Abstract
Background
Helicobacter pylori gastritis may lead to impairment of the production of pepsinogen and acid, which are essential to cobalamin absorption. In turn, cobalamin deficiency leads to hyperhomocysteinaemia, a risk factor for cardio and cerebrovascular diseases.
Aim
To evaluate the effect of H pylori eradication on plasma homocysteine levels in elderly patients.
Patients
Sixty‐two H pylori‐positive elderly patients with cobalamin deficiency were prospectively studied.
Methods
Homocysteine and cobalamin concentrations were determined before, 6 and 12 months after H pylori eradication.
Results
Corpus atrophy was observed in a few patients; otherwise, in most of them, the degree of corpus gastritis was moderate to severe. The initial homocysteine mean (SD) levels decreased from 41.0 (27.1) to 21.6 (10.1) μmol/l at the 6 month follow‐up (p<0.001) and to 13.1 (3.8) μmol/l 12 months after H pylori eradication (p<0.001). Conversely, initial cobalamin mean levels increased from 145.5 (48.7) pmol/l to 209.8 (87.1) pmol/l and to 271.2 (140.8) pmol/l, 6 and 12 months after treatment, respectively (p<0.001 for both). Although the erythrocyte mean corpuscular volume was within reference intervals, it decreased significantly 6 (p = 0.002) and 12 (p<0.001) months after treatment.
Conclusions
The results of the current study demonstrated that the eradication of H pylori in elderly patients with cobalamin deficiency is followed by increasing of cobalamin and decreasing of homocysteine blood levels.
Keywords: helicobacter pylori , hyperhomocysteinaemia, cobalamin, elderly
Cardiovascular and cerebrovascular diseases are considered a serious public health problem that is expected to increase in parallel with aging of the world's population.1 Hyperhomocysteinaemia has been considered an important factor associated with the development of these disorders, which include coronary artery disease, stroke, peripheral vascular disease, venous thrombosis and dementia.1,2 Abnormally high plasma levels of homocysteine have been found to be influenced by renal function, use of medications and genetic defects of enzymes involved in the methionine metabolism, but the most common cause is vitamin deficiency, folate, B6 or B12 (cobalamin).1 The cobalamin is an essential co‐factor in the reaction in which a methyl group of tetrametil‐folate is transferred to homocysteine to form methionine. Therefore, cobalamin deficiency leads to hyperhomocysteinaemia due to an impaired homocysteine remethylation.1,3 It has been demonstrated that cobalamin deficiency increases with age, being present in 10 to 15% of elderly people.3
Recent studies showing that eradication of Helicobacter pylori restores the cobalamin‐deficient state4,5 point to infection playing a role in the vitamin deficiency. Since H pylori infection is mainly acquired in childhood and persists lifelong unless treated, its prevalence is high among the elderly. Inflammation, due to lifelong infection, may progress in some individuals to gastric atrophy, leading to impairment of the production of pepsinogen and acid6 which are essential to cobalamin absorption by releasing the vitamin from food proteins.3 Taking into account that the cobalamin deficiency is one of the most common causes of hyperhomocysteinaemia, it is reasonable to hypothesise that H pylori infection may be associated with an increased level of plasma homocysteine. However, there are few studies on this subject, and their results are discordant. Some authors demonstrated that the plasma concentration of homocysteine is higher in infected than in uninfected subjects.7,8 Otherwise, no significant association between plasma homocysteine levels and H pylori infection was observed by Whincup et al9 and Leung et al.10 The Leung study also demonstrated that the bacterium eradication had no impact on the homocysteine concentration, but one of its limitations was the inclusion of subjects with normal baseline homocysteine levels.10
Our aim was to evaluate the level of homocysteine before and after H pylori eradication in elderly patients with cobalamin deficiency.
Methods
This study was approved by the Ethics Committee of the Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, and all patients provided written informed consent.
Patients
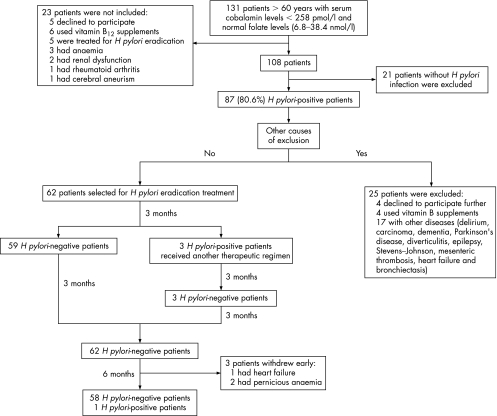
Between February 2002 and March 2004, we screened 131 consecutive outpatients over 60 years old (95 women, 36 men, mean (SD) age 72.8 (7.9) years). Each had been referred to the Geriatric Service of the Hospital das Clínicas, Universidade Federal de Minas Gerais and fulfilled the inclusion criteria of the study in that they had a cobalamin serum concentration of <258.0 pmol/l and a normal serum folate level (6.8–38.4 nmol/l). The cut‐off point we adopted for cobalamin was based on the study by Lindenbaum et al11 who recommended this value for geriatric patients in order to capture those otherwise unrecognised cases of presumptive cobalamin deficiency. The exclusion criteria were: the presence of conditions associated with hyperhomocysteinaemia, such as the use of metformin, lipid‐modifying drugs, levodopa, methrotrexate, cyclosporine, colchicin, niacin, theophylline, phenytoin, and cholestiramine and alcohol intake >60 g daily; disorders such as pancreatitis, neoplasia, myeloproliferative diseases, chronic diarrhea, intestinal surgery, Parkinson's disease, and severe or terminal illness; renal failure (serum creatinine levels >106.8 μmol/l in women and >132.6 μmol/l in men); hyperglycemia (fasting glucose plasma level (>5.5 mmol/l); hypo‐ or hyperthyroidism (serum thyroid‐stimulating hormone <0.4 μIU/ml or >4.0 μIU/ml) and hypoalbuminemia (serum albumin <350.0 g/l). Other exclusion criteria were: anaemia (haemoglobin values <120.0 g/l in women and <135.0 g/l in men); neurological symptoms; previous treatment for H pylori infection; and use of cobalamin. Twenty‐three patients were not included because six had been treated with cobalamin and five with antimicrobial drugs for H pylori eradication, three had anaemia, two had renal dysfunction, one had rheumatoid arthritis, one had a cerebral aneurism and five declined to participate. The other 108 patients (79 women, 29 men, mean age 72.1 (7.9) years) agreed to participate and at this time they did not have any exclusion condition. As a part of the service routine procedures to clarify the origin of the cobalamin deficiency, patients were submitted to endoscopy to obtain gastric and duodenal mucosa fragments for histological evaluation. At endoscopy, fragments of gastric mucosa were also obtained for H pylori culture (antrum and corpus) and preformed urease test (antrum and corpus). Other tests for H pylori diagnosis included carbolfuchsin‐stained histological section, 13C‐urea breath test (13C‐UBT) and serology.12 The patients were considered to be H pylori‐positive if the culture was positive or if two among the other tests were positive.
Eighty‐seven patients were H pylori‐positive. Among them, serology was positive in 84 (96.6%), urea breath test in 82 (94.3%), histology in 81 (93.1%), preformed urease test in 72 (82.8%) and culture in 62 (71.3%). Twenty‐one patients did not achieve the criterion for H pylori‐positivity (15 had negative results in all the tests; the preformed urease test was the only positive test in two patients, the histology in another two patients and two patients had positive results only on serology). Cyanocobalamin replacement therapy was administered to the H pylori‐negative patients and to 25 H pylori‐positive patients who were further excluded from the study because, between initial inclusion and the beginning of H pylori therapy, four declined to participate, four used cobalamin supplement and 17 had intercurrent disorders (delirium, carcinoma, dementia, Parkinson's disease, diverticulitis, epilepsy, Stevens–Johnson syndrome, mesenteric thrombosis, heart failure and bronchiectasis).
Sixty‐two H pylori‐positive patients (51 women, 11 men, mean (SD) age 71.1 (7.0) years, range 60–86 years) remained in the study. They received 20 mg of omeprazole once daily, 500 mg of clarithromycin twice daily and 200 mg of furazolidone twice daily for 1 week.13 A second regimen (20 mg of omeprazole once daily, 150 mg of bismuth subcitrate thrice daily, 500 mg of oxytetracycline thrice daily and 150 mg of furazolidone thrice daily for 10 days) was required by three patients to achieve H pylori eradication. The patients were re‐evaluated for bacterium eradication by the 13C‐urea breath test 3, 6 and 12 months after the end of treatment, and on these occasions they were submitted to neurological and cognitive evaluation (fig 1). As the result of the 13C‐UBT was negative in three patients at the beginning of the study, they were submitted to endoscopy at 3 and 12 months after the end of treatment to evaluate H pylori eradication. The patients were also clinically evaluated monthly and none of them used cobalamin supplement during the period of the study.
Figure 1 Design and outcome of the study.
Six patients among nine whose homocysteine did not return to normal levels one year after treatment agreed to be submitted to another endoscopy to evaluate their H pylori status, the histology of the gastric mucosa and to obtain fluid from the stomach and proximal intestine to investigate bacterium overgrowth by quantitative aerobic and anaerobic culture.
Controls
In order to evaluate variability in homocysteine plasma levels, we determined the plasma homocysteine level of 20 apparently healthy subjects older than 60 years (16 women, 4 men, mean (SD) age 72.4±7.4 years) with normal cobalamin serum concentration (>258.0 pmol/l) at entrance and one year later.
Laboratory parameters
For all determinations, blood samples were obtained from patients after a minimum of 10 h fasting and the specimens were stored at ‐20°C before testing.
The concentrations of cobalamin and folate were determined by a chemiluminescent immunometric assay using the Immulite Analyser (Diagnostic Products Corporation, Los Angeles, California, USA) in serum samples kept protected from UV light.
For the determination of homocysteine, the blood sample collected in EDTA was centrifuged at 2000 g, at 4°C immediately after venipuncture. Total plasma homocysteine values were determined by high‐performance liquid chromatography with fluorimetric detection and isocratic elution according to Pfeiffer et al.14 The adopted reference interval was 5–15 μmol/l.
The serum concentrations of pepsinogen I and pepsinogen II were determined by a specific ELISA (Biohit., Helsinki, Finland), and of gastrin by radioimmunoassay, using the GammaDab Gastrin 125I (DiaSorin, Stillwater, Minnesota, USA) according to recommendations of the manufacturers.
The presence of antiparietal cell and anti‐intrinsic factor antibodies was evaluated by an indirect immunofluorescent assay employing the Autoantibody Screen (Diagnostic & Technical Services, Johannesburg, South Africa) and by radioimmunoassay employing the Solid Phase Intrinsec Factor Blocking Antibody (Diagnostic Products Corporation, Los Angeles, California, USA), respectively.
Homocysteine and cobalamin blood concentrations, as well as antiparietal cell and anti‐intrinsic factor antibodies, were evaluated before, 6 and 12 months after the treatment.
The other blood biochemical analyses were performed by routine laboratory methods. All test results before treatment were within reference intervals. The mean (SD) values and reference intervals were 41.0 (4.0) g/l and 35.0–50.0 g/l for albumin, 5.2l (0.04) mmol/l and 3.9–5.5 mmol/l for fasting glucose, 1.8 (1.9) μIU/ml and 0.4–4.0 μIU/ml for Thyroid‐Stimulating Hormone, 79.5 (17.7) μmol/l and 70.7–132.6 μmol/l in men and 61.9–106.8 μmol/l in women for creatinine, 140.0 (11.0) g/l and >130 g/l in men and >120 g/l in women for haemoglobin and 15.4 (8.4) nmol/l and 6.8–38.5 nmol/l for folate. The mean values and reference intervals of mean corpuscular volume (MCV) were 90.1 (5.7) fl and 80–96 fl, respectively.
Histology
Single fragments obtained from the lesser curvature of the antrum, the lesser curvature of the angular region, the lesser curvature of the gastric body and greater curvature of the gastric body, as well as one fragment from the duodenum, were routinely processed for histology. The degree of mononuclear cells, polymorphonuclear cells and atrophy of the oxyntic mucosa were scored according to the revised Sydney System as: 0, absent; 1, mild; 2, moderate; and 3, marked. The villous height, crypt depth, villous/crypt ratio, number of intraepithelial lymphocytes and number of mononuclear cells in the lamina propria, and the presence of granuloma were evaluated in duodenal mucosa.
Quantitative culture of gastric and intestinal fluid
Quantitative culture was performed in the gastric (n = 6) and proximal intestinal (n = 4) fluid from six patients whose homocysteine did not return to normal levels one year after H pylori eradication. The number of colony forming units (CFU) per millilitre aspirate was obtained by serial dilution through the 10th tube (−1010). A 0.1‐mL aliquot of each dilution was plated on blood agar plates and incubated in an anaerobic chamber and in a CO2 jar at 37°C for 48 h.15
Statistical analysis
Data were analysed with SPSS V.10.0. In addition to visual examination of histograms and box plots, the Kolmogorov‐Smirnov goodness‐of‐fit test was used to assess the normality of the data. Significant departures from normality were detected for homocysteine and cobalamin levels, which were log‐transformed before analysis. After log‐transformation all parameters became normally distributed (p = 0.20). Paired two‐tailed Student's t test was used to assess differences between the pre‐ and post‐treatment values, whereas two‐sample two‐tailed Student's t test was used to detect differences between sub‐groups (mean (SD)). For correlations, Pearson's correlation (r) coefficient was used. The level of significance was set at p⩽0.05.
Results
During the intervening period, all patients remained off neurological and cognitive dysfunctions.
Before treatment
Histology
On histological examination all patients had antral gastritis in activity and 93.4% had corpus gastritis in activity. The presence of atrophy in the oxyntic mucosa was seen in nine (14.8%) of 61 patients (table 1).
Table 1 Histological changes of the antral and oxyntic mucosa of 61 patients.*.
| Variables | Absent n (%) | Mild n (%) | Moderate n (%) | Marked n (%) |
|---|---|---|---|---|
| Antral mucosa | ||||
| MN | 0 | 06 (9.8) | 32 (52.5) | 23 (37.7) |
| PMN | 0 | 15 (24.6) | 34 (55.7) | 12 (19.7) |
| Oxyntic mucosa | ||||
| MN | 02 (3.3) | 12 (19.7) | 31 (50.8) | 16 (26.2) |
| PMN | 04 (6.6) | 14 (22.9) | 36 (59.0) | 07 (11.5) |
| atrophy | 52 (85.2) | 02 (3.3) | 03 (4.9) | 04 (6.6) |
*Fragments of gastric mucosa from one patient were not adequate for analysis; n = number; MN = mononuclear cells; PMN = polymorphonuclear cells
Histological changes suggestive of celiac disease, such as villous atrophy, reduced folds, infiltration of mononuclear cells in the lamina propria or a greater number of intraepithelial lymphocytes, were not seen in any duodenal mucosa fragment. The presence of granuloma was not observed in the duodenal mucosa from all patients.
Pepsinogen and gastrin levels
Pepsinogen I levels ⩽25 μg/l, pepsinogen I/pepsinogen II ratio ⩽2.5 and gastrin levels >200 ng/l were observed in four, five and two patients, respectively.
Antiparietal cell and anti‐intrinsic factor antibodies
Antiparietal cell antibodies were present in six patients. Anti‐intrinsic factor antibodies were also detected in two of them.
13C‐UBT Δ over baseline (DOB) values and correlation between DOB and cobalamin or homocysteine levels
The mean DOB (SD) level was 27.4 (19.6)‰. The DOB levels did not correlate with either cobalamin (r = 0.07, p = 0.60) or homocysteine (r = −0.17, p = 0.21) concentrations.
After treatment
Eradication of bacterium
All patients completed the course of treatment. Mild side effects were observed in 32 patients, but they were self‐limiting and none of them interrupted the treatment. The bacterium was eradicated in 59 of 62 patients with the first treatment and in all patients after the use of the second schedule. One year after the end of the treatment, 58 of 59 patients who were evaluated remained H pylori‐negative.
Antiparietal cell and anti‐intrinsic factor antibodies
At 6 months follow‐up, antiparietal cell antibodies became negative in two of six patients, but anti‐intrinsic factor antibodies remained unchanged in the two previously positive patients.
Cobalamin levels
The initial cobalamin mean levels increased from 145.5 (48.7) pmol/l to 209.8 (87.1) pmol/l (p<0.001) 6 months after eradication of the bacterium. The mean levels returned to normal values (271.2 (140.8) pmol/l, p<0.001) 12 months after treatment.
Mean corpuscular volume
As an additional indication of the cobalamin increase, the MCV mean values, although within normal ranges before treatment (90.1 (5.0) fl), decreased significantly 6 (89.4 (5.3) fl, p = 0.002) and 12 (88.6 (4.7) fL, p<0.001) months after the treatment.
Homocysteine levels
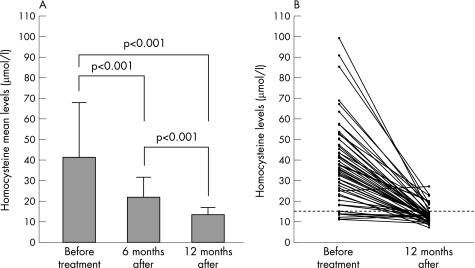
The initial homocysteine mean (SD) levels changed significantly (p<0.001) from 41.0 (27.1) μmol/l to 21.6 (10.1) μmol/l 6 months after treatment. The mean levels returned to normal values (13.3 (3.8) μmol/l, p<0.001) within 12 months following bacterium eradication (fig 2A). When patients were stratified according to pre‐treatment homocysteine levels, higher or lower than 15 μmol/l, a significant decrease (p<0.001) from 45.2 (26.6) μmol/l to 13.7 (3.9) μmol/l was observed in the first group. However, although the concentration of homocysteine decreased from 13.0 (1.6) μmol/l to 11.5 (2.0) μmol/l in the second group, it did not achieve statistical significance (p = 0.19).
Figure 2 A. Plasma homocysteine mean levels of patients with cobalamin deficiency before (n = 62), 6 (n = 62) and 12 (n = 59) months after H pylori eradication. B. Individual plasma homocysteine levels of 59 patients before and 12 months after H pylori eradication. The dot line indicates the cut‐off value for homocysteine.
Individual evaluation of the homocysteine levels at 1 year follow‐up
Individual evaluation showed that in 50 of 59 (84.8%) patients, the homocysteine concentration was within the normal range at 1 year follow‐up. Otherwise, although the homocysteine level had significantly decreased (p<0.001) in eight of nine patients, it was not within the reference intervals (mean 20.7 (3.2) μmol/l; range 16.7–27.2 μmol/l) (fig 2B). The haemoglobin levels of all these patients were within the reference values and no difference between pre‐ (142.0 (14.8) g/l) and one‐year post‐treatment (144.0 (14.0) g/l) mean values was seen (p = 0.63). Conversely, the mean concentration of cobalamin increased significantly (p = 0.003) from 164.2 (49.0) pmol/l to 219.7 (67.6) pmol/l and the MCV values also changed significantly (p = 0.02) from 93.6 (4.7) fl to 91.9 (3.9) fl 1 year after the end of treatment. Six of these nine patients agreed to under go another endoscopy. The results of the tests for H pylori diagnosis were negative and no bacterial growth >103 CFU/ml in aerobic and anaerobic conditions was observed in the gastric or intestinal fluid. Furthermore, an almost complete normalisation of the antral mucosa (mild mononuclear infiltration was seen in two patients) and complete normalisation of the corpus mucosa were observed at histology. The serum creatinine level increased above the reference values in three patients (from 97.2 μmol/l and 61.9 μmol/l to 114.9 μmol/l and 123.8 μmol/l, respectively in two womens and from 123.8 μmol/l to 141.4 μmol/l in one man). When all patients were analysed, no correlation between creatinine and homocysteine levels before treatment was observed (p = 0.24). Otherwise, one year after the end of treatment a significant correlation between the two variables was seen (r = 0.5, p<0.001). The mean concentration of creatinine was significantly higher in the patient with hyperhomocysteinaemia (97.2 (26.1) μmol/l) than in those with normal homocysteine concentrations (80.6 (18.0) μmol/l) one year after H pylori eradication (p = 0.02). Cyanocobalamin replacement therapy was administered to these patients at the end of the study.
Patients who were not evaluated at 1 year follow‐up
Pernicious anaemia could not be ruled out in two patients who had positive antiparietal and anti‐intrinsic factor antibodies and corpus atrophic gastritis at histology. Pepsinogen I levels of the patients were 6.0 and 13.0 μg/l, whereas their pepsinogen I/pepsinogen II ratios were 0.7 and 1.4, respectively. Gastrin levels were 630.0 ng/l and 361.0 ng/l. Since they neither had anaemia, nor other clinical signs of pernicious anaemia, they were included in the study. In these patients, an increase of cobalamin levels from 100.3 pmol/l to116.6 pmol/l and 73.8 pmol/l to 127.6 pmol/l was seen at the 6 month follow‐up. The homocysteine concentrations decreased from 109.4 μg/l to 34.2 μg/l in one of them and did not change in the other (22.6 and 22.5 μg/l). The presence of antiparietal cell and anti‐intrinsic factor antibodies did not change in both patients. At this time, they received cobalamin and were excluded from the study.
At 8 months after treatment, another patient was also excluded due to heart failure. She also received cobalamin replacement.
Controls
The homocysteine mean levels of the control subjects did not change significantly (p = 0.28) between the first (13.5 (2.5) μmol/l, range from 7.7 to 18.3 μmol/l) and second evaluation one year later (13.9 (2.8) μmol/l, range 8.5–19.5 μmol/l).
Discussion
There is increasing evidence linking H pylori infection to cobalamin deficiency in the elderly. The rationale is that long‐term H pylori infection may alter various gastric functions such as acid/pepsin secretion6 which is essential for releasing cobalamin from the food protein to become available for absorption mediated by the intrinsic factor.3,16 Acid also is needed for controlling gastric and duodenal bacterial overgrowth that may contribute to cobalamin deficiency by limiting vitamin bioavailability.15 Therefore, in some patients with long‐term H pylori infection, cobalamin chronic malabsorption may lead to depletion of the vitamin storage that culminates in its deficiency.16,17 Since cobalamin is an essential co‐factor in the reaction that methylates homocysteine in methionine, its deficiency is followed by hyperhomocysteinaemia.1,3
In the present study, we aimed to evaluate the impact of H pylori eradication on plasma homocysteine concentrations in elderly patients with cobalamin deficiency.
After bacterium eradication, homocysteine levels decreased significantly in parallel with increasing cobalamin levels and decreasing MCV values. However, in the sub‐groups of patients with normal homocysteine concentrations, the levels did not change significantly after bacterium eradication, resulting in agreement with the results of Leung et al.10 Therefore, in as much as no other apparent cause for hyperhomocysteinaemia could be demonstrated in this study, one may speculate that the hyperhomocysteinaemia observed in most of the patients we evaluated is attributed to long‐term H pylori infection that may progress to atrophy leading to gastric secretion dysfunction with consequent cobalamin malabsorption. However, most of our patients exhibited either minimal or no atrophy in the corpus gastric mucosa. Furthermore, biochemical markers of corpus atrophy (serum pepsinogen I levels ⩽25 μg/l, pepsinogen I/pepsinogen II ratio ⩽2.5 and gastrin levels >200 ng/l)18,19 were identified only in a few patients. Otherwise, since more than 70% of the patients had moderate to severe corpus gastritis that has been seen to be independently associated with hypochlorhydria,20 we may hypothesise that it is not necessary for complete gastric atrophy with achlorhydria to impair the absorption of cobalamin. In fact, Carmel's group recently demonstrated severe vitamin malabsorption in the absence of atrophic gastritis.21
In most patients, homocysteine levels became normal after H pylori eradication. However, in nine patients, although homocysteine levels decreased significantly, values were not within the reference ranges 12 months after bacterium eradication. The mean homocysteine levels before treatment were higher in these patients than in the others. One year after treatment, in three patients creatinine values increased above the reference value. Also, the serum creatinine significantly correlated with the homocysteine level. Taking these findings together, and considering also that renal clearance capacity substantially declines with age and the level of homocysteine is markedly influenced by renal excretion,22 we cannot rule out that the high homocysteine levels we observed in this sub‐group of patients before treatment was due to concomitant, previously undetected renal dysfunction.
In as much as no other cause for the presence of antiparietal cell antibodies could be identified in four patients in whom the cobalamin and homocysteine levels became normal after H pylori eradication, and considering that the presence of antibodies was not detected in two of them after the treatment, one may speculate that molecular mimicry between epitopes of H pylori proteins and epitopes of human H+K+‐ATPase of gastric parietal cells may have occurred.23
Since some authors consider that UBT values correlate with bacterial density in the stomach,24 in an attempt to evaluate whether the extent of H pylori infection in our patients correlated with their blood levels of cobalamin or homocysteine, we calculated the correlations between DOB values and cobalamin and homocysteine concentrations. The results, however, were not significant.
In interpreting data from this study, several limitations and potential sources of bias should be considered. First, most patients we studied were women. However, this is an expected finding since women live longer than men in almost all areas of the world. In Brazil, women make up approximately two‐thirds of the population over age 75.25 Second, we did not include a placebo control group for ethical reasons. Otherwise, although it is difficult to select elderly patients without any causes of hyperhomocysteinaemia, we determined the plasma homocysteine level at entrance and one year later in 20 patients with normal cobalamin serum concentration. Since a non‐significant increase was seen, we may conclude that the results we observed in the patients treated for H pylori eradication were neither due to chance, nor to a great variability in the homocysteine plasma levels. Another argument against chance is that there was a stepwise and regular decrease in homocysteine levels in all the patients with hyperhomocysteinaemia that we treated (fig 2).
Another point that deserves consideration is that instead of a long‐term H pylori infection, intestinal bacterial overgrowth (that could be reduced by antimicrobial treatment) could be the cause of cobalamin deficiency in the patients we studied. Against this last possibility it may be argued that none of the patients had chronic diarrhoea or other malnutrition signals to clinically diagnose intestinal bacterial overgrowth. Also, since no other intervention was carried out in addition to one‐week antimicrobial therapy, causes for bacterial overgrowth other than marked corpus gastritis with hypochlorydria are unlikely to be consistently resolved in most patients, as we observed.
In conclusion, the results of the current study demonstrated that the eradication of H pylori in elderly patients with cobalamin deficiency is followed by increasing cobalamin and decreasing homocysteine blood levels.
Acknowledgements
We are indebted to Dr Rogério Beato Lopes for neurological evaluation of patients, Dr Climene Mendonça for neuropsychiatry assessment of patients, Dr Edgar Nunes Moraes and Dr Anielo Greco Rodrigues dos Santos from the Núcleo de Geriatria/Gerontologia (NUGG), Universidade Federal de Minas Gerais, for the inclusion of patients in this study.
Abbreviations
CFU - colony forming units
MCV - mean corpuscular volume
13C‐UBT - 13C‐urea breath test
DOB - delta over baseline
Footnotes
Supported by research grants from Fundação de Apoio a Pesquisa do Estado de Minas Gerais–FAPEMIG and Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq/Brazil
Competing interests: None.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non‐exclusive for government employees) on a worldwide basis to the BMJ Pbulishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://gut.bmjjournals.com/ifora/licence.dtl).
References
- 1.Stanger O, Herrmann W, Pietrzik K.et al Consensus paper on the rational clinical use of homocysteine, folic acid, and B‐vitamins in cardiovascular and thrombotic diseases–guidelines and recommendations. Clin Chem Lab Med 2003411392–1403. [DOI] [PubMed] [Google Scholar]
- 2.Seshadri S, Beiser A, Selhub J.et al Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med 2002346476–483. [DOI] [PubMed] [Google Scholar]
- 3.Wolters M, Ströhle A, Hahn A. Cobalamin: a critical vitamin in the elderly. Prev Med 2004391256–1266. [DOI] [PubMed] [Google Scholar]
- 4.Kaptan K, Beyan C, Ural A U.et alHelicobacter pylori – is it a novel causative agent in vitamin B12 deficiency? Arch Inter Med 20001601349–1353. [DOI] [PubMed] [Google Scholar]
- 5.Serin E, Gümurdülü Y, Özer B.et al Impact of Helicobacter pylori on the development of vitamin B12 deficiency in the absence of gastric atrophy. Helicobacter 20027337–341. [DOI] [PubMed] [Google Scholar]
- 6.Sipponen P, Laxén F, Huotari K.et al Prevalence of low vitamin B12 and high homocysteine in serum in an elderly men population: association with atrophic gastritis and Helicobacter pylori infection. Scand J Gastroenterol 2003381209–1216. [DOI] [PubMed] [Google Scholar]
- 7.Tamura A, Fujioka T, Nasu M. Relation of Helicobacter pylori to plasma vitamin B12, folic acid and homocysteine levels in patients who underwent diagnostic coronary arteriography. Am J Gastroenterol 200297861–866. [DOI] [PubMed] [Google Scholar]
- 8.Khaled M A, Cornwell P E. Hyperhomocysteinaemia due to Helicobacter pylori. Atherosclerosis 2004172199–200. [DOI] [PubMed] [Google Scholar]
- 9.Whincup P, Danesh J, Walker M.et al Prospective study of potentially virulent strains of Helicobacter pylori and coronary heart disease in middle‐aged men. Circulation 20001011647–1652. [DOI] [PubMed] [Google Scholar]
- 10.Leung W K, Ma P K, Choi P C L.et al Correlation between Helicobacter pylori infection, gastric inflammation and serum homocysteine concentration. Helicobacter 20016146–150. [DOI] [PubMed] [Google Scholar]
- 11.Lindenbaum J, Savage D G, Stabler S P.et al Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol 19903499–107. [DOI] [PubMed] [Google Scholar]
- 12.Queiroz D M M, Guerra J B, Rocha G A.et alIL1B and IL1RN polymorphic genes and Helicobacter pylori cagA strains decrease the risk of reflux esophagitis. Gastroenterology 200412773–79. [DOI] [PubMed] [Google Scholar]
- 13.Dani R, Queiroz D M M, Dias M G M.et al Omeprazole, clarithromycin and furazolidone for the eradication of Helicobacter pylori in patients with duodenal ulcer. Aliment Pharmacol Ther 1999131647–1652. [DOI] [PubMed] [Google Scholar]
- 14.Pfeiffer C M, Huff D L, Gunter E W. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 199945290–292. [PubMed] [Google Scholar]
- 15.Suter P M, Golner B B, Goldin B R.et al Reversal of protein‐bound vitamin B12 malabsorption with antibiotics in atrophic gastritis. Gastroenterology 19911011039–1045. [DOI] [PubMed] [Google Scholar]
- 16.Carmel R, Perez‐Perez G I, Blaser M J.Helicobacter pylori infection and food‐cobalamin malabsorption. Dig Dis Sci 199439309–314. [DOI] [PubMed] [Google Scholar]
- 17.Carmel R, Aurangzeb I, Qian D. Associations of food‐cobalamin malabsorption with ethnic origin, age, Helicobacter pylori infection, and serum markers of gastritis. Am J Gastroenterol 20019663–70. [DOI] [PubMed] [Google Scholar]
- 18.Sipponen P, Ranta P, Helske T.et al Serum Levels of amidated gastrin‐17 and pepsinogen I in atrophic gastritis: an observational case‐control study. Scan JGastroenterol200237785–791. [PubMed] [Google Scholar]
- 19.Walsh J H, Grossman M I. Gastrin. New Eng J Med 19752921324–1334. [DOI] [PubMed] [Google Scholar]
- 20.Furuta T, El Omar E M, Xiao F.et al Interleukin 1 β polymorphisms increase the risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology 200212392–105. [DOI] [PubMed] [Google Scholar]
- 21.Cohen H, Weinstein W M, Carmel R. Heterogeneity of gastric histology and function in food cobalamin malabsorption: absence of atrophic gastritis and achlorhydria in some patients with severe malabsorption. Gut 200047638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann W, Quast S, Ulrich M.et al Hyperhomocysteinaemia in high‐aged subjects: relation of B‐vitamins, folic acid, renal function and the methylenetetrahydrofolate reductase mutation. Atherosclerosis 199914491–101. [DOI] [PubMed] [Google Scholar]
- 23.D'Elios M M, Appelmelk B J, Amedei A.et al Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med 200410316–323. [DOI] [PubMed] [Google Scholar]
- 24.Gisbert J P, Pajares J M.13C‐urea breath test in the management of Helicobacter pylori infection. Dig Liv Disease 200537899–906. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization Department of Health promotion, non‐communicable disease prevention and surveillance. Health and ageing: a discussion paper http://whqlibdoc, who.int/hq/2001/WHO_NMH_HPS_01.1.pdf