Abstract
Background
Recent studies have shown the presence of vascular endothelial growth factor (VEGF)‐dependent splanchnic angiogenesis in experimental models of portal hypertension, and the role of such neovascularisation on the development of both portosystemic collaterals and hyperdynamic splanchnic circulation. However, the mechanisms modulating angiogenesis in portal hypertension are unknown. Experimental evidence indicates that NAD(P)H oxidase is required for VEGF‐induced angiogenesis. Interestingly, we have recently shown that splanchnic NAD(P)H oxidase activity is significantly increased in portal hypertensive rats. Therefore, it could be possible that activated NAD(P)H oxidases modulate angiogenesis in portal hypertension.
Aim
To determine the effects of chronic NAD(P)H oxidase inhibition on angiogenesis and splanchnic haemodynamics in portal hypertensive rats.
Methods
Partial portal vein‐ligated and sham‐operated rats were treated with the NAD(P)H oxidase inhibitor apocynin, or with vehicle for 5 days. Then, the expression of angiogenesis markers (western blotting), the formation of portosystemic collaterals (radioactive microspheres) and the production of superoxide anion (lucigenin‐enhanced chemiluminescence) were determined. Mean arterial pressure, portal pressure, and superior mesenteric arterial blood flow and resistance were also measured.
Results
In portal hypertensive rats, NAD(P)H oxidase blockade significantly decreased portosystemic collateral formation, and superior mesenteric arterial flow. It also reduced the splanchnic expression of VEGF, VEGF receptor‐2 and CD31, and attenuated the increased production of superoxide, compared with vehicle.
Conclusions
NAD(P)H oxidase plays an important role in experimental portal hypertension, modulating splanchnic angiogenesis, the formation of portosystemic collaterals and the development of splanchnic hyperdynamic circulation. These results suggest that NAD(P)H oxidase may represent a new target in the treatment of portal hypertension.
Portal hypertension is a frequent and severe complication of chronic liver diseases.1,2 It is characterised by a pathological increase in portal venous pressure through an increase hepatic resistance to portal blood flow, and by the subsequent development of an extensive network of portosystemic collateral blood vessels. Even though these vessels aim to lower portal pressure, portal hypertension is maintained by an increased blood flow into the portal system, which thus becomes hyperdynamic.1,2
Recent studies from our laboratory (Hepatic Hemodynamic Laboratory, Liver Unit, Hospital Clinic, Institut d́Investigacions, Biomediques August Pi i Sunyer, Barcelona, Spain) on experimental models of portal hypertension suggest that the development of portosystemic collateral vessels is, at least in part, an active angiogenic process modulated by vascular endothelial growth factor (VEGF).3,4 These studies further showed that the increased splanchnic blood flow characteristic of portal hypertension is not only due to splanchnic vasodilatation, but it is also mediated through an increased VEGF‐dependent splanchnic vascularity.4
The precise mechanisms by which angiogenesis‐associated responses are modulated in portal hypertension remain to be defined. Experimental evidence indicates that NAD(P)H oxidases, a major source of reactive oxygen species (ROS) in the vasculature,5,6 are required for VEGF‐dependent angiogenesis.7,8,9,10,11 Interestingly, we have recently shown that NAD(P)H oxidase activity is significantly increased in splanchnic organs from portal hypertensive rats, as compared with sham‐operated animals.12 Previous studies have also suggested that the portal hypertensive syndrome is associated with increased oxidative stress.13,14 Therefore, it could be possible that activated NAD(P)H oxidases play a role in angiogenesis‐associated responses in portal hypertension. To test this hypothesis, we determined the effects of chronic inhibition of NAD(P)H oxidase on the splanchnic neovascularisation, the extent of portosystemic collateral vessels, and the development of hyperdynamic splanchnic circulation in portal hypertensive rats and in sham‐operated control animals.
Materials and methods
Animals and experimental groups
Experimental procedures were approved by the “Laboratory Animal Care and Use Committee” of the Institut d́Investigacions, Biomediques August Pi i Sunyer, at the University of Barcelona, and performed according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH publication 86–23, revised 1985).
Male Sprague‐Dawley rats (350–400 g body weight; Charles River, Cambridge, Massachusetts, USA) were used in this study. Portal hypertension was induced by partial portal vein ligation, as described previously.15 Briefly, under ketamine and xylacine anaesthesia (80 and 12 mg/kg body weight, respectively, intramuscularly), the portal vein was isolated, and a stenosis was created by a single ligature of 3–0 silk placed around both the portal vein and a 20 gauge blunt‐tipped needle. The needle was then removed, leaving a calibrated constriction of the portal vein. In sham‐operated control animals, the portal vein was isolated and similarly manipulated, but not ligated. Studies were performed 5 days after the initial surgery.
Rats received daily intraperitoneal injections of the NAD(P)H oxidase inhibitor apocynin (30 mg/kg/day; partial portal vein ligated rats: n = 10; sham‐operated rats: n = 5; Sigma Chemical, St Louis, Missouri, USA), or its vehicle (0.3 ml dimethyl sulfoxide; partial portal vein ligated rats: n = 10; sham‐operated rats: n = 5; Sigma), starting immediately after surgery, and then every day for a total of 5 days. The dose of apocynin used has been previously shown to be effective in inhibiting NAD(P)H oxidase activity.16
Haemodynamic studies
Under anaesthesia with ketamine and xylacine, a tracheotomy was performed and a polyethylene PE‐240 tubing was inserted into the trachea to ensure a patent airway. PE‐50 catheters were introduced into the femoral artery, to record arterial pressure (mm Hg), and into the portal vein through an ileocolic vein, to measure portal pressure (mm Hg). Then, the superior mesenteric artery was carefully dissected free from the connective tissue, and a non‐constrictive perivascular transit‐time ultrasonic flowprobe (1PR, 1‐mm diameter; Transonic Systems, Ithaca, New York, USA) was placed around this vessel close to its aortic origin. The flow probe was connected to a flowmeter, to measure the superior mesenteric arterial blood flow (ml/min/100 g).17 Resistance in the superior mesenteric artery (mm Hg/ml/min/100 g) was calculated as: (mean arterial pressure–portal pressure)/superior mesenteric arterial blood flow. Blood pressures and flows were registered on a multichannel computer‐based recorder (PowerLab; ADInstruments, Colorado Springs, Colorado, USA). The external zero reference point was placed at the midportion of the animal. Studies were performed after a 30‐min stabilisation period.
Determination of the extent of portosystemic collateral formation
The extent of portosystemic collateral vessels was quantified using 51Cr‐labelled radioactive microspheres (Perkin‐Elmer; Boston, Massachusetts, USA) injected into the portal vein via a distal ileocolic vein.18 The radioactivity in the liver and lungs was determined in a γ‐scintillation counter. This allows quantification of the degree of collateral formation in a 0–100% scale, by the equation: collateralisation (%) = [lung radioactivity/(lung radioactivity + liver radioactivity)] × 100. Approximately 30 000 51Cr‐labelled microspheres (diameter 15 (3) µm; specific activity 41 mCi/g) were injected into the distal ileocolic vein of each animal.
Quantification of NAD(P)H oxidase‐dependent superoxide production
NAD(P)H oxidase‐dependent superoxide anion production was assessed in the rat mesentery by lucigenin‐enhanced chemiluminescence.19 Briefly, tissues were excised, immediately snap‐frozen in liquid nitrogen and stored at −80°C for analysis. Tissues were minced thoroughly with mortar under liquid nitrogen. A 10% homogenate was prepared by homogenising the obtained powder in 1 ml of Krebs‐HEPES buffer, containing 0.01 mM EDTA and 0.01 mM ethylene glycol tetraacetic acid (pH 7.4; Sigma), by using a glass‐to‐glass homogeniser. The homogenate was centrifuged at 1000 g for 10 min, to remove unbroken cells and debris. Protein quantification was performed by the Lowry method, and the final concentration adjusted to 10 μg/ml. For the chemiluminescence assay, 100 μl aliquots were added to 400 μl of a Krebs‐HEPES assay solution containing lucigenin (5 μM; Sigma) as the electron acceptor. After equilibration and background counts, NAD(P)H (0.1 mM; Sigma) was added as the substrate, and the luminescence counts (relative light units) were monitored continuously over a 3‐min period in a luminometer, at 37°C. Then, superoxide dismutase (400 U/ml; Sigma) was added and counts were measured again for 3 min.
Western blot analysis
Protein expressions of VEGF, VEGF receptor‐2 (VEGFR‐2) and CD31 in the rat intestine were determined by western blotting, as described previously.3,4 In brief, tissues were homogenised in ice‐cold lysis buffer containing 50 mM Tris‐HCl (pH 7.4), 0.1 mM ethylene glycol tetraacetic acid, 0.1 mM EDTA, 2 µM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1% Nonidet P40, 0.1% sodium dodecyl sulphate and 0.1% deoxycholate (Sigma). The resultant lysates were centrifuged at 10 000 g for 30 min at 4°C. The supernatants were collected and total protein was quantified using a colorimetric assay (Bio‐Rad Laboratories; Hercules, California, USA). Samples (100 µg protein) were mixed with double‐strength sample buffer (250 mM Tris‐HCl, 4% sodium dodecyl sulphate, 10% glycerol, 2% β‐mercaptoethanol and 0.006% bromophenol blue (pH 6.8)), boiled for 5 min and subjected to 7.5% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes (Bio‐Rad), and stained with Ponceau S solution (Sigma), to check for equal loading of protein in each lane. Membranes were blocked in Tris‐buffered saline (TBS) with 0.05% Tween 20 (TBS‐T buffer) containing 10% (wt/vol) non‐fat dry milk. The membranes were then incubated, overnight at 4°C, with polyclonal monospecific antibodies against VEGF, VEGFR‐2, and CD31 (1:200 dilution in TBS‐T with 5% milk; Santa Cruz Biotechnology, Santa Cruz, California, USA). Blots were subsequently washed in TBS‐T and incubated with horseradish peroxidase‐conjugated antibody against rabbit IgG (1:10 000 dilution in TBS‐T with 5% milk; Stressgen, Sidney, Canada) for 30 min at room temperature. Immunoreactive bands were detected using the enhanced chemiluminescence western blotting system (Santa Cruz). Molecular weight was calculated with prestained standards (Bio‐Rad). The western blot analysis was repeated to confirm reproducibility using the samples collected from three individual rats of each experimental group.
Statistical analysis
Results are expressed as mean (SEM). Data were normally distributed, and, therefore, we used parametric statistical procedures (Student's test for unpaired data, and 2‐way analysis of variance followed by the Tukey‐Kramer test for multiple comparisons). Differences were considered significant at p<0.05.
Results
All animals had a similar body weight at the time of the studies. Treatments were well tolerated and no side effects were observed during the treatment period.
Effects of chronic NAD(P)H oxidase inhibition on the expression of angiogenesis markers
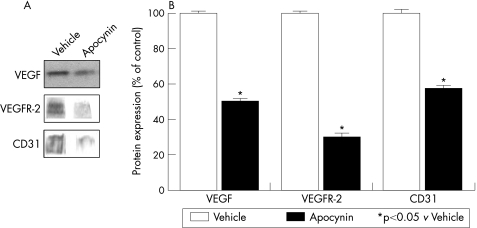
We determined the effects of chronic NAD(P)H oxidase inhibition on intestinal VEGF, VEGFR‐2 and CD31 protein expression in portal hypertensive rats, and in sham‐operated control animals. NAD(P)H oxidase blockade significantly reduced the expression of the angiogenic factor VEGF and its receptor VEGFR‐2 in rats with portal hypertension, as compared with vehicle‐treated portal hypertensive rats (fig 1). In addition, the expression of CD31, which is widely used as a marker of neovascularisation,20 was also significantly lower in portal hypertensive rats treated with the NAD(P)H oxidase inhibitor than in those receiving vehicle (fig 1). In sham‐operated rats, blockade of NAD(P)H oxidase had no significant effects on the protein expression of angiogenesis markers (data not shown).
Figure 1 Effects of chronic NAD(P)H oxidase inhibition on vascular endothelial growth (VEGF), VEGF receptor‐2 (VEGFR‐2), and CD31 protein expression in the intestine of portal hypertensive rats. (A) Representative western blots for VEGF, VEGFR‐2, and CD31 in portal hypertensive rats treated with the NAD(P)H oxidase inhibitor apocynin or with vehicle. (B) Densitometric quantification of VEGF, VEGFR‐2, and CD31 protein expression (expressed as % of the vehicle‐treated portal hypertensive group, as control; mean (SEM) of three different experiments). *p<0.05 vs vehicle‐treated portal hypertensive rats.
Effects of chronic NAD(P)H oxidase inhibition on the formation of portosystemic collateral vessels
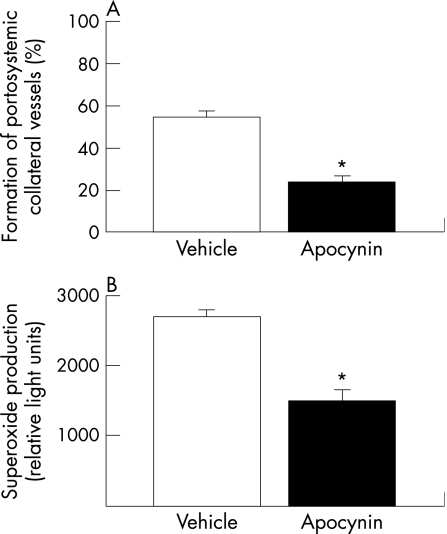
As expected, vehicle‐treated portal hypertensive rats had a higher degree of collateral vessel formation than vehicle‐treated sham‐operated animals (55.1% (7.0%) vs 0.08% (0.01%), p<0.001). Interestingly, chronic NAD(P)H oxidase blockade caused a marked and significant inhibition (by 55%) of the formation of portosystemic collateral vessels in portal hypertensive rats, compared with the vehicle‐treated group (fig 2A). In sham‐operated control animals, blockade of NAD(P)H oxidase had no significant effects on the formation of portosystemic collaterals (data not shown). These results were also confirmed in additional studies performed at a later time point during the evolution of portal hypertension. Thus, the degree of collateral formation was significantly lower in portal hypertensive rats receiving the NAD(P)H oxidase inhibitor for 8 days (26.06% (9.45%); n = 7) than in vehicle‐treated portal hypertensive rats (83.38% (6.82%); n = 6, p = 0.00075).
Figure 2 Effects of chronic NAD(P)H oxidase inhibition (A) on the formation of portosystemic collateral vessels, and (B) on the mesenteric production of superoxide anion in portal hypertensive rats. Results are shown as mean (SEM). *p<0.05 vs vehicle‐treated portal hypertensive rats.
Effects of chronic NAD(P)H oxidase inhibition on mean arterial pressure and splanchnic haemodynamics
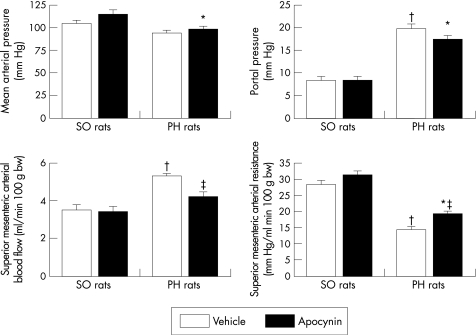
Portal hypertensive rats had significantly higher portal pressure and superior mesenteric arterial blood flow, and significantly lower superior mesenteric arterial resistance than sham‐operated rats (fig 3).
Figure 3 Effects of chronic NAD(P)H oxidase inhibition on mean arterial pressure and splanchnic haemodynamics in portal hypertensive (PH) and sham‐operated (SO) rats. Results are shown as mean (SEM). * p<0.05 vs apocynin‐treated sham‐operated rats; † p<0.05 vs vehicle‐treated sham‐operated rats; ‡ p<0.05 vs vehicle‐treated portal hypertensive rats.
In portal hypertensive rats, NAD(P)H oxidase inhibition caused a significant decrease in superior mesenteric arterial blood flow, increasing the superior mesenteric arterial resistance, in comparison with portal hypertensive animals receiving vehicle (fig 3). Portal pressure decreased after NAD(P)H oxidase blockade in portal hypertensive rats, but the difference did not reach statistical significance (p = 0.081 vs vehicle‐treated portal hypertensive rats). Mean arterial pressure was not significantly modified after inhibition of NAD(P)H oxidase in portal hypertensive rats (fig 3).
In sham‐operated rats, chronic NAD(P)H oxidase blockade had no significant haemodynamic effects (fig 3).
Effects of chronic NAD(P)H oxidase inhibition on superoxide anion production
To show the efficacy of the apocynin treatment in inhibiting NAD(P)H oxidase activity in portal hypertensive rats, we determined mesenteric superoxide anion levels using the lucigenin‐enhanced chemiluminescence method. Superoxide levels were significantly lower in apocynin‐treated portal hypertensive rats than in those receiving vehicle (fig 2B). In all assays, NAD(P)H did not evoke lucigenin chemiluminescence in the absence of homogenate. In addition, to ensure that the chemiluminescence signal obtained in the presence of NAD(P)H was attributable to superoxide, we examined the effects of a selective superoxide scavenger. NAD(P)H‐dependent chemiluminescence was completely abolished by superoxide dismutase, confirming the specificity of the assay.
Discussion
This study shows that chronic NAD(P)H oxidase inhibition markedly and significantly attenuates the formation of portosystemic collateral vessels, and the development of hyperdynamic splanchnic circulation in portal hypertensive rats. To our knowledge, this study is the first demonstration that NAD(P)H oxidase plays a pathophysiological role in experimental portal hypertension. These findings also suggest that inhibition of NAD(P)H oxidase activity might have therapeutic potential in the treatment of portal hypertension.
The effects of NAD(P)H oxidase blockade attenuating the extent of collateralisation and the splanchnic hyperaemia of portal hypertensive rats were most probably mediated through inhibition of VEGF‐induced angiogenesis. This is based on the following considerations: first, in recent studies performed on experimental models of portal hypertension, we have shown that these animals have a marked and progressive overexpression of VEGF, VEGFR‐2 and CD31 in splanchnic tissues.3,4 Inhibition of such splanchnic neovascularisation, using either a monoclonal antibody against VEGFR‐2, or a specific inhibitor of VEGFR‐2, resulted in a significant decrease in the formation of portosystemic collateral vessels, as well as in an attenuation of the hyperdynamic splanchnic circulation.3,4 These results suggest that the formation of collaterals in portal hypertension is not only due to the opening of pre‐existing blood vessels because of the increase in portal venous pressure, as it has been traditionally thought,1 but it is also mediated by a VEGF‐dependent angiogenic process. In addition, our recent data indicate that an increased splanchnic neovascularisation significantly contributes to the splanchnic hyperaemia associated with portal hypertension, on top of the well‐established role of splanchnic vasodilatation.1 Second, our results show that chronic NAD(P)H oxidase inhibition significantly decreased the protein expression of the angiogenic factor VEGF and its receptor VEGFR‐2 in splanchnic organs from portal hypertensive rats. The expression of CD31, as an index of vascular density,20 was also significantly reduced after NAD(P)H oxidase blockade in rats with portal hypertension. In addition, the excessive splanchnic ROS production, observed in splanchnic tissues from portal hypertensive rats,12 was also significantly attenuated after treatment with the NAD(P)H oxidase inhibitor, as compared with the vehicle‐treated group. Taken together, these results suggest that NAD(P)H oxidase‐derived ROS are critically important in VEGF‐induced angiogenesis in portal hypertensive rats. At this point, we cannot rule out a possible role of VEGF‐independent mechanisms mediating the effects of NAD(P)H oxidase blockade in portal hypertensive rats.
The underlying molecular mechanisms by which activation of NAD(P)H oxidase stimulates angiogenesis are incompletely understood.7,8,9,10,11 In this regard, it has been shown that ROS produced by NAD(P)H oxidases can activate multiple signalling pathways, such as mitogen‐activated protein kinases, tyrosine kinases and transcription factors,21 which have been implicated in VEGF‐induced neovascularisation.22,23,24 Accumulating evidence suggests that ROS can also modulate angiogenesis via induction of VEGF and VEGFR‐2 expression.25,26 Interestingly, our results show that NAD(P)H oxidase blockade significantly decreases VEGF and VEGFR‐2 protein expressions in portal hypertensive rats. Therefore, two potential mechanisms by which NAD(P)H oxidase stimulates angiogenesis in portal hypertensive rats could be by increasing the expression of VEGF and/or that of its receptor. It should be noted that, as VEGFR‐2 is exclusively expressed on vascular endothelial cells,24 its down regulation may also reflect the overall decrease in splanchnic vascularity (ie, CD31 expression) observed in portal hypertensive rats after NAD(P)H oxidase inhibition.
Recently, we have also shown that the increased baseline oxidative stress observed in portal hypertensive rats is, at least in part, responsible for the upregulation of the enzyme heme oxygenase‐1 in this experimental model.12,27,28 Thus, when oxidative stress was reduced in portal hypertensive rats by chronic inhibition of NAD(P)H oxidase, heme oxygenase‐1 protein expression was also significantly down regulated.12 In addition, another salient finding of our previous study is that chronic heme oxygenase inhibition markedly and significantly decreases (by 74%) VEGF protein expression in the mesentery of portal hypertensive rats, suggesting that heme oxygenase enzymatic activity is an important stimulus for VEGF production in portal hypertension. Taken together, these results suggest that oxidative stress modulates angiogenesis in portal hypertension directly by activating the intracellular VEGF signalling pathway, and indirectly by stimulating the induction of heme oxygenase‐1, which, in turn, increases the expression of VEGF.
Multiple physiological agents, which may be increased during portal hypertension, including cytokines, shear stress and ischaemia, have been shown to activate NAD(P)H oxidase.5,6,29 Interestingly, the production of ROS has also been shown to be triggered secondarily to stimulation by growth factors, such as VEGF, indicating that NAD(P)H oxidase may also act downstream from VEGF signalling.11 Whether one or more of these factors stimulate NAD(P)H oxidases in portal hypertensive rats requires further investigation. In addition to portal hypertension, NAD(P)H oxidases contribute to excessive ROS production in other diseases such as hypertension,30,31 atherosclerosis,32 diabetic vascular diseases,33 cardiac hypertrophy and heart failure.34,35
In summary, this study provides compelling evidence that NAD(P)H oxidase plays an important role modulating angiogenesis‐dependent processes (ie, formation of portosystemic collaterals, increased splanchnic vascularity and development of hyperdynamic splanchnic circulation) in portal hypertensive rats. These findings suggest that NAD(P)H oxidase may serve as a novel therapeutic target for portal hypertension.
Acknowledgements
This study was supported in part by grants from the Ministerio de Ciencia y Tecnologia (SAF2002‐01461, and SAF2005‐05825) and Instituto de Salud Carlos III (PI02739, PI040655, and CO3/02). BA is a recipient of the Sheila Sherlock Fellowship of the European Association for the Study of the Liver (EASL), and MF of a contract from the Programa Ramon y Cajal, Ministerio de Ciencia y Tecnologia, Spain. The authors thank Dr Carlos Piera, Nieves Campos, and Franz Koening for technical and statistical assistance, and Dr Raul Mendez for helpful discussions.
Abbreviations
ROS - reactive oxygen species
TBS - Tris‐buffered saline
VEGF - vascular endothelial growth factor
VEGFR‐2 - VEGF receptor‐2
Footnotes
Competing interests: None.
References
- 1.Bosch J, Pizcueta P, Feu F.et al Pathophysiology of portal hypertension. Gastroenterol Clin North Am 1992211–14. [PubMed] [Google Scholar]
- 2.Bosch J, Garcia‐Pagan J C. Complications of cirrhosis. I. Portal hypertension. J Hepatol 200032141–156. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez M, Vizzutti F, Garcia‐Pagan J C.et al Anti‐VEGF receptor‐2 monoclonal antibody prevents portal‐systemic collateral vessel formation in portal hypertensive mice. Gastroenterology 2004126886–894. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez M, Mejias M, Angermayr B.et al Inhibition of VEGF‐receptor 2 decreases the development of hyperdynamic splanchnic circulation and portal‐systemic collateral vessels in portal hypertensive rats. J Hepatol 20054398–103. [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Griendling K K, Harrison D G. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 200324471–478. [DOI] [PubMed] [Google Scholar]
- 6.Griendling K K, Sorescu D, Ushio‐Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 200086494–501. [DOI] [PubMed] [Google Scholar]
- 7.Abid M R, Kachra Z, Spokes K C.et al NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 2000486252–256. [DOI] [PubMed] [Google Scholar]
- 8.Ushio‐Fukai M, Tang Y, Fukai T.et al Novel role of gp91(phox)‐containing NAD(P)H oxidase in vascular endothelial growth factor‐induced signaling and angiogenesis. Circ Res 2002911160–1167. [DOI] [PubMed] [Google Scholar]
- 9.Lelkes P I, Hahn K L, Sukovich D A.et al On the possible role of reactive oxygen species in angiogenesis. Adv Exp Med Biol 1998454295–310. [DOI] [PubMed] [Google Scholar]
- 10.Colavitti R, Pani G, Bedogni B.et al Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor‐2/KDR. J Biol Chem 20022773101–3108. [DOI] [PubMed] [Google Scholar]
- 11.Ushio‐Fukai M, Alexander R W. Reactive oxygen species as mediators of angiogenesis signaling. Role of NAD(P)H oxidase. Mol Cell Biochem 200426485–97. [DOI] [PubMed] [Google Scholar]
- 12.Angermayr B, Mejias M, Gracia‐Sancho J.et al Heme oxygenase attenuates oxidative stress and inflammation, and increases VEGF expression in portal hypertensive rats. J Hepatol 2006441033–1039. [DOI] [PubMed] [Google Scholar]
- 13.Fernando B, Marley R, Holt S.et al N‐acetylcysteine prevents development of the hyperdynamic circulation in the portal hypertensive rat. Hepatology 199828689–694. [DOI] [PubMed] [Google Scholar]
- 14.Bomzon A, Ljubuncic P. Oxidative stress and vascular smooth muscle cell function in liver disease. Pharmacol Ther 200189295–308. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez M, Garcia‐Pagan J C, Casadevall M.et al Evidence against a role for inducible nitric oxide synthase in the hyperdynamic circulation of portal hypertensive rats. Gastroenterology 19951081487–1495. [DOI] [PubMed] [Google Scholar]
- 16.Cotter M A, Cameron N E. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci 2003731813–1824. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez M, Garcia‐Pagan J C, Casadevall M.et al Acute and chronic cyclooxygenase blockage in portal‐hypertensive rats: influence in nitric oxide biosynthesis. Gastroenterology 19961101529–1535. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez M, Pizcueta P, Garcia‐Pagan J C.et al Effects of ritanserin, a selective and specific S2‐serotonergic antagonist, on portal pressure and splanchnic hemodynamics in rats with long‐term bile duct ligation. Hepatology 199318389–393. [PubMed] [Google Scholar]
- 19.Janiszewski M, Souza H P, Liu X.et al Overestimation of NADH‐driven vascular oxidase activity due to lucigenin artifacts. Free Rad Biol Med 200232446–453. [DOI] [PubMed] [Google Scholar]
- 20.Newman P J, Berndt M C, Gorski J.et al PECAM‐1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 19902471219–1222. [DOI] [PubMed] [Google Scholar]
- 21.Maulik N, Das D K. Redox signaling in vascular angiogenesis. Free Radic Biol Med 2002331047–1060. [DOI] [PubMed] [Google Scholar]
- 22.Leung D W, Cachiane G, Kuang W J.et al Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 19892461306–1309. [DOI] [PubMed] [Google Scholar]
- 23.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular growth factor family. Cardiovasc Res 200149568–581. [DOI] [PubMed] [Google Scholar]
- 24.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem 199727232521–32527. [DOI] [PubMed] [Google Scholar]
- 25.Arbiser J L, Petros J, Klafter R.et al Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA 200299715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandes R P, Miller F J, Beer S.et al The vascular NADPH oxidase subunit p47phox is involved in redox‐mediated gene expression. Free Radic Biol Med 2002321116–1122. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez M, Bonkovsky H L. Increased heme oxygenase‐1 gene expression in liver cells and splanchnic organs from portal hypertensive rats. Hepatology 1999291672–1679. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez M, Lambrecht R W, Bonkovsky H L. Increased heme oxygenase activity in splanchnic organs from portal hypertensive rats: role in modulating mesenteric vascular reactivity. J Hepatol 200134812–817. [DOI] [PubMed] [Google Scholar]
- 29.Grote K, Flach I, Luchtefeld M.et al Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase‐2 (MMP‐2) via NAD(P)H oxidase‐derived reactive oxygen species. Circ Res 20039280–86. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopalan S, Kurz S, Munzel T.et al Angiotensin II‐mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 1996971916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zalba G, Beaumont F J, San Jose G.et al Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 2000351055–1061. [DOI] [PubMed] [Google Scholar]
- 32.Warnholtz A, Nickenig G, Schulz E.et al Increased NADH‐oxidase‐mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin‐angiotensin system. Circulation 1999992027–2033. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y K, Lee M S, Son S M.et al Vascular NADH oxidase is involved in impaired endothelium‐dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes 200251522–527. [DOI] [PubMed] [Google Scholar]
- 34.Li J M, Gall N P, Grieve D J.et al Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 200240477–484. [DOI] [PubMed] [Google Scholar]
- 35.Gu W, Weihrauch D, Tanaka K.et al Reactive oxygen species are critical mediators of coronary collateral development in a canine model. Am J Physiol Heart Circ Physiol 2003285H1582–H1589. [DOI] [PubMed] [Google Scholar]