdon J V. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr 20031333275S–84S. [DOI] [PubMed] [Google Scholar]
- 35.Joe B, Vijaykumar M, Lokesh B R. Biological properties of curcumin‐cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr 20044497–111. [DOI] [PubMed] [Google Scholar]
- 36.Bengmark S. Curcumin, an atoxic antioxidant and natural NF{kappa}B, cyclooxygenase‐2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr 20063045–51. [DOI] [PubMed] [Google Scholar]
- 37.Dias A S, Porawski M, Alonso M.et al Quercetin decreases oxidative stress, NF‐{kappa}B activation, and iNOS overexpression in liver of streptozotocin‐induced diabetic rats. J Nutr 20051352299–2304. [DOI] [PubMed] [Google Scholar]
- 38.Delhalle S, Blasius R, Dicato M.et al A beginner's guide to NF‐kappa B signaling pathways. Ann N Y Acad Sci 200410301–13. [DOI] [PubMed] [Google Scholar]
- 39.Kaga S, Zhan L, Matsumoto M.et al Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin‐1, heme oxygenase‐1 and vascular endothelial growth factor. J Mol Cell Cardiol 200539813–822. [DOI] [PubMed] [Google Scholar]
- 40.Gaedeke J, Noble N A, Border W A. Curcumin blocks fibrosis in anti‐Thy 1 glomerulonephritis through up‐regulation of heme oxygenase 1. Kidney Int 2005682042–2049. [DOI] [PubMed] [Google Scholar]
- 41.Surh Y J, Kundu J K, Na H K.et al Redox‐sensitive transcription factors as prime targets for chemoprevention with anti‐inflammatory and antioxidative phytochemicals. J Nutr 2005135(Suppl 12)2993S–3001S. [DOI] [PubMed] [Google Scholar]
- 42.Moskaug J O, Carlsen H, Myhrstad M C.et al Polyphenols and glutathione synthesis regulation. Am J Clin Nutr 200581(Suppl 1)277S–283S. [DOI] [PubMed] [Google Scholar]
- 43.Lee J M, Johnson J A. An important role of Nrf2‐ARE pathway in the cellular defense mechanism. J Biochem Mol Biol 200437139–143. [DOI] [PubMed] [Google Scholar]
- 44.Lipman T O. The chicken soup paradigm and nutrition support: rethinking terminology. JPEN J Parenter Enteral Nutr 20032793–94. [DOI] [PubMed] [Google Scholar]
- 45.Grimble R F. Immunonutrition. Curr Opin Gastroenterol 200521216–222. [DOI] [PubMed] [Google Scholar]
- 46.Olah A, Belagyi T, Issekutz A.et al Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg 2002891103–1107. [DOI] [PubMed] [Google Scholar]
- 47.Fell J M. Control of systemic and local inflammation with transforming growth factor beta containing formulas. JPEN J Parenter Enteral Nutr 200529(Suppl 4)S126–S128. [DOI] [PubMed] [Google Scholar]
- 48.Bengmark S. Synbiotics and the mucosal barrier in critically ill patients. Curr Opin Gastroenterol 200521712–716. [DOI] [PubMed] [Google Scholar]
- 49.Grimble R. Fatty acid profile of modern lipid emulsions: scientific considerations for creating the ideal composition. Clin Nutr Suppl 200519–15. [Google Scholar]
- 50.Meyskens F L, Jr, Szabo E. Diet and cancer: the disconnect between epidemiology and randomized clinical trials. Cancer Epidemiol Biomarkers Prev 2005141366–1369. [DOI] [PubMed] [Google Scholar]
- 51.Zingarelli B, Sheehan M, Wong H R. Nuclear factor‐kappa B as a therapeutic target in critical care medicine. Crit Care Med 200331(Suppl 1)S105–S111. [DOI] [PubMed] [Google Scholar]
- 52.Wright J G, Christman J W. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med 20032211–219. [DOI] [PubMed] [Google Scholar]
- 53.Foulds S, Galustian C, Mansfield A O.et al Transcription factor NF kappa B expression and postsurgical organ dysfunction. Ann Surg 200123370–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guttridge D C. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care 20047443–450. [DOI] [PubMed] [Google Scholar]
- 55.Schottelius A J, Baldwin A S., Jr A role for transcription factor NF‐kappa B in intestinal inflammation. Int J Colorectal Dis 19991418–28. [DOI] [PubMed] [Google Scholar]
- 56.Algul H, Tando Y, Schneider G.et al Acute experimental pancreatitis and NF‐kappa B/Rel activation. Pancreatology 20022503–509. [DOI] [PubMed] [Google Scholar]
- 57.Friedman S, Blumberg R S. Inflammatory bowel disease. In: Fauci ASBraunwald EIsselbacher KJ, et al eds. Harrison's principles of internal medicine. 16th edn. New York: McGraw Hill Professional 2005
- 58.Kleinman R E, Baldassano R N, Caplan A.et al Nutrition support for pediatric patients with inflammatory bowel disease: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 20043915–27. [DOI] [PubMed] [Google Scholar]
- 59.Lochs H, Dejong C, Hammarqvist F.et al ESPEN guidelines on enteral nutrition: gastroenterology. Clin Nutr 200625260–274. [DOI] [PubMed] [Google Scholar]
- 60.Martin A R, Villegas I, La Casa C.et al Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol 2004671399–1410. [DOI] [PubMed] [Google Scholar]
- 61.Martin A R, Villegas I, Sanchez‐Hidalgo M.et al The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol 2006147873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazzon E, Muia C, Paola R D.et al Green tea polyphenol extract attenuates colon injury induced by experimental colitis. Free Radic Res 2005391017–1025. [DOI] [PubMed] [Google Scholar]
- 63.Oz H S, Chen T S, McClain C J.et al Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem 200516297–304. [DOI] [PubMed] [Google Scholar]
- 64.Varilek G W, Yang F, Lee E Y.et al Green tea polyphenol extract attenuates inflammation in interleukin‐2‐deficient mice, a model of autoimmunity. J Nutr 20011312034–2039. [DOI] [PubMed] [Google Scholar]
- 65.Jian Y T, Mai G F, Wang J D.et al Preventive and therapeutic effects of NF‐kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol 2005111747–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ukil A, Maity S, Karmakar S.et al Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid‐induced colitis. Br J Pharmacol 2003139209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugimoto K, Hanai H, Tozawa K.et al Curcumin prevents and ameliorates trinitrobenzene sulfonic acid‐induced colitis in mice. Gastroenterology 20021231912–1922. [DOI] [PubMed] [Google Scholar]
- 68.Zhang M, Deng C, Zheng J.et al Curcumin inhibits trinitrobenzene sulphonic acid‐induced colitis in rats by activation of peroxisome proliferator‐activated receptor gamma. Int Immunopharmacol 200661233–1242. [DOI] [PubMed] [Google Scholar]
- 69.Jiang H, Deng C S, Zhang M.et al Curcumin‐attenuated trinitrobenzene sulphonic acid induces chronic colitis by inhibiting expression of cyclooxygenase‐2. World J Gastroenterol 2006123848–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salh B, Assi K, Templeman V.et al Curcumin attenuates DNB‐induced murine colitis. Am J Physiol Gastrointest Liver Physiol 2003285G235–G243. [DOI] [PubMed] [Google Scholar]
- 71.Kim H, Kong H, Choi B.et al Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm Res 2005221499–1509. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez de Medina F, Vera B.et al Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci 2002703097–3108. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez de Medina F, Galvez J.et al Effect of quercitrin on acute and chronic experimental colitis in the rat. J Pharmacol Exp Ther 1996278771–779. [PubMed] [Google Scholar]
- 74.Comalada M, Camuesco D, Sierra S.et al In vivo quercitrin anti‐inflammatory effect involves release of quercetin, which inhibits inflammation through down‐regulation of the NF‐kappaB pathway. Eur J Immunol 200535584–592. [DOI] [PubMed] [Google Scholar]
- 75.Kwon K H, Murakami A, Tanaka T.et al Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium‐induced experimental colitis in mice: attenuation of pro‐inflammatory gene expression. Biochem Pharmacol 200569395–406. [DOI] [PubMed] [Google Scholar]
- 76.Camuesco D, Comalada M, Rodriguez‐Cabezas M E.et al The intestinal anti‐inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol 2004143908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W P, Guo X, Koo M W.et al Protective role of heme oxygenase‐1 on trinitrobenzene sulfonic acid‐induced colitis in rats. Am J Physiol Gastrointest Liver Physiol 2001281G586–G594. [DOI] [PubMed] [Google Scholar]
- 78.Berberat P O, A‐Rahim Y I, Yamashita K.et al Heme oxygenase‐1‐generated biliverdin ameliorates experimental murine colitis. Inflamm Bowel Dis 200511350–359. [DOI] [PubMed] [Google Scholar]
- 79.Siddiqui A M, Cui X, Wu R.et al The anti‐inflammatory effect of curcumin in an experimental model of sepsis is mediated by up‐regulation of peroxisome proliferator‐activated receptor‐gamma. Crit Care Med 2006342009–2011. [DOI] [PubMed] [Google Scholar]
- 80.Zheng S, Chen A. Activation of PPAR gamma is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J 2004384149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol 2005288G447–G456. [DOI] [PubMed] [Google Scholar]
- 82.Adachi M, Kurotani R, Morimura K.et al PPAR{gamma} in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 2006551067–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhong Z, Connor H D, Froh M.et al Free radical‐dependent dysfunction of small‐for‐size rat liver grafts: prevention by plant polyphenols. Gastroenterology 2005129652–664. [DOI] [PubMed] [Google Scholar]
- 84.Chueh S C, Lai M K, Liu I S.et al Curcumin enhances the immunosuppressive activity of cyclosporine in rat cardiac allografts and in mixed lymphocyte reactions. Transplant Proc 2003351603–1605. [DOI] [PubMed] [Google Scholar]
- 85.Wu S L, Pan C E, Yu L.et al Immunosuppression by combined use of cyclosporine and resveratrol in a rat liver transplantation model. Transplant Proc 2005372354–2359. [DOI] [PubMed] [Google Scholar]
- 86.Shoskes D, Lapierre C, Cruz‐Corerra M.et al Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation 2005801556–1559. [DOI] [PubMed] [Google Scholar]
- 87.Wyke S M, Russell S T, Tisdale M J. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF‐kappaB activation. Br J Cancer 2004911742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holt P R, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci 2005502191–2193. [DOI] [PubMed] [Google Scholar]
- 89.Hanai H, Iida T, Takeuchi K.et al Koide Curcumin, a promising drug for long‐term maintenance therapy in patients with ulcerative colitis: results from a multicenter, randomized double‐blind placebo‐controlled clinical trial. Gastroenterology 2006130A84. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell R M, Byrne M F, Baillie J. Pancreatitis. Lancet 20033611447–1455. [DOI] [PubMed] [Google Scholar]
- 91.Meier R, Ockenga J, Pertkiewicz M.et al ESPEN guidelines on enteral nutrition: pancreas. Clin Nutr 200625275–284. [DOI] [PubMed] [Google Scholar]
- 92.Ma Z H, Ma Q Y. Resveratrol: a medical drug for acute pancreatitis. World J Gastroenterol 2005113171–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawinski M, Sledzinski Z, Kubasik‐Juraniec J.et al Does resveratrol prevent free radical‐induced acute pancreatitis? Pancreas 20053143–47. [DOI] [PubMed] [Google Scholar]
- 94.Meng Y, Ma Q Y, Kou X P.et al Effect of resveratrol on activation of nuclear factor kappa‐B and inflammatory factors in rat model of acute pancreatitis. World J Gastroenterol 200511525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma Z H, Ma Q Y, Wang L C.et al Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm Res 200554522–527. [DOI] [PubMed] [Google Scholar]
- 96.Li Z D, Ma Q Y, Wang C A. Effect of resveratrol on pancreatic oxygen free radicals in rats with severe acute pancreatitis. World J Gastroenterol 200612137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szabolcs A, Varga I S, Varga C.et al Beneficial effect of resveratrol on cholecystokinin‐induced experimental pancreatitis. Eur J Pharmacol 2006532(1–2)187–193. [DOI] [PubMed] [Google Scholar]
- 98.Meng Y, Zhang M, Xu J.et al Effect of resveratrol on microcirculation disorder and lung injury following severe acute pancreatitis in rats. World J Gastroenterol 200511433–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takabayashi F, Harada N. Effects of green tea catechins (polyphenon 100) on cerulein‐induced acute pancreatitis in rats. Pancreas 199714276–279. [DOI] [PubMed] [Google Scholar]
- 100.Takabayashi F, Harada N, Hara Y. The effects of green tea catechins (polyphenon) on DL‐ethionine‐induced acute pancreatitis. Pancreas 199511127–131. [DOI] [PubMed] [Google Scholar]
- 101.Gulcubuk A, Sonmez K, Gurel A.et al Pathologic alterations detected in acute pancreatitis induced by sodium taurocholate in rats and therapeutic effects of curcumin, ciprofloxacin and metronidazole combination. Pancreatology 20055(4–5)345–353. [DOI] [PubMed] [Google Scholar]
- 102.Gulcubuk A, Altunatmaz K, Sonmez K.et al Effects of curcumin on tumour necrosis factor‐alpha and interleukin‐6 in the late phase of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin Med 20065349–54. [DOI] [PubMed] [Google Scholar]
- 103.Gukovsky I, Reyes C N, Vaquero E C.et al Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol 2003284G85–G95. [DOI] [PubMed] [Google Scholar]
- 104.Nakamichi I, Habtezion A, Zhong B.et al Hemin‐activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase‐1 induction. J Clin Invest 20051153007–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mojzis J, Hviscova K, Germanova D.et al Protective effect of quercetin on ischemia/reperfusion‐induced gastric mucosal injury in rats. Physiol Res 200150501–506. [PubMed] [Google Scholar]
- 106.Giakoustidis A E, Giakoustidis D E, Iliadis S.et al Attenuation of intestinal ischemia/reperfusion induced liver and lung injury by intraperitoneal administration of (‐)‐Epigallocatechin‐3‐gallate. Free Radic Res 200640103–110. [DOI] [PubMed] [Google Scholar]
- 107.Rahman S H, Ammori B J, Holmfield J.et al Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg 2003726–35. [DOI] [PubMed] [Google Scholar]
- 108.Kolkman J J, Mensink P B. Non‐occlusive mesenteric ischaemia: a common disorder in gastroenterology and intensive care. Best Pract Res Clin Gastroenterol 200317457–473. [DOI] [PubMed] [Google Scholar]
- 109.Singer P, Shapiro H, Cohen J. Brain death and organ damage: the modulating effects of nutrition. Transplantation 2005801363–1368. [DOI] [PubMed] [Google Scholar]
- 110.Bradamante S, Barenghi L, Piccinini F.et al Resveratrol provides late‐phase cardioprotection by means of a nitric oxide‐ and adenosine‐mediated mechanism. Eur J Pharmacol 2003465115–123. [DOI] [PubMed] [Google Scholar]
- 111.Imamura G, Bertelli A A, Bertelli A.et al Pharmacological preconditioning with resveratrol: an insight with iNOS knockout mice. Am J Physiol Heart Circ Physiol 2002282H1996–H2003. [DOI] [PubMed] [Google Scholar]
- 112.Hung L M, Su M J, Chu W K.et al The protective effect of resveratrols on ischaemia‐reperfusion injuries of rat hearts is correlated with antioxidant efficacy. Br J Pharmacol 20021351627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fiorini R N, Donovan J L, Rodwell D.et al Short‐term administration of (‐)‐epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl 200511298–308. [DOI] [PubMed] [Google Scholar]
- 114.Zhong Z, Froh M, Connor H D.et al Prevention of hepatic ischemia‐reperfusion injury by green tea extract. Am J Physiol Gastrointest Liver Physiol 2002283G957–G964. [DOI] [PubMed] [Google Scholar]
- 115.Yeh C H, Lin Y M, Wu Y C.et al Inhibition of NF‐kappaB activation can attenuate ischemia/reperfusion‐induced contractility impairment via decreasing cardiomyocytic proinflammatory gene up‐regulation and matrix metalloproteinase expression. Cardiovasc Pharmacol 200545301–309. [DOI] [PubMed] [Google Scholar]
- 116.Madan B, Ghosh B. Diferuloylmethane inhibits neutrophil infiltration and improves survival of mice in high‐dose endotoxin shock. Shock 20031991–96. [DOI] [PubMed] [Google Scholar]
- 117.Yang F, de Villiers W J, McClain C J.et al Green tea polyphenols block endotoxin‐induced tumor necrosis factor‐production and lethality in a murine model. J Nutr 19981282334–2340. [DOI] [PubMed] [Google Scholar]
- 118.Takahashi K, Morikawa A, Kato Y.et al Flavonoids protect mice from two types of lethal shock induced by endotoxin. FEMS Immunol Med Microbiol 20013129–33. [DOI] [PubMed] [Google Scholar]
- 119.Camuesco D, Comalada M, Concha A.et al Intestinal anti‐inflammatory activity of combined quercitrin and dietary olive oil supplemented with fish oil, rich in EPA and DHA (n‐3) polyunsaturated fatty acids, in rats with DSS‐induced colitis. Clin Nutr . 2006;25466–476. [DOI] [PubMed]
- 120.Wei Y Q, Zhao X, Kariya Y.et al Induction of apoptosis by quercetin: involvement of heat shock protein. Cancer Res 1994544952–4957. [PubMed] [Google Scholar]
- 121.Singleton K D, Serkova N, Beckey V E.et al Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med 2005331206–1213. [DOI] [PubMed] [Google Scholar]
- 122.Singleton K D, Serkova N, Banerjee A.et al Glutamine attenuates endotoxin‐induced lung metabolic dysfunction: potential role of enhanced heat shock protein 70. Nutrition 200521214–223. [DOI] [PubMed] [Google Scholar]
- 123.Warzecha Z, Dembinski A, Ceranowicz P.et al Ischemic preconditioning inhibits development of edematous cerulein‐induced pancreatitis: involvement of cyclooxygenases and heat shock protein 70. World J Gastroenterol 2005115958–5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dunsmore K E, Chen P G, Wong H R. Curcumin, a medicinal herbal compound capable of inducing the heat shock response. Crit Care Med 2001292199–2204. [DOI] [PubMed] [Google Scholar]
- 125.Crowell J A, Korytko P J, Morrissey R L.et al Resveratrol‐associated renal toxicity. Toxicol Sci 200482614–619. [DOI] [PubMed] [Google Scholar]
- 126.Isbrucker R A, Edwards J A, Wolz E.et al Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short‐term toxicity studies. Food Chem Toxicol 200644636–650. [DOI] [PubMed] [Google Scholar]
- 127.Isbrucker R A, Bausch J, Edwards J A.et al Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: genotoxicity. Food Chem Toxicol 200644626–635. [DOI] [PubMed] [Google Scholar]
- 128.Chow H H, Cai Y, Hakim I A.et al Pharmacokinetics and safety of green tea polyphenols after multiple‐dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res 200393312–3319. [PubMed] [Google Scholar]
- 129.Cheng A L, Hsu C H, Lin J K.et al Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high‐risk or pre‐malignant lesions. Anticancer Res 2001212895–2900. [PubMed] [Google Scholar]
- 130.He N, Edeki T. The inhibitory effects of herbal components on CYP2C9 and CYP3A4 catalytic activities in human liver microsomes. Am J Ther 200411206–212. [DOI] [PubMed] [Google Scholar]
- 131.Van Assche G, Vermeire S, Rutgeerts P. Medical treatment of inflammatory bowel diseases. Curr Opin Gastroenterol 200521443–447. [PubMed] [Google Scholar]
- 132.Choi J S, Choi B C, Choi K E. Effect of quercetin on the pharmacokinetics of oral cyclosporine. Am J Health Syst Pharm 2004612406–2409. [DOI] [PubMed] [Google Scholar]
- 133.Donovan J L, Chavin K D, Devane C L.et al Green tea (Camellia sinensis) extract does not alter cytochrome p450 3A4 or 2D6 activity in healthy volunteers [correction appears in Drug Metab Dispos 2004;32:1331]. Drug Metab Dispos 200432906–908. [DOI] [PubMed] [Google Scholar]
- 134.Chang E J, Mun K C. Effect of epigallocatechin gallate on renal function in cyclosporine‐induced nephrotoxicity. Transplant Proc 2004362133–2134. [DOI] [PubMed] [Google Scholar]
- 135.Anjaneyulu M, Tirkey N, Chopra K. Attenuation of cyclosporine‐induced renal dysfunction by catechin: possible antioxidant mechanism. Ren Fail 200325691–707. [DOI] [PubMed] [Google Scholar]
- 136.Satyanarayana P S, Singh D, Chopra K. Quercetin, a bioflavonoid, protects against oxidative stress‐related renal dysfunction by cyclosporine in rats. Methods Find Exp Clin Pharmacol 200123175–181. [DOI] [PubMed] [Google Scholar]
- 137.Chander V, Tirkey N, Chopra K. Resveratrol, a polyphenolic phytoalexin protects against cyclosporine‐induced nephrotoxicity through nitric oxide dependent mechanism. Toxicology 200521055–64. [DOI] [PubMed] [Google Scholar]
- 138.Mohamadin A M, El‐Beshbishy H A, El‐Mahdy M A. Green tea extract attenuates cyclosporine A‐induced oxidative stress in rats. Pharmacol Res 20055151–57. [DOI] [PubMed] [Google Scholar]
- 139.Shi S H, Zheng S S, Jia C K.et al Inhibitory effect of tea polyphenols on transforming growth factor‐beta1‐expression in rat with cyclosporine A‐induced chronic nephrotoxicity. Acta Pharmacol Sin 20042598–103. [PubMed] [Google Scholar]
- 140.Tirkey N, Kaur G, Vij G.et al Curcumin, a diferuloylmethane, attenuates cyclosporine‐induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol 200555–15. [DOI] [PMC free article] [PubMed] [Google Scholar]