Abstract
Background
The recommended treatment for patients infected with hepatitis C virus genotype 2 (HCV2) is pegylated interferon (peginterferon) and ribavirin for 24 weeks.
Aim
To assess whether a shorter 16‐week treatment is as effective as a standard 24‐week treatment.
Methods
Patients with HCV2 infection were randomised in a 1:2 ratio to either 16 weeks (n = 50) or 24 weeks (n = 100) of treatment with peginterferon α‐2a (180 μg/week) and weight‐based ribavirin 1000–1200 mg/day, with a 24‐week follow‐up period. A rapid virological response (RVR) was defined as seronegative for HCV RNA at 4 weeks of treatment, and the primary end point, sustained virological response (SVR), as seronegative for HCV RNA at the 24‐week follow‐up.
Results
The rate of RVR and SVR was 86% (43/50, 95% confidence interval (CI) 76% to 96%) and 94% (47/50, CI 87% to 100%), respectively, in the 16‐week group, which was comparable to 87% (87/100, CI 80% to 94%) and 95% (95/100, CI 91% to 99%) in the 24‐week group. Patients with RVR had a significantly higher SVR rate than patients without RVR in both 16‐week (100% vs 57%, p = 0.015) and 24‐week groups (98% vs 77%, p = 0.002). Multivariate analysis showed that RVR and age were independent factors associated with SVR. Both treatment arms were equally well tolerated. The incidence of alopecia was significantly higher in the 24‐week group (49%) than in the 16‐week group (20%, p = 0.001).
Conclusion
16 weeks and 24 weeks of peginterferon treatment with weight‐based ribavirin at a dose of 1000–1200 mg/day provided equal efficacy in patients with HCV2 who achieved RVR at 4 weeks.
Hepatitis C virus (HCV) can be classified into six distinct genotypes, numbered 1–6.1 The HCV genotype has an important role in the response to antiviral treatment.2,3 Patients infected with HCV genotypes 2 and 3 (HCV2 and HCV3) have a significantly higher sustained virological response (SVR) rate to interferon‐based treatment than those with HCV1 infection.4,5 Pegylated interferon (peginterferon) and ribavirin combination treatment has been recommended for all patients infected with HCV, but the treatment duration varies depending on the HCV genotype. For patients infected with HCV1, the recommended treatment duration is 48 weeks, whereas for patients infected with HCV2 or HCV3, the recommended treatment duration is 24 weeks.5,6
Although treatment with peginterferon and ribavirin for 24 weeks could achieve an SVR rate of 80–93% in patients with HCV2 or HCV3,5,7,8,9 side effects are common and sometimes serious, and increase with the length of the treatment, leading to premature termination of treatment in a large number of patients.10,11,12 Hence, it is desirable to tailor the treatment regimen to a shorter duration without compromising on efficacy to reduce the cost of treatment and the incidence of adverse events. Recent studies from Europe13,14,15 have shown that for patients with HCV2 or HCV3, who had a rapid virological response (RVR) at 4 weeks, a shorter duration of treatment over 12–16 weeks is as effective as a 24‐week treatment regimen. However, racial and geographical factors may influence the treatment outcome for chronic hepatitis C (CHC),16 thus, more studies are required to substantiate these findings in patients of various ethnicities.17 Moreover, previous studies of short duration13,14,15 have included patients infected with HCV2 or HCV3; infection with HCV3 is associated with lower rates of SVR than with HCV2, hence, virological response for both genotypes cannot be assessed together. This randomised, controlled study is the first study in Asia to report the efficacy of 16 weeks of treatment with peginterferon and ribavirin for Taiwanese patients infected with HCV2.
The primary aim of this study was to evaluate whether treatment with peginterferon and ribavirin for 16 weeks is sufficient to achieve SVR comparable to that achieved after the standard treatment duration of 24 weeks in patients infected with HCV2. The secondary aim was to evaluate the role of RVR at 4 weeks in the treatment efficacy in both treatment groups.
Methods
Selection of patients
Eligible patients were previously untreated Taiwanese patients with CHC, aged 18–65 years, who (1) were seropositive for HCV antibodies (third‐generation enzyme immunoassay; Abbott Laboratories, North Chicago, Illinois, USA) and for HCV RNA PCR; (2) had undergone a liver biopsy within 1 year before entry, the result of which was consistent with chronic hepatitis; (3) displayed an increased serum alanine transaminase level, defined as ⩾1.5 times the upper limit of the normal range for at least two measurements within 6 months preceding the trial entry; and (4) had HCV2 infection. Other eligibility criteria included neutrophil count >1500/mm3, platelet count >9×104/mm3, haemoglobin concentration >12 g/dl for men and 11 g/dl for women, serum creatinine concentration <1.5 mg/dl, no pregnancy or lactation, and the use of a reliable method of contraception for women.
Patients with an HCV genotype infection other than type 2 infection, hepatitis B surface antigen, HIV infection, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, Wilson's disease, α1‐antitrypsin deficiency, decompensated cirrhosis (Child–Pugh class B or C), overt hepatic failure, current alcohol misuse or history of alcohol misuse (⩾20 g/day), psychiatric condition, previous liver transplantation, or evidence of hepatocellular carcinoma were excluded from the study.
Study design
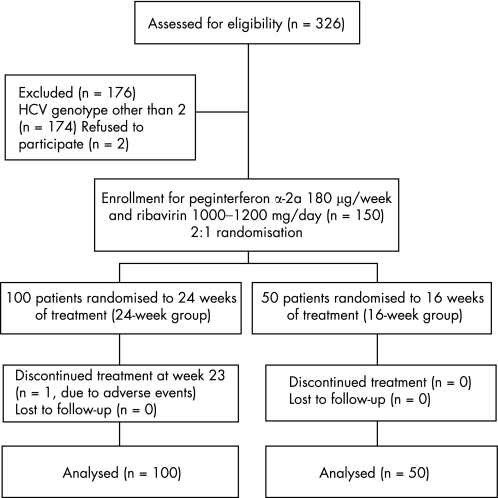
This study was an investigator‐initiated study. This randomised, open‐label, active‐controlled trial was carried out in a medical centre and three regional core hospitals in Taiwan from September 2003 to December 2005. The study was approved by the ethics committees at the participating hospitals and carried out according to the guidelines of the International Conference on Harmonisation for Good Clinical Practice. All patients gave written informed consent before enrolment. A total of 326 patients were screened and 150 protocol‐eligible patients were assigned randomly by computer coding with a 1:2 randomisation ratio (fig 1). Fifty patients (16‐week group) received peginterferonα‐2a (Pegasys, Hoffmann‐La Roche, Basal, Switzerland) at a dose of 180 μg/week subcutaneously, and oral ribavirin 1000–1200 mg/day in two divided doses for 16 weeks. The other 100 patients (24‐week group) received a comparable 24‐week course of peginterferon and ribavirin. In both groups of patients, the dose of ribavirin was based on body weight (1000 mg ribavirin for weight ⩽75 kg and 1200 mg ribavirin for weight >75 kg).
Figure 1 Trial profile. HCV, hepatitis C virus.
All patients were monitored for a further 24 weeks after the end of the treatment. They had biweekly outpatient visits during the first month and monthly visits during the rest of the treatment period and during the 24‐week follow‐up period. At each visit, they underwent a physical examination, and adverse events were recorded. Biochemical and haematological testing was performed by commercial assays. HCV genotypes 1a, 1b, 2a, 2b and 3a were determined by the method described by Okamoto et al.18 Serum HCV RNA levels at baseline and during treatment week 4 were measured using the branched DNA assay (Versant HCV RNA 3.0, Bayer, Tarrytown, New Jersey, USA; quantification limit: 615 IU/ml). Serum HCV RNA at baseline, during treatment weeks 4 and 12, at the end of treatment and at 24 weeks after treatment was determined by standardised automated qualitative PCR (Cobas Amplicor Hepatitis C Virus Test, V.2.0; Roche Diagnostics, Branchburg, New Jersey, USA; detection limit: 50 IU/ml). Liver histology was graded and staged according to the scoring system described by Scheuer19 and Knodell,20 by a single pathologist who was blinded to the treatment of each patient. Hepatic steatosis was scored from 0 (none) to 3 (severe).21
Dose modifications
Adverse events were graded as mild, moderate, severe or potentially lifethreatening. The dose of peginterferon was decreased by 50% and the dose of ribavirin lowered to 600 mg/day when severe adverse events occurred or when laboratory results showed haemoglobin concentraion<10 g/dl in patients with no cardiac disease, haemoglobin decrease >2 g/dl in those with cardiac disease, white cell counts <3000/mm3 or platelet counts <50 000/mm3. Full doses could be resumed when the event resolved. If the event persisted, both drugs were discontinued. Treatment was permanently discontinued for life‐threatening events or when laboratory results showed a haemoglobin concentration <7.5 g/dl in patients with no cardiac disease, haemoglobin concentration <12 g/dl in those with cardiac disease, after 4 weeks of dose reduction, white cell count <1500/mm3, platelet count <30 000/mm3 or serum creatinine concentration >2 mg/dl.
Assessment of efficacy
The primary end point of this study was to assess SVR, which was defined as PCR‐negative serum HCV RNA by the end of treatment and throughout the follow‐up period. Patients not achieving this were classified as non‐responders. RVR was defined by PCR‐negative serum HCV RNA at 4 weeks of treatment. End‐of‐treatment virological response (ETVR) was defined as PCR‐negative serum HCV RNA at end of treatment. Relapse was defined as reappearance of HCV RNA during the follow‐up period in patients who achieved an ETVR.
Statistical analyses
Randomisation sequence with 1:2 ratio using a computer‐generated code was generated by a contract research organisation independent of the study, centrally accessed through telephone or direct office visit. The details of the series were contained in a set of sealed envelopes and were unknown to any of the investigators who enrolled the patients for the study. At the time of conceiving this study plan, no published reports were available for the efficacy of 16 weeks of treatment. Assuming an SVR rate of 82% when treated for 24 weeks,5 and no SVR if untreated, the study was powered to detect a difference of ⩾24.6% with 80% power, anticipating a 10% dropout rate. The margin we proposed is equivalent to other published data.15 Evaluation of efficacy of antiviral treatment was based on an intention‐to‐treat analysis. All patients receiving a treatment dose of peginterferon or ribavirin were analysed.
Frequency was compared between groups using the χ2 test, with the Yates correction, or Fisher's exact test. Group means, presented as mean values (standard deviation (SD)) were compared using analysis of variance and the Student's t test, or nonparametric Mann–Whitney U test when appropriate. Serum HCV RNA levels were expressed after logarithmic transformation of original values. Stepwise logistical regression was used to analyse which variables had a better predictive value for SVR. The procedures were performed using the SPSS V.12.0 statistical package. All statistical analyses were based on two‐sided hypothesis tests with a significance level of p<0.05.
Results
Patient demographic and other baseline characteristics
All 150 randomised patients completed the study and were included in the final analysis (fig 1). Patients in the two treatment groups were well matched for baseline characteristics (table 1). The mean doses of peginterferon and ribavirin were comparable between the two groups. The 16‐week group tended to have higher rate of 80/80/80 adherence (86%; 95% confidence interval (CI) 76% to 96%), defined as patients who had received ⩾80% of expected peginterferon and ribavirin doses and completed at least 80% of the expected duration,12 than the 24‐week group (73%; 95% CI 64% to 82%).
Table 1 Basic demographical, virological, and clinical features and doses of peginterferon and ribavirin of the patients.
| 24‐week group | 16‐week group | p Value | |
|---|---|---|---|
| Patients (n) | 100 | 50 | |
| Mean (SD) age (years) | 49.9 (10.69) | 50.8 (9.74) | 0.621 |
| Sex, n (%) | 0.596 | ||
| Male | 58 (58) | 32 (64) | |
| Female | 42 (42) | 18 (36) | |
| Mean (SD) body weight (kg) | 65.8 (10.49) | 67.7 (9.42) | 0.261 |
| Mean (SD) body mass index (kg/m2)* | 24.8 (3.08) | 25.1 (2.7) | 0.62 |
| Mean (SD) alanine aminotransferase (IU/l) | 108.9 (68.75) | 107 (64.6) | 0.857 |
| Mean (SD) aspartate aminotransferase (IU/l) | 173 (109.6) | 161.9 (110.7) | 0.536 |
| Liver histopathology | |||
| Mean (SD) necroinflammatory activity | 4.84 (2.34) | 5.48 (3.32) | 0.226 |
| Fibrosis, n (%) | 0.832 | ||
| F 0–2 | 80 (80) | 39 (78) | |
| F 3–4 | 20 (20) | 11 (22) | |
| Steatosis, n (%) | 1 | ||
| None (0) | 67 (67) | 34 (68) | |
| Mild (1) | 28 (28) | 13 (26) | |
| Moderate to severe (2–3) | 5 (5) | 3 (6) | |
| Pretreatment HCV viral load (log IU/ml) | 4.88 (1.07) | 4.98 (1.08) | 0.62 |
| Mean (SD) dose of peginterferon (μg/kg/week) | 2.77 (0.46) | 2.68 (0.36) | 0.208 |
| Mean (SD) dose of ribavirin (mg/kg/day) | 15.3 (3.2) | 15.8 (3.13) | 0.408 |
| 80/80/80 adherence, n (%)† | 73 (73) | 43 (86) | 0.073 |
HCV, hepatits C virus.*The body mass index is the weight in kilograms divided by the square of the height in metres.
†Patients who had received >80% of expected peginterferon and ribavirin doses, and completed at least 80% of expected duration.
Virological response
At week 4, RVR was achieved by 86% of patients (43/50; 95% CI 76% to 96%) in the 16‐week group and by 87% of patients (87/100, 95% CI 80% to 94%) in the 24‐week group (fig 2). An ETVR was achieved by all patients in the 16‐week group and by 98% (98/100; 95% CI 95% to 100%) of patients in the 24‐week group. The primary end point, SVR, was achieved by 94% of patients (47/50; 95% CI 87% to 100%) in the 16‐week group and by 95% of patients (95/100; 95% CI 91% to 99%) in the 24‐week group, without any significant between‐group difference (difference −1%; 95% CI 9% to 7%). The relapse rate was higher in the 16‐week group (6%; 3/50; 95% CI 0%–7%) than in the 24‐week group (3.1%; 3/98; 95% CI −1% to 13%). However, the difference did not reach significance (95% CI −10.4% to 4.5%).
Figure 2 The rates of rapid virological response (RVR), end‐of‐treatment virological response (ETVR), sustained virological response (SVR) and relapse rate (RR). The rates of RVR (defined as seronegative for hepatitis C virus (HCV) RNA at 4 weeks of treatment), ETVR (defined as seronegative for HCV RNA at end of treatment), SVR (defined as seronegative for HCV RNA at end of treatment and throughout the follow‐up period) and RR (defined as HCV RNA reappearance during the follow‐up period for patients with ETVR) of patients infected with HCV genotype 2 treated for 24 or 16 weeks with peginterferon α and ribavirin.
Factors affecting SVR and relapse rate
The influence of prognostic factors on the SVR rate was analysed in both groups. They included baseline demographical features, liver histopathology, viral loads, 80/80/80 adherence and received doses of peginterferon and ribavirin, and on‐treatment response (table 2). Patients who achieved RVR at week 4 had a significantly higher SVR rate than those who did not in both the 16‐week (100% vs 57%; p = 0.002) and 24‐week groups (98% vs 77%; p = 0.015). Sustained virological responders were significantly younger than non‐responders in the 24‐week group (p = 0.041). The independent predictive value of age, sex, body weight, body mass index, necroinflammatory activity, fibrosis and steatosis of liver histopathology, pretreatment serum concentrations of alanine aminotransferase and HCV RNA, mean doses of peginterferon and ribavirin, adherence, RVR at week 4 and regimens (16 vs 24 weeks) for the achievement of SVR was determined by using stepwise logistic regression analysis. The factors significantly associated with SVR in 150 patients were RVR at week 4 and patient's age, with an odds ratio (OR, 95% CI) of 40.76 (5.964 to 278.6) and 0.834 (0.721 to 0.965), respectively, whereas the treatment duration was not associated with SVR (OR 1.241; 95% CI 0.186 to 8.279).
Table 2 Factors associated with sustained virological response.
| 24‐week treatment group | 16‐week treatment group | |||||
|---|---|---|---|---|---|---|
| Sustained virological response, n = 100 | p Value | Sustained virological response, n = 50 | p Value | |||
| (+) | (−) | (+) | (−) | |||
| Patients, n (%) | 95 (95) | 5 (5) | 47 (94) | 3 (6) | ||
| Mean (SD) age (years) | 49.4 (10.7) | 59.4 (5.7) | 0.041* | 50.2 (9.7) | 59.7 (6.8) | 0.103 |
| Male sex, n (%) | 57 (60) | 1 (20) | 0.158 | 31 (66) | 1 (33) | 0.291 |
| Mean (SD) body weight (kg) | 66.2 (10.5) | 58.1 (7.7) | 0.092 | 67.7 (9.7) | 67.2 (4.3) | 0.926 |
| Mean (SD) body mass index (kg/m2)† | 24.9 (3.1) | 23.7 (2.7) | 0.394 | 25.1 (2.8) | 24.3 (0.9) | 0.614 |
| Mean (SD) alanine aminotransferase (IU/l) | 174 (112) | 151 (65) | 0.641 | 164 (113) | 125 (52) | 0.553 |
| Liver histopathology | ||||||
| Necroinflammatory activity | 4.79 (2.37) | 5.80 (1.3) | 0.349 | 5.32 (3.34) | 8 (2) | 0.178 |
| Fibrosis score, n (%) | 1 | 0.534 | ||||
| F 0–2 | 76 (80) | 4 (80) | 37 (79) | 2 (67) | ||
| F 3–4 | 19 (80) | 1 (80) | 10 (21) | 1 (33) | ||
| Steatosis, n (%) | 1 | 1 | ||||
| None (0) | 63 (66) | 4 (80) | 32 (68) | 2 (67) | ||
| Mild (1) | 28 (30) | 0 (0) | 12 (26) | 1 (33) | ||
| Moderate to severe (2–3) | 4 (4) | 1 (20) | 3 (6) | 0 (0) | ||
| Mean (SD) baseline HCV RNA level (log IU/ml) | 4.86 (1.08) | 5.33 (0.55) | 0.342 | 4.93 (1.1) | 5.63 (0.35) | 0.283 |
| Mean (SD) mean dose of peginterferon (μg/kg/week) | 2.75 (0.46) | 3.14 (0.41) | 0.065 | 2.68 (0.37) | 2.69 (0.16) | 0.969 |
| Mean (SD) mean dose of ribavirin (mg/kg/day) | 15.4 (3.12) | 14.2 (4.73) | 0.451 | 15.9 (3.12) | 13.0 (1.97) | 0.113 |
| 80/80/80 adherence, n (%)‡ | 0.61 | 0.37 | ||||
| Yes | 70 (74) | 3 (60) | 41 (87) | 2 (67) | ||
| No | 25 (26) | 2 (40) | 6 (13) | 1 (33) | ||
| RVR at 4 weeks, n (%)§ | 0.015* | 0.002* | ||||
| Yes | 85 (90) | 2 (40) | 43 (92) | 0 (0) | ||
| No | 10 (11) | 3 (60) | 4 (9) | 3 (100) | ||
HCV, hepatitis C virus; RVR, rapid virological response
*significant.
†The body mass index is the weight in kilograms divided by the square of the height in metres.
‡Patients who had received ⩾80% of expected peginterferon and ribavirin doses, and completed at least 80% of expected duration.
§RVR, rapid virological response, seronegative of HCV RNA at week 4 of treatment.
Between‐group difference in relapse and SVR rates was analysed by stratification of baseline and on‐treatment factors (table 3). For patients without RVR at week 4, the relapse rate was higher in the 16‐week (42.9%; 95% CI −7% to 92%) group than in the 24‐week group (9.1%; 95% CI −11% to 29%), and the SVR rate was lower in the 16‐week group (57%; 95% CI 20% to 94%) than in the 24‐week group (77%; 95% CI 54% to 99%). However, the differences of both relapse rate and SVR rate between the 16‐week and 24‐week groups did not reach significance. For patients who did not achieve 80/80/80 adherence, received ribavirin dose < 13 mg/kg/day, had a dose modification of peginterferon and/or ribavirin and had dose modification of ribavirin, the relapse rate tended to be higher in the 16‐week group (14.3%, 16.7%, 11.5% and 13%, respectively) than in the 24‐week group (3.8%, 4.5%, 3.8% and 4%, respectively). Nevertheless, all the differences were not significant.
Table 3 Between‐group difference in relapse rate and sustained virological response (SVR) rate.
| Relapse rate, % (n/N) | p Value | SVR rate, % (n/N) | p Value | ||||
|---|---|---|---|---|---|---|---|
| 24‐week group, n = 98 | 16‐week group, n = 50 | 24‐week group, n = 100 | 16‐week group, n = 50 | ||||
| Age | – | ||||||
| <50 years | 0 (0/46) | 0 (0/19) | – | 100 (46/46) | 100 (19/19) | – | |
| >50 years | 5.8 (3/52) | 9.7 (3/31) | 0.666 | 91 (49/54) | 90 (28/31) | 1 | |
| Sex | |||||||
| Female | 5 (2/40) | 11.1 (2/18) | 0.581 | 91 (38/42) | 89 (16/18) | 1 | |
| Male | 1.7 (1/58) | 3.1 (1/32) | 1 | 98 (57/58) | 97 (31/32) | 1 | |
| Body‐mass index* | |||||||
| <25 kg/m2 | 5.8 (3/52) | 7.4 (2/27) | 1 | 93 (49/53) | 93 (25/27) | 1 | |
| >25 kg/m2 | 0 (0/46) | 4.3 (1/23) | 0.333 | 98 (46/47) | 96 (22/23) | 1 | |
| Hepatic fibrosis | |||||||
| F 0–2 | 3.8 (3/79) | 5.1 (2/39) | 1 | 95 (76/80) | 95 (37/39) | 1 | |
| F 3–4 | 0 (0/19) | 9.1 (1/11) | 0.367 | 95 (19/20) | 91 (10/11) | 1 | |
| Hepatic steatosis | |||||||
| No | 3.1 (2/65) | 5.9 (2/34) | 0.605 | 94 (63/67) | 94 (32/34) | 1 | |
| Yes | 3 (1/33) | 6.3 (1/16) | 1 | 97 (32/33) | 94 (15/16) | 1 | |
| HCV RNA level | |||||||
| <800 000 IU/ml | 3.6 (3/84) | 4.9 (2/41) | 1.000 | 95 (81/85) | 95 (39/41) | 1 | |
| >800 000 IU/ml | 0 (0/14) | 11.1 (1/9) | 0.391 | 93 (14/15) | 89 (8/9) | 1 | |
| RVR at 4 weeks | |||||||
| Yes | 2.3 (2/87) | 0 (0/43) | 0.554 | 98 (85/87) | 100 (43/43) | 1 | |
| No | 9.1 (1/11) | 42.9 (3/7) | 0.245 | 77 (10/13) | 57 (4/7) | 0.610 | |
| 80/80/80 adherence† | |||||||
| Yes | 2.8 (2/72) | 4.7 (2/43) | 0.629 | 96 (70/73) | 95 (41/43) | 1 | |
| No | 3.8 (1/26) | 14.3 (1/7) | 0.384 | 93 (25/27) | 86 (6/7) | 0.511 | |
| Mean ribavirin dose | |||||||
| <13 mg/kg/day | 4.5 (1/22) | 16.7 (2/12) | 0.279 | 91 (21/23) | 83 (10/12) | 0.594 | |
| >13 mg/kg/day | 2.6 (2/76) | 2.6 (1/38) | 1 | 96 (74/77) | 97 (37/38) | 1 | |
| Dose modification | |||||||
| Peginterferon and/or ribavirin | |||||||
| Yes | 3.8 (2/53) | 11.5 (3/26) | 0.324 | 94 (51/54) | 89 (23/26) | 0.384 | |
| No | 2.2 (1/45) | 0 (0/24) | 1 | 96 (44/46) | 100 (24/24) | 0.543 | |
| Peginterferon | |||||||
| Yes | 0 (0/9) | 0 (0/4) | – | 100 (9/9) | 100 (4/4) | – | |
| No | 3.4 (3/89) | 6.5 (3/46) | 0.409 | 95 (86/91) | 94 (43/46) | 1 | |
| Ribavirin | |||||||
| Yes | 4 (2/50) | 13 (3/23) | 0.317 | 94 (48/51) | 87 (20/23) | 0.536 | |
| No | 2.1 (1/48) | 0 (0/27) | 1 | 96 (47/49) | 100 (27/27) | 0.367 | |
HCV, hepatitis C virus; RVR, rapid virological response; SVR, sustained virological response.
*The body‐mass index is the weight in kilograms divided by the square of the height in meters.
† Patients who had received at least 80% of total peginterferon dose, at least 80% of total ribavirin dose and completed at least 80% of total study duration.
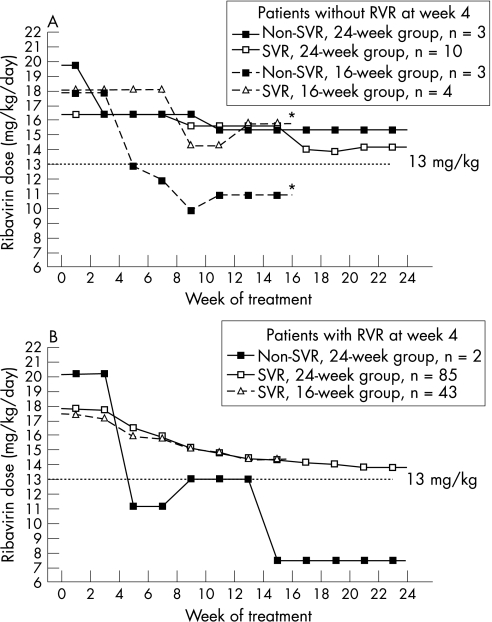
As RVR at week 4 was the most important factor associated with SVR in both the 16‐week and 24‐week groups, we stratified patients by RVR to evaluate the influence of ribavirin dose expressed per kilogram of body weight on SVR. For patients without RVR at week 4, the mean dose of ribavirin was comparable between sustained responders and non‐responders of the 24‐week group throughout the treatment period; however, the mean dose of ribavirin from 5th to 16th weeks of treatment was significantly lower in non‐responders of the 16‐week group than in sustained responders of the 16‐week group (11.3 (2.5) vs 16.1 (0.8) mg/kg/day; p = 0.034; fig 3A). For patients with RVR at week 4, all patients achieved SVR in the 16‐week group; the mean dose of ribavirin from 5th to 16th weeks of treatment was lower in non‐responders of the 24‐week group (9.7 (8.9) mg/kg/day) than in sustained responders of the 24‐week group (14.8 (3.7) mg/kg/day). However, the difference was not significant (p = 0.395, Mann–Whitney U test; fig 3B).
Figure 3 Mean ribavirin dose throughout the treatment period, stratified by rapid virological response (RVR) at week 4 (seronegative for hepatitis C virus RNA at 4 weeks of treatment), sustained virological response (SVR) and treatment regimen. Mean ribavirin dose was expressed per kilogram of body weight for patients without RVR at week 4 (A) and with RVR at week 4 (B). *For patients without RVR, the mean dose of ribavirin from the 5th to 16th weeks of treatment was significantly lower in non‐responders of the 16‐week group than in sustained responders of the 16‐week group (11.3 (2.5) vs 16.1 (0.8) mg/kg/day; p = 0.034, Mann–Whitney U test); for patients without RVR, the mean dose of ribavirin from the 5th to 16th weeks of treatment in non‐responders of the 24‐week group was 9.7 (8.9) mg/kg/day, compared with 14.8 (3.7) mg/kg/day in sustained responders of the 24‐week group (p = 0.395, Mann–Whitney U test).
Safety
No serious adverse event was reported. One patient in the 24‐week group discontinued treatment because of anaemia and leucopenia at week 23. A total of 4 (8%) patients in the 16‐week group and 9 (9%) in the 24‐week groups required dose reduction of peginterferon because of adverse events (5 patients), leucopenia (3), anaemia (4) and thrombocytopenia (1). Adverse events were typical of those previously reported for peginterferon and ribavirin combination treatment (table 4). Incidence rates for most adverse events were similar between the 16‐week and 24‐week treatment groups, except that the incidence of alopecia was significantly higher in the 24‐week group (49%) than the 16‐week group (20%; p = 0.001).
Table 4 Adverse events and dose modifications according to the treatment group.
| 24‐week group, n = 100, n (%) | 16‐week group, n = 50, n (%) | p Value | ||
|---|---|---|---|---|
| Discontinuation | 1 (1) | 0 (0) | 1 | |
| Dose modification for adverse events or laboratory abnormalities | ||||
| Peginterferon α‐2a | 9 (9) | 4 (8) | 1 | |
| Ribavirin | 51 (51) | 23 (46) | 0.564 | |
| Peginterferon α‐2a or ribavirin | 54 (54) | 26 (52) | 0.817 | |
| Influenza‐like symptoms | ||||
| Fever | 55 (55) | 29 (58) | 0.727 | |
| Chills | 28 (28) | 12 (24) | 0.602 | |
| Headache | 39 (39) | 21 (42) | 0.724 | |
| Gastrointestinal symptoms | ||||
| Anorexia | 46 (46) | 20 (40) | 0.601 | |
| Nausea | 15 (15) | 3 (6) | 0.181 | |
| Diarrhoea | 9 (9) | 5 (10) | 1 | |
| Psychiatric symptoms | ||||
| Anxiety | 7 (7) | 4 (8) | 1 | |
| Depression | 10 (10) | 3 (6) | 0.545 | |
| Insomnia | 57 (57) | 23 (46) | 0.227 | |
| Dermatological symptoms | ||||
| Hair loss | 49 (49) | 10 (20) | 0.001* | |
| Skin rash | 54 (54) | 22 (44) | 0.248 | |
| Haematological abnormality | ||||
| Leucopenia (white cell count <1500/mm3) | 2 (2) | 1 (2) | 1 | |
| Aanemia (haemoglobin level <10 g/dl) | 53 (53) | 27 (54) | 0.908 | |
| Thrombocytopaenia (<50 000/mm3) | 1 (1) | 0 (0) | 1 | |
| Abnormal thyroid function tests | 13 (13) | 4 (8) | 0.362 | |
*Significant.
Discussion
In this study, we showed that a shorter course of 16‐week peginterferon and weight‐based ribavirin 1000–1200 mg/day is as effective as a standard 24‐week course in Taiwanese patients infected with chronic HCV2 who achieved RVR at week 4. Achievement of RVR at week 4 and patient's age, but not treatment duration, were independent factors associated with SVR. The shorter duration of treatment resulted in a lower incidence of adverse events such as alopecia and in lower cost of treatment. An insufficient dose of ribavirin after 4 weeks of treatment may compromise the response to a 16‐week treatment in patients without RVR at week 4.
For patients infected with HCV2, peginterferon/ribavirin for 24 weeks is the standard recommended treatment,5 which could achieve SVR rates similar to those treated with a 48‐week course.8 Subsequently, three European studies13,14,15 have shown that a shorter course of treatment over 12–16 weeks with peginterferon/ribavirin is as effective as a 24‐week course for patients with HCV2 with RVR at 4 weeks. These previous studies used RVR at week 4 as an indicator for choosing the treatment period. Only patients who had RVR were assigned to groups that had shorter treatment periods.13,14,15 Thus, no data were available for shorter courses of treatment in patients who did not achieve RVR. In our study, Taiwanese patients with chronic HCV2 infection were randomly assigned to either 16 or 24 weeks of treatment with peginterferon/ribavirin regardless of their RVR. Our results clearly showed that a rapid response at week 4 is an important factor that can significantly affect the outcome of treatment in both the 16‐week and 24‐week groups. Consistent with previous European studies,13,14,15 this study also showed that 16 and 24 weeks of peginterferon/ribavirin treatment provided equal efficacy for patients with HCV2 who achieved RVR at week 4, with an SVR rate of 98–100%. Recent data also suggest that 24 weeks of treatment is effective and sufficient for achieving SVR in patients with HCV1 or HCV4 who exhibit RVR at 4 weeks, compared with a recommended standard course of 48 weeks of treatment.22,23,24 These findings emphasise the importance of RVR at 4 weeks in the individualised treatment for CHC.
Other studies examining shorter treatment durations in patients with HCV2 and HCV3 have shown higher relapse rates with the shorter treatment duration.14,15 We also observed that the 16‐week group had a lower SVR rate and a higher relapse rate than the 24‐week group for patients who did not achieve RVR. However, too few patients had a relapse for this to be a meaningful comparison. The between‐group difference showed a tendency towards ribavirin dose reduction with a higher relapse rate and lower SVR rate. We also found that a significantly lower mean dose of ribavirin by body weight after 4 weeks of treatment was observed in non‐responders who did not achieve RVR at week 4 after 16 weeks treatment, even with a limited number of cases. Significant incremental virological response rates with adherence were not detected for patients with HCV2 or HCV3 with 24 or 48 weeks of treatment.12 Our observation suggested that ribavirin adherence and ribavirin dose by body weight after 4 weeks of treatment may play a role in the response to a shorter course of treatment for patients with HCV2 without RVR at week 4. Further large‐scale studies are needed to confirm these findings.
For patients without RVR, a 16‐week regimen is obviously suboptimum. Nevertheless, the efficacy remains unsatisfactory in this relatively difficult‐to‐treat subgroup even with a 24‐week course of treatment (SVR rate 77% in this study and 50–72% in European studies13,14). Whether an extended course of treatment >24 weeks is of benefit for this subgroup remains to be studied.
The SVR rate of patients with HCV2 with RVR at 4 weeks using a shorter course of peginterferon and ribavirin is slightly higher in our study than observed in previous studies conducted in Europe.13,14,15,25 A greater response to peginterferon/ribavirin in Taiwanese patients with chronic HCV‐1b than in Caucasian patients was also observed in previous studies.26,27 There may be a few reasons for this diffference. Firstly, racial factors have been shown to influence the virological response even when peginterferon/ribavirin are given for the standard duration of treatment.16,17 Ethnic and racial differences in immunogenetics, peginterferon pharmacokinetics, signal transduction pathway or viral kinetics may play a role in determining the outcome of HCV infections.3,28,29 Secondly, geographical variations of HCV by the emergence of quasispecies may have influenced the virological response.30 Thirdly, the mean body weight was lower in our study population than in previous European populations, with a mean difference of 3–10 kg. The higher average doses of peginterferon or ribavirin per kilogram body weight in our patients might contribute to a higher SVR.31,32
Previous study showed that peginterferon and ribavirin at a dose of 800 mg/day is as effective as peginterferon and ribavirin at a dose of 1000–1200 mg/day in patients with HCV2 or HCV3 infections with a 24‐week regimen.33 Thus, peginterferon and ribavirin at a dose of 800 mg/day for 24 weeks is recommended for patients with HCV2 or HCV3.6 In our study we used a higher ribavirin dose of 1000–1200 mg/day to lower the relapse rate in patients treated for 16 weeks. More recently, the ACCELERATE Trial, evaluating 16 or 24 weeks of peginterferon α‐2a 180 μg/week and ribavirin 800 mg/day for patients with HCV2 and HCV3 with quantifiable serum HCV RNA (>600 IU/ml), showed that SVR was significantly greater with 24 rather than 16 weeks of treatment for patients with HCV2 (82% vs 65%; p<0.001).25 In the ACCELERATE Trial, the mean body weight of patients with HCV2 was around 84 kg, with a mean ribavirin dose of 9.52 mg/kg/day for a daily dose of 800 mg, in contrast to 15.3 mg/kg/day in our study. The difference may explain the conflict in the results between the ACCELERATE Trial and this study. Whether a higher weight‐based dose of ribavirin is required for a shorter duration of treatment without compromising the efficacy for treating patients with HCV2 through the reduction of the relapse rate remains to be studied.
A shorter treatment period can benefit patients by reducing the cost of treatment and the incidence of adverse events.14 As the mean doses of peginterferon and ribavirin were not different between the groups in our study, the reduced incidence of adverse events can be considered to be a benefit of shorter treatment duration. Shorter treatment duration can, in particular, reduce the incidence of delayed side effects of peginterferon treatment. In our study, the incidence of alopecia, a common late side effect of peginterferon,34 was significantly less in the short‐duration treatment group.
In conclusion, this study shows that for patients with CHC infected with HCV2 who achieved RVR at 4 weeks, the efficacy of peginterferon α‐2a and weight‐based ribavirin at a dose of 1000–1200 mg/day administered for 16 weeks is comparable with treatment duration of 24 weeks. A shorter duration of treatment can reduce the incidence of adverse events and the cost of treatment. Our results provide information necessary for decision making of individualised treatment based on response at 4 weeks in CHC patients with HCV2 infections.
Abbreviations
CHC - chronic hepatitis C
ETVR - end‐of‐treatment virological response
HCV - hepatitis C virus
RVR - rapid virological response
SVR - sustained virological response
Footnotes
Funding: Taiwan Liver Research Foundation.
Competing interests: None.
References
- 1.Bukh J, Miller R H, Purcell R H. Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin Liver Dis 19951541–63. [DOI] [PubMed] [Google Scholar]
- 2.Poynard T, Yuen M F, Ratziu V.et al Viral hepatitis C. Lancet 20033622095–2100. [DOI] [PubMed] [Google Scholar]
- 3.Dai C Y, Chuang W L, Chang W Y.et al Tumor necrosis factor‐alpha promoter polymorphism at position ‐308 predicts response to combination therapy in hepatitis C virus infection. J Infect Dis 200619398–101. [DOI] [PubMed] [Google Scholar]
- 4.Fried M W, Shiffman M L, Reddy K R.et al Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002347975–982. [DOI] [PubMed] [Google Scholar]
- 5. NIH NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements 2002191–46. [PubMed] [Google Scholar]
- 6.Strader D B, Wright T, Thomas D L.et al Diagnosis, management, and treatment of hepatitis C. Hepatology 2004391147–1171. [DOI] [PubMed] [Google Scholar]
- 7.Chuang W L, Yu M L, Dai C Y.et al Treatment of chronic hepatitis C in southern Taiwan. Intervirology 20064999–106. [DOI] [PubMed] [Google Scholar]
- 8.Zeuzem S, Hultcrantz R, Bourliere M.et al Peginterferon alfa‐2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol 200440993–999. [DOI] [PubMed] [Google Scholar]
- 9.Chuang W L, Dai C Y, Chang W Y.et al Viral interaction and responses in chronic hepatitis C and B coinfected patients with interferon‐alpha plus ribavirin combination therapy. Antivir Ther 200510125–133. [PubMed] [Google Scholar]
- 10.Yu M L, Dai C Y, Lee L P.et al Outcome of chronic hepatitis C patients who required early termination of peginterferon‐alfa plus ribavirin combination therapy. Antivir Ther 2006111015–1019. [PubMed] [Google Scholar]
- 11.Fattovich G, Giustina G, Favarato S.et al A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol 19962438–47. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison J G, Manns M, Patel K.et al Adherence to combination therapy enhances sustained response in genotype‐1‐infected patients with chronic hepatitis C. Gastroenterology 20021231061–1069. [DOI] [PubMed] [Google Scholar]
- 13.Dalgard O, Bjoro K, Hellum K B.et al Treatment with pegylated interferon and ribavirin in HCV infection with genotype 2 or 3 for 14 weeks: a pilot study. Hepatology 2004401260–1265. [DOI] [PubMed] [Google Scholar]
- 14.Mangia A, Santoro R, Minerva N.et al Peginterferon alfa‐2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med 20053522609–2617. [DOI] [PubMed] [Google Scholar]
- 15.von Wagner M, Huber M, Berg T.et al Peginterferon‐alpha‐2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology 2005129522–527. [DOI] [PubMed] [Google Scholar]
- 16.Reddy K R, Hoofnagle J H, Tong M J.et al Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group. Hepatology 199930787–793. [DOI] [PubMed] [Google Scholar]
- 17.Muir A J, Bornstein J D, Killenberg P G. Peginterferon alfa‐2b and ribavirin for the treatment of chronic hepatitis C in blacks and non‐Hispanic whites. N Engl J Med 20043502265–2271. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto H, Tokita H, Sakamoto M.et al Characterisation of the genomic sequence of type V (or 3a) hepatitis C virus isolates and PCR primers for specific detection. J Gen Virol 199374(Pt 11)2385–2390. [DOI] [PubMed] [Google Scholar]
- 19.Scheuer P J. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 199113372–374. [DOI] [PubMed] [Google Scholar]
- 20.Knodell R G, Ishak K G, Black W C.et al Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 19811431–435. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner D E, Brunt E M, Van Natta M.et al Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005411313–1321. [DOI] [PubMed] [Google Scholar]
- 22.Ferenci P, Laferl H, Elisabethinnen K.et al Is shorter treatment with peginterferon alfa‐2a (40KD) (Pegasys) plus ribavirin (Copegus) possible in HCV genotype 1 ‘super‐responders'? Preliminary results of a prospective randomized clinical trial [abstract]. Hepatology 200542(Suppl 1)218 [Google Scholar]
- 23.Zeuzem S, Buti M, Ferenci P.et al Efficacy of 24 weeks treatment with peginterferon alfa‐2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol 20064497–103. [DOI] [PubMed] [Google Scholar]
- 24.Jensen D, Morgan T, Marcellin P.et al Rapid virological response at week 4 (RVR) of peginterferon alfa‐2a (40KD) (Pegasys) plus ribavirin (RBV, Copegus) treatment predicts sustained virological response (SVR) after 24 weeks in genotype 1 patients [abstract]. Hepatology 200542(Suppl 1)650 [Google Scholar]
- 25.Shiffman M L, Pappas S, Nyberg L.et al Peginterferon alfa‐2a (Pegasys) plus ribavirin (Copegus) for 16 or 24 weeks in patients with HCV genotype 2 or 3. Final results of the accelerate Trial [abstract]. J Hepatol 200644(Suppl 2)271 [Google Scholar]
- 26.Lee S D, Yu M L, Cheng P N.et al Comparison of a 6‐month course peginterferon alpha‐2b plus ribavirin and interferon alpha‐2b plus ribavirin in treating Chinese patients with chronic hepatitis C in Taiwan. J Viral Hepat 200512283–291. [DOI] [PubMed] [Google Scholar]
- 27.Yu M L, Dai C Y, Lin Z Y.et al A randomized trial of 24‐ vs. 48‐week courses of PEG interferon alpha‐2b plus ribavirin for genotype‐1b‐infected chronic hepatitis C patients: a pilot study in Taiwan, Liver Int 20062673–81. [DOI] [PubMed] [Google Scholar]
- 28.Thio C L, Thomas D L, Goedert J J.et al Racial differences in HLA class II associations with hepatitis C virus outcomes. J Infect Dis 200118416–21. [DOI] [PubMed] [Google Scholar]
- 29.Yu M L, Dai C Y, Chen S C.et al Human leukocyte antigen class I and II alleles and response to interferon‐alpha treatment, in Taiwanese patients with chronic hepatitis C virus infection. J Infect Dis 200318862–65. [DOI] [PubMed] [Google Scholar]
- 30.Lam N P. Hepatitis C: natural history, diagnosis, and management. Am J Health Syst Pharm 199956961–973. [DOI] [PubMed] [Google Scholar]
- 31.Shiffman M L, Wilson M, Salvatori J.et al Treatment of chronic hepatitis C virus (HCV) genotype 1 with peginterferon alfa‐2b (PEGIFN), high weight based dose ribavirin (RVN) and epoetin alfa (EPO) enhances sustained virologic response (SVR) [abstract]. Hepatology 200542(Suppl 1)217 [Google Scholar]
- 32.Manns M P, McHutchison J G, Gordon S C.et al Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001358958–965. [DOI] [PubMed] [Google Scholar]
- 33.Hadziyannis S J, Sette H, Jr, Morgan T R.et al Peginterferon‐alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004140346–355. [DOI] [PubMed] [Google Scholar]
- 34.van Zonneveld M, Flink H J, Verhey E.et al The safety of pegylated interferon alpha‐2b in the treatment of chronic hepatitis B: predictive factors for dose reduction and treatment discontinuation. Aliment Pharmacol Ther 2005211163–1171. [DOI] [PubMed] [Google Scholar]