Abstract
Background
In chronic pancreatitis, obstruction of the main pancreatic duct (MPD) may contribute to the pathogenesis of pain. Pilot studies suggest that extracorporeal shock wave lithotripsy (ESWL) alone relieves pain in calcified chronic pancreatitis.
Aim
To compare ESWL alone with ESWL and endoscopic drainage of the MPD for treatment of pain in chronic pancreatitis.
Subjects
Patients with uncomplicated painful chronic pancreatitis and calcifications obstructing the MPD.
Methods
55 patients were randomised to ESWL alone (n = 26) or ESWL combined with endoscopy (n = 29).
Results
2 years after trial intervention, 10 (38%) and 13 (45%) patients of the ESWL alone and ESWL combined with endoscopy group, respectively, had presented pain relapse (primary outcome) (OR 0.77; 95% CI 0.23 to 2.57). In both groups, a similar decrease was seen after treatment in the MPD diameter (mean decrease 1.7 mm; 95% CI 0.9 to 2.6; p<0.001), and in the number of pain episodes/year (mean decrease, 3.7; 95% CI 2.6 to 4.9; p<0.001). Treatment costs per patient were three times higher in the ESWL combined with endoscopy group compared with the ESWL alone group (p = 0.001). The median delay between the onset of chronic pancreatitis and persistent pain relief for both groups was 1.1 year (95% CI 0.7 to 1.6), as compared with 4 years (95% CI 3 to 4) for the natural history of chronic pancreatitis in a reference cohort (p<0.001).
Conclusions
ESWL is a safe and effective preferred treatment for selected patients with painful calcified chronic pancreatitis. Combining systematic endoscopy with ESWL adds to the cost of patient care, without improving the outcome of pancreatic pain.
Pain is the symptom in chronic pancreatitis that most often requires treatment.1 It may be related to an increased pressure within the ductal system and/or parenchyma secondary to outflow obstruction of the main pancreatic duct (MPD) and other mechanisms (eg, perineural fibrosis).2,3,4,5 We have developed the application of extracorporeal shock wave lithotripsy (ESWL) to pancreatic stones to facilitate their removal during therapeutic endoscopy.6 Long‐term follow‐up studies have shown that ESWL combined with endoscopic drainage of the MPD relieves pain and may avoid the need for surgery in approximately two‐thirds of patients on an intention‐to‐treat basis.7 This has become a successful alternative to surgery for treating patients with painful obstructive chronic pancreatitis in Europe.8,9 However, its use has remained limited to highly specialised tertiary referral centres as it is technically demanding.
Ohara et al10 have reported a pilot study showing that the application of ESWL alone to pancreatic calcifications was followed by a spontaneous clearance of stone fragments and complete relief of pain in 79% of the patients. Therefore, we conducted a randomised trial comparing pain relief after ESWL alone with ESWL combined with endoscopic drainage of the MPD in patients with painful calcified chronic pancreatitis.
Methods
Patients
Enrolment of participants began on 24 March 1998 and was completed on 17 September 2002 in Brussels, Belgium (centre 1) and Roma, Italy (centre 2). Patients were considered eligible if they had painful chronic pancreatitis with at least one calcification >4 mm in the pancreatic head or body with upstream dilation of the MPD and no previous intervention on the pancreas. Exclusion criteria included the presence of a pancreatic fluid collection >2 cm, serum alkaline phosphatases greater than twice the normal value or cholangitis, age <18 years or pregnancy or lactation, and unwillingness to participate. All patients gave written informed consent for participation in the study. The study complied with the Declaration of Helsinki regarding investigation in humans, and was approved by the institutional ethics committee. There was no involvement of industry in the design, conduct, financial support or analysis of the study.
Randomisation, intervention and follow‐up
Randomisation was performed on the day of ESWL by opening a sequentially numbered opaque sealed envelope. The random allocation schedule, stratified according to centres, had been generated by a study nurse not involved in the trial using blocks of 6 and a 1:1 allocation ratio, using a table of random digits. If patients in the ESWL alone group had persisting or recurrent pain after treatment, crossover to the ESWL combined with endoscopy group was offered. One or more sessions of ESWL were performed in all patients using the Lithostar Plus (Siemens, Ehrlangen, Germany) until the obstructive stones were broken into fragments <2 mm, as measured by x ray.6 In addition to this, patients in the ESWL combined with endoscopy group underwent an endoscopic retrograde pancreatography (ERP) immediately after the last ESWL session with attempted extraction of stone fragments and insertion of 10‐French plastic pancreatic stents (Wilson‐Cook) if pancreatic strictures were identified.7,8,9,10,11 Stents were exchanged every 6 months (or earlier in case of suspected stent dysfunction), and were removed after 12–24 months, if stricture dilatation was judged satisfactory.12 Treatment approaches were agreed upon and were standardised before the trial.
Follow‐up consisted of clinical examination 1 month after treatment (supplemented with secretin‐enhanced magnetic resonance cholangio‐pancreatography (S‐MRCP) in centre 1),13 and every 6 months thereafter. Data collected included pain relapses, weight change, intake of drugs, performance of ESWL, endoscopic or surgical procedures and any other seemingly unrelated medical treatments. No additional ancillary care (eg, referral to a multidisciplinary team specialised in pain treatment, prescription of drugs with a possible effect against pain in chronic pancreatitis such as octreotide/combined antioxidant preparations) was provided, except for preparations of pancreatic enzymes, which were systematically prescribed in case of steatorrhoea (regardless of the allocation group). In June 2005, a final follow‐up was made by a gastroenterologist (EG) blinded to the patients' allocation group, who reviewed with the patients the data collected prospectively (these were instructed at the beginning of the interview not to disclose their allocation group). Pairs of S‐MRCP performed in centre 1 before and 1 month after the trial intervention were reviewed by an experienced radiologist (Monia Bali) unaware of the details of the trial and blinded to the imaging sequence.
Study end points and definitions
The primary end point was the incidence of pain relapse at 2 years. Secondary end points were MPD decompression at 1 month as assessed by S‐MRCP, complications, treatment‐related costs, and the delay between the onset of chronic pancreatitis and persistent pain relief as compared with the natural history of chronic pancreatitis.
The diagnosis of calcified chronic pancreatitis was based on clinical and radiological criteria.14 It was confirmed during follow‐up in all but one patient, who died from a pancreatic cancer (18 months after randomisation, and 7 years after the onset of chronic pancreatitis). The onset of chronic pancreatitis was defined as the first documented episode of pancreatitis or the first episode of typical abdominal pain (whichever occurred first).15 Alcoholism was defined as daily alcohol intake >80 g for at least 5 years. The intensity of pain on admission or during the last painful episode (if absent at the time of admission) was graded on a 10‐point visual analogue scale by all the patients (regardless of whether the pain was continuous/present after every meal or not). Obstructive calcifications were defined at CT scan as calcifications with upstream dilation of the MPD adjacent to them. The number, location (head, body or tail) and diameter of obstructive calcifications were assessed at CT scan. If multiple obstructive stones were detected, the largest diameter was noted. Duration of hospital stay was calculated starting on the day of the first ESWL, or of readmission in case of relapsing pain. Pain recurrence was defined as relapsing pain with an intensity >2 on a 10‐point scale.11 Persistent pain relief was defined as the absence of pain recurrence for ⩾2 years without intake of analgesics.16
A comparison with the natural history of chronic pancreatitis was performed for the subgroup of patients who presented <2 years after the onset of chronic pancreatitis. For this subgroup of patients, the delay between the onset of chronic pancreatitis and of persistent pain relief was compared with that observed in a cohort that has served as a reference for the natural history of pain in chronic pancreatitis (data on individual patients provided by RW Ammann).16 Other patients were excluded from this subgroup analysis because they presented too late after the onset of chronic pancreatitis to experience a considerable modification in the timing of persistent pain relief compared with the natural history of chronic pancreatitis (persistent pain relief spontaneously developed a mean of 4 years after the onset of chronic pancreatitis in the cohort chosen for comparison). Except for this latter comparison, all analyses were performed on an intention‐to‐treat basis.
Hospital costs were analysed during initial treatment (starting on the day of the first ESWL) and during follow‐up. Costs not directly related to the treatment of pain or of procedure‐related complications (eg, diabetes), as well as costs induced by the study protocol (eg, S‐MRCP), were disregarded. For all patients, the calculation of costs was based on the rate for Belgian public healthcare insurance. These include all hospital, doctor and paramedical costs. To allow for recalculation according to different national prices, main relevant Belgian prices (2003 values) are listed below: hospitalisation, 389€/day; ESWL, 639€; ERP with pancreatic sphincterotomy alone, 675€; ERP with stone extraction (including sphincterotomy if required), 1039€; ERP with stent insertion (including sphincterotomy and stone extraction if required), 1351€; coeliac block, 325€; surgical pancreaticojejunostomy, 3052€.
Statistics
Continuous variables were described by their mean (standard deviation (SD)), or their median with the ranges under parentheses if they were found to be non‐normally distributed after Shapiro–Wilk normality test using a p value <0.05 (except costs and durations of hospital stay, for which means were found to be more clinically relevant).
The sample‐size calculation was based on the assumption that ESWL alone would be ineffective to treat pain, so that 90% of patients in the ESWL alone group would have presented pain recurrence at 2 years,15,16,17,18,19 compared with 50% in the ESWL combined with endoscopy group.11 On the basis of 0.8 power, 50 patients were required to detect a significant difference (p = 0.05). Expecting that 10% of the patients would be lost to follow‐up, we included 55 patients in the study.
All analyses were performed on an intention‐to‐treat basis including the 55 patients, except for the comparison with the natural history of chronic pancreatitis, which included the subgroup of 42 patients who presented <2 years after the onset of chronic pancreatitis. Comparisons between the groups were performed with the Pearson χ2 test or Fisher's exact test for categorical data, the two‐sample unpaired t test for continuous approximately normally distributed variables and the Wilcoxon signed rank test for continuous non‐normally distributed variables. Comparisons between data before and after treatment in each group were performed with the paired t test. We also examined which factors—including group allocation, number of pain episodes during the year before treatment, presence of pain at the time of inclusion in the study, stone number, size and location (head only or not), duration of chronic pancreatitis before inclusion in the study, sex and age—were associated with pain relapse at 2 years, using multiple logistic regression analysis. In Kaplan–Meier analysis, patients who died or were lost to follow‐up without having presented pain relapse were censored at the time of last contact. All p tests were two‐sided, and p values <0.05 were considered as significant. Analyses were performed with JMP software V.5.1.2. Confidence intervals (CIs) for the treatment effect were calculated with an exact method using StatXact sofware V.5.
Results
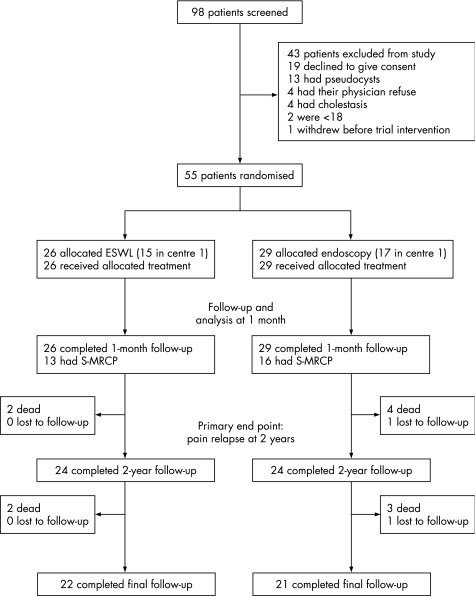
In all, 55 patients were enrolled in the study (fig 1) after a pretherapeutic investigation including CT scan without contrast medium injection (n = 55) and an S‐MRCP (n = 48). All patients received intended treatment only at baseline (pain disappeared or sufficiently decreased to allow discharge of all patients without crossover from the ESWL alone group to the ESWL combined with endoscopy group or use of other therapeutic modalities). Table 1 shows the baseline characteristics of the study population and procedural characteristics.
Figure 1 Screening, enrolment and outcomes.
Table 1 Characteristics of the patients at baseline and of trial intervention*.
| ESWL alone (n = 26) | ESWL combined with endoscopy (n = 29) | |
|---|---|---|
| Age (years) | 51.8 (12.3) | 49 (10.1) |
| Male sex, n (%) | 22 (85) | 21 (72) |
| Alcoholism, n (%) | 19 (73) | 20 (69) |
| Number of pain episodes during the year before treatment | 2.5 (1–13) | 3 (1–12) |
| Intensity of pain on a 10‐point visual analogue scale | 7.2 (2) | 7.3 (1.7) |
| Pain present at the time of inclusion, n (%) | 11 (42) | 20 (69) |
| Pain continuous or present after every meal, n (%) | 10 (38) | 8 (34) |
| Diabetes, n (%) | 6 (23) | 4 (14) |
| Number of obstructive calcifications | 3.0 (1–10) | 1 (1–20) |
| Diameter of obstructive calcification (mm) | 10.5 (5–26) | 8 (3.5–20) |
| Diameter of the main pancreatic duct (mm) | 8.2 (2.9) | 7.8 (2.3) |
| Obstructive calcifications in the head of the pancreas only, n (%) | 19 (73) | 24 (83) |
| Number of ESWL sessions | 2 (1–3) | 2 (1–4) |
| Number of endoscopy sessions | 0 | 2 (1–4) |
| Patients with procedure‐related complication, n (%) | 0 | 1 (3) |
| Hospital stay duration (days) | 2.2 (1.5) | 7.3 (4.9)† |
ESWL, extracorporeal shock wave lithotripsy.
*Values are means (SD) (normally distributed data); values with ranges under parentheses are medians (non‐normally distributed data)
†p<0.001 for comparison with the ESWL alone group; the other comparisons regarding trial intervention (ie, ESWL and endoscopy sessions, as well as procedure‐related complications) showed a p value >0.05.
ESWL was performed in the 55 patients, and all obstructive calcifications were broken into fragments <2 mm in thickness. All patients in the ESWL combined with endoscopy group immediately underwent pancreatic sphincterotomy, followed by stone fragment extraction (associated with pancreatic stent insertion in 13 (45%) cases). By June 2005, a mean (SD) follow‐up of 51.3 (21.5) months was available. In all, 11 (20%) patients died during the whole follow‐up period, at a median of 18 months (interquartile range 14–47) after the trial intervention. Causes of death were unrelated to chronic pancreatitis (with the exception of one case of pancreatic cancer), and included lung cancer (n = 2), bronchopneumonia (n = 2), traumatism (n = 2), chronic renal failure (n = 2), stroke (n = 1) and suicide (n = 1).
Clinical and radiological outcome
Two years after treatment, 10 (38%) patients in the ESWL alone group had presented pain relapse (primary outcome), as compared with 13 (45%) in the ESWL combined with endoscopy group (OR 0.77; 95% CI, 0.23 to 2.57; table 2). The number of pain episodes during the year after treatment was markedly decreased compared with the year before treatment, by a mean of 3.8 (95% CI 2 to 5.6; p<0.001) in the ESWL alone group, and of 3.7 (95% CI 2.1 to 5.2; p<0.001) in the ESWL combined with endoscopy group. The difference between groups was not significant (p = 0.759).
Table 2 Evolution during follow‐up*.
| ESWL alone (n = 26) | ESWL combined with endoscopy (n = 29) | p Value | |
|---|---|---|---|
| Follow‐up duration (months) | 52 (19.3) | 50.7 (23.6) | 0.460 |
| Patients with pain relapse, n (%) | |||
| At 2 years | 10 (38) | 13 (45) | 0.633 |
| During the whole follow‐up | 11 (42) | 13 (45) | 0.851 |
| Number of pain episodes during the year after trial intervention | 0 (0–5)† | 0 (0–4)‡ | 0.759 |
| Intensity of relapsing pain on a 10‐point visual analogue scale | 5.7 (2.1) | 5.7 (1.3) | 0.963 |
| Weight increase (kg) | 3.9 (4.9) | 3.5 (6.4) | 0.846 |
| Patients with therapeutic procedure§, n (%) | 8 (31) | 18 (62) | 0.02 |
| Endoscopic retrograde pancreatography | 8 (31) | 18 (62) | 0.02 |
| ESWL | 7 (27) | 7 (24) | 0.813 |
| Coeliac block | 0 | 3 (10) | 0.238 |
| Surgery | 1 (4) | 3 (10) | 0.613 |
| Duration of hospital stay (days) | 3.1 (5.3) | 8.6 (16.5) | 0.099 |
ESWL, extracorporeal shock wave lithotripsy.
*Values are means (SD) (normally distributed data); values with ranges under parentheses are medians (non‐normally distributed data).
†p<0.001 and ‡p<0.001 compared with the year before trial intervention for the ESWL alone and ESWL combined with endoscopy groups, respectively.
§Some patients had >1 therapeutic procedure; in particular coeliac blocks and surgery were performed in a total of five patients who had presented pain relapses.
Kaplan–Meier estimates indicate that pain relapse rates were, for the ESWL alone and ESWL combined with endoscopy group, respectively, 35.2% and 42.6% by 1 year, 39.3% and 46.7% by 2 years, and remained stable at 43.3% and 46.7% from 3 to 7 years (fig 2). Pain relapse did not differ between groups (log‐rank test, p = 0.651). A logistic regression analysis found that the location of obstructive calcifications in the head of the pancreas was the only single factor that was independently associated with the absence of pain relapse (p = 0.013).
Figure 2 Kaplan–Meier cumulative‐event curves for pain relapse for the ESWL alone and ESWL combined with endoscopy group.
During follow‐up, pain relapses were mild and managed with analgesics for a few days only in 6 of 24 (25%) patients (three in each group). Table 2 lists the additional treatments performed during follow‐up in the other patients. In the ESWL alone group, pain relapsed and was treated by endoscopy in eight patients who received ERP at a mean of 15.5 (11.6) months after the trial intervention (no patient had further intervention on the pancreas in the absence of pain relapse). In the ESWL combined with endoscopy group, ERP during follow‐up was motivated by scheduled stent exchange in patients without symptoms in eight cases. At the end of follow‐up, among the 20 patients of both groups who had received pancreatic stents, 1 (5%) still had a stent in place and 1 (5%) had been lost to follow‐up. Coeliac blocks and surgery were performed in a total of five patients (both groups), for relapsing pain in all cases. The effect of these procedures on the end points was as follows: (1) 1‐month end points, none (the first of these procedures was performed 2 months after the trial intervention); (2) persistent pain relief, minimal (pain relief was achieved in only 1 of these patients, 38 months after the onset of chronic pancreatitis); (3) costs, complete.
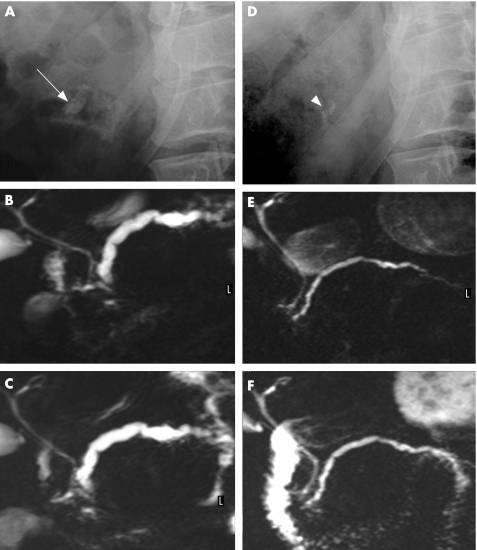
One month after treatment, the MPD diameter (as assessed by S‐MRCP at baseline and 1 month after treatment) had significantly decreased in both groups, by a mean of 1.7 mm (95% CI 0.9 to 2.6; p<0.001; fig 3). The difference between groups was not significant (p = 0.391).
Figure 3 Treatment by extracorporeal shock wave lithotripsy (ESWL) alone: decompression of the main pancreatic duct and restoration of the outflow of pancreatic juice into the duodenum (D–F) after ESWL, as compared with before (A–C) ESWL. Before treatment, (A) a single stone (arrow) is detected in the area of the head of the pancreas at abdominal plain film, (B) the main pancreatic duct is severely dilated and (C) no pancreatic juice appears in the duodenum after intravenous injection of secretin, as evidenced by magnetic resonance. After ESWL alone, (D) few stone fragments (arrowhead) are detected in the pancreatic area, (E) the main pancreatic duct is much thinner, and (F) pancreatic juice (in white) fills the duodenum shortly after intravenous injection of secretin.
Complications and mortality were assessed at 1 month. There was no procedure‐related mortality, but one procedure‐related complication (30‐day complication rate in the ESWL alone and ESWL combined with endoscopy groups, 0 and 3%, respectively; p = 1). This consisted of a pseudocyst related to a ruptured secondary branch of the MPD; endoscopic treatment was successful, and the patient was discharged 13 days after readmission (complication graded as severe).20
Comparison with the natural history of chronic pancreatitis
In all, 42 (76%) of the 55 patients received one of the trial interventions during the 2 years after the onset of chronic pancreatitis. They experienced persistent pain relief 3.1 years (95% CI 2.4 to 3.8, p<0.001) earlier after the onset of chronic pancreatitis compared with the natural history of chronic pancreatitis (fig 4).16
Figure 4 Kaplan–Meier cumulative‐event curves for persistent pain relief in the 42 patients included in this trial within 2 years from the onset of chronic pancreatitis, as compared with the cohort of patients that served as a reference for the natural history of pain in chronic pancreatitis (individual data kindly provided by RW Ammann).16 Persistent pain relief was defined as the absence of pain recurrence for ⩾2 years.
Costs of treatment
Costs of treatment per patient during the whole study were about three times higher in the ESWL combined with endoscopy group compared with the ESWL alone group (p = 0.001; table 3). The difference between groups was significant during the initial treatment (because of shorter hospital stay and absence of endoscopy‐related costs in the ESWL alone group), but not during follow‐up (except for endoscopy‐related costs, which were 2.5 times higher in the ESWL combined with endoscopy group during this period, p = 0.047). The duration of hospital stay over the whole study period was longer for patients in the ESWL combined with endoscopy group compared with the ESWL alone group (mean difference, 10.6 days; 95% CI 3.3 to 17.9; p = 0.006).
Table 3 Costs of treatment expressed in € per patient*.
| ESWL alone (n = 26) | ESWL combined with endoscopy (n = 29) | p Value | |
|---|---|---|---|
| Initial treatment | |||
| Hospital stay | 838 (579) | 2830 (1913) | <0.001 |
| ESWL | 1229 (476) | 1189 (504) | 0.769 |
| Endoscopy | 0 | 2152 (982) | NA |
| Procedure‐related complications | 0 | 363 (1955) | 0.348 |
| Total | 2067 (852) | 6535 (3160) | <0.001 |
| Follow‐up | |||
| Hospital stay | 1199 (2050) | 3327 (6403) | 0.1 |
| ESWL | 296 (549) | 286 (606) | 0.957 |
| Endoscopy | 831 (1619) | 2110 (2898) | 0.047 |
| Other† | 117 (598) | 383 (1116) | 0.271 |
| Total | 2443 (4157) | 6106 (10 068) | 0.087 |
| Total | 4509 (4080) | 12 641 (11 531) | 0.001 |
ESWL, extracorporeal shock wave lithotripsy; NA, not applicable.
*Values are means (SD).
†Coeliac block and pancreaticojejunostomy.
Discussion
Our findings indicate that ESWL considerably reduces the number of pain episodes in chronic pancreatitis with obstructive calcifications, with more than half of the patients having no pain relapse at all during a median follow‐up of 4 years. Similar findings were made in the ESWL combined with endoscopy group, but at a higher cost. Hospital stay duration was shorter and invasive procedures were less frequent in the ESWL alone compared with the ESWL combined with endoscopy group, during both trial intervention and follow‐up. It should be emphasised that this was obtained in selected patients who had painful chronic pancreatitis with a MPD obstruction and no severe complication (ie, pancreatic fluid collection or common bile duct obstruction).
The patients who received one of the trial interventions during the 2 years after onset of chronic pancreatitis experienced persistent pain relief (defined as the absence of relapsing pain during 2 years) a median of 1 year after the onset of chronic pancreatitis. This delay was considerably shorter compared with that observed in a well‐characterised cohort of patients who received only supportive measures in case of uncomplicated chronic pancreatitis.16 This cohort was chosen for comparison among five cohorts published to date (table 4) as it is the cohort that disclosed the shortest delay between the onset of chronic pancreatitis and pain relief, and it is the one most often cited (individual data on patients with dates of pain relief were necessary to allow for statistical comparison, and these were kindly provided by RW Ammann).15,17,18,19
Table 4 Published cohorts on the “natural” history of chronic pancreatitis and present study.
| First author, year | Patients, n | Prospective study | Country | Follow‐up (years) | Alcoholic CP (%) | Pancreatic surgery (%) | Definition of pain relief | Years between onset of CP and pain relief for 50% of patients |
|---|---|---|---|---|---|---|---|---|
| Miyake, 198719 | 125 | Y | Japan | 6 | 62 | 31 | Relief or improvement | 5 |
| Lankisch, 199317 | 335 | NA | Germany | 10 | 73 | 27 | Pain‐free>1 year | NA (35% with pain relief at the end of follow‐up) |
| Layer, 199418 | 315 | N | USA | 15 | 79 | 40 | Pain decreased or disappeared | NA (64–77% with pain relief after 13–27 years) |
| Cavallini, 199815 | 715 | Y | Italy | 10 | 75 | 63 | Pain free ⩾1 year | 8 |
| Ammann, 199916 | 207 | Y | Switzerland | 17 | 100 | 56 | Pain free ⩾2 years | 4 |
| Present study | 55 | Y | Belgium, Italy | 4 | 70 | 8 | Pain free ⩾2 years | 1 |
CP, chronic pancreatitis; NA, not available; Y, yes.
In this cohort,16 the onset of chronic pancreatitis was defined as the first documented episode of pancreatitis, as compared with the first documented episode of pancreatitis or the first episode of typical abdominal pain (whichever occurred first) in the present series. This strengthens our finding that the development of persistent pain relief was accelerated in our cohort compared with the natural course of chronic pancreatitis. However, we recognise that the addition of a no‐treatment group to assess the natural history of chronic pancreatitis in this trial would have been of greater scientific value. We decided not to include such a group for ethical and practical reasons. Published guidelines do not recommend non‐specific supportive measures to wait for spontaneous pain relief in chronic pancreatitis,21 and many patients are referred to us after failure of such measures. Given the non‐randomised nature of the comparison with historical controls, interpretation of our findings regarding the efficacy of either intervention compared with a control group should be made cautiously. It is not possible to rule out the possibility that an unmeasured factor, other than the interventions themselves, can explain the large differences in pain relief reported in our trial and in the cohort studies. Patients' unblinding is another limitation of this study; however, we think that it has not favoured the ESWL alone group, whose patients understood that they received only a portion of the treatment usually performed in our institutions and for which they were initially referred.
Costs of treatment observed during trial intervention were about three times lower in the ESWL alone compared with the ESWL combined with endoscopy group, due to the absence of baseline endoscopic treatment and shorter hospital stay (even though hospital stay was short in the ESWL combined with endoscopy group compared with the number of hospitalisation days commonly reported).8 Longer hospital duration in the ESWL combined with endoscopy group compared with the ESWL alone group was related to our routine practice to hospitalise patients after treatment of chronic pancreatitis, to assess whether pain decreases and to facilitate clinical and biological surveillance after invasive procedures. Only 5 (19%) of the 26 patients in the ESWL alone group had ambulatory treatment, a proportion which could be increased to further decrease costs, as ESWL alone was found to be extremely well tolerated. It should be emphasised that the total cost of the initial treatment in the ESWL alone group was about 10% that observed for pylorus‐preserving pancreaticoduodenectomy (an operation commonly performed to achieve pain relief in patients with chronic pancreatitis).22,23 During follow‐up, costs remained similarly lower in the ESWL alone compared with the ESWL combined with endoscopy group, as fewer patients required therapeutic procedures.
Despite the relatively high drop‐out rate (7/55, 12.7%) for the analysis of the primary end point (pain relapse at 2 years), an intention‐to‐treat analysis could be performed because 3 of the 7 patients lost to follow‐up had presented pain relapse before drop‐out (so that these had been included in the count of 23 patients with pain relapse at 2 years), and the other four patients were uniformly distributed between groups (1/26 v 3/29, ESWL alone v ESWL combined with endoscopy group, respectively; p = 0.613).24 Furthermore, among these four patients with no pain relapse before drop‐out, two were lost to follow‐up 1 and 4 months after the trial intervention (one in each group), and the remaining two patients were both lost to follow‐up 18 months after ESWL combined with endoscopy. The probability of presenting a first episode of pain relapse during the 6‐month time frame after drop‐out at 18 months is very low (<10%).7,8,9,10,11
The estimate of the proportion of patients with pain relapse at 2 years used for sample‐size calculation was far from the observed value for the ESWL alone group (90 v 38%). This was related to our assumption that ESWL alone would be ineffective to treat pain (removal of stone fragments after ESWL was advocated by all experts in the field except some Japanese authors),25 and that we would thus observe the natural course of chronic pancreatitis for this group (the 90% value was mainly derived from Talamini et al).26 Although the observed rates of pain relapse were similar in both groups (38 v 45% at 2 years, p = 0.633), this does not show that both treatments are equivalent, as shown by the large 95% CI for the treatment effect (0.23 to 2.57). Indeed, our calculated probability of being wrong in claiming such an equivalence based on our findings is about 37% (using a clinically reasonable relevant effect size of 20%). It should be emphasised that a randomised study showing such an equivalence would be extremely difficult to perform as this would require >300 patients (based on an effect size of 20%), and these patients are notably difficult to recruit (in the five randomised trials of any form of invasive treatment for chronic pancreatitis published to date, the numbers of patients included ranged from 22 to 72).27,28,29,30,31
This is the first randomised controlled trial assessing ESWL for painful calcifying chronic pancreatitis. The safety profile of ESWL was found to be excellent; no significant side effects were noted in the ESWL alone group. Acute pancreatitis attributed to ESWL has been reported in 6.3–12.5% of patients after ESWL “alone” for the treatment of calcified chronic pancreatitis.10,32 However, most of the patients included in these studies had received ERP as part of the initial treatment (including therapeutic procedures in 18–43% of cases).10,32 Our findings about the safety profile of ESWL are consistent with those reported in a large series of patients treated by ESWL alone for urological stones, which showed side effects (mainly injuries to adjacent organs) in <1% of cases.33
Pain is a major determinant of poor health status perception in patients with chronic pancreatitis,34,35,36 and greatly favours social deprivation, unemployment and early retirement, which affect up to 40–50% of patients with chronic pancreatitis.17 The evolution of pain in an individual with chronic pancreatitis cannot be reliably predicted,21 and persistent pain relief has even been reported to be absent in most of the patients after 10 years of follow‐up.17 Therefore, we believe that the option of proposing analgesics only to patients with painful uncomplicated calcified chronic pancreatitis should be carefully balanced in view of the advantages of ESWL found in this trial (ie, non‐invasiveness, efficiency, innocuousness and low cost) and discussed with the patient. Treatment with ESWL alone as a first option does not impede further endoscopic or surgical treatment, and patients' selection for ESWL can be based on fully non‐invasive tests, as shown in this study. Patients with calcifications confined to the head of the pancreas are the best candidates for benefiting from this treatment.
Surgery has been shown to be more effective compared with endoscopy for the treatment of painful chronic pancreatitis in terms of pain relief in two studies.27,37 However, one of these studies experienced methodological and technical drawbacks (eg, allocation to study groups in alternating fashion, treatment = pancreatic sphincterotomy (a treatment known to be ineffective) with neither stone extraction nor pancreatic stenting in one‐third of patients, no ESWL available),27 and the other one has been published in abstract form only. Even if the superiority of surgery in terms of pain relief for unselected patients is confirmed, we think that an intermediate step (between analgesics and surgery) is desirable due to the drawbacks of surgery (ie, invasiveness, cost and possibility of pain relapse even after major surgical procedures (up to 56% of operated patients)).16
In patients with uncomplicated calcified chronic pancreatitis, ESWL is a safe and effective preferred treatment. Combining systematic endoscopy with ESWL adds to the cost of patient care, at the same time not probably improving the outcome of pancreatic pain. Comparison with current data on the natural history of chronic pancreatitis suggests that treatment with ESWL endoscopy might significantly accelerate the development of persistent pain relief.
Acknowledgements
We thank RW Ammann for the provision of individual data on the patients included in a cohort on the natural history of chronic pancreatitis,16 Monia Bali for the analysis of secretin‐enhanced magnetic resonance cholangio‐pancreatography, Norman Godin for proofreading the manuscript, and the paramedical, medical and surgical digestive teams in the Erasmus and Gemelli Hospitals for making this study possible.
Abbreviations
ERP - endoscopic retrograde pancreatography
ESWL - extracorporeal shock wave lithotripsy
MPD - main pancreatic duct
S‐MRCP - secretin‐enhanced magnetic resonance cholangio‐pancreatography
Footnotes
Competing interests: None.
References
- 1.Steer M L, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med 19953321482–1490. [DOI] [PubMed] [Google Scholar]
- 2.Ebbehoj N, Borly L, Bulow J.et al Evaluation of pancreatic tissue fluid pressure and pain in chronic pancreatitis. A longitudinal study. Scand J Gastroenterol 199025462–466. [DOI] [PubMed] [Google Scholar]
- 3.Jalleh R P, Aslam M, Williamson R C. Pancreatic tissue and ductal pressures in chronic pancreatitis. Br J Surg 1991781235–1237. [DOI] [PubMed] [Google Scholar]
- 4.Karanjia N D, Widdison A L, Leung F.et al Compartment syndrome in experimental chronic obstructive pancreatitis: effect of decompressing the main pancreatic duct. Br J Surg 199481259–264. [DOI] [PubMed] [Google Scholar]
- 5.Di Sebastiano P, Fink T, Weihe E.et al Immune cell infiltration and growth‐associated protein 43 expression correlate with pain in chronic pancreatitis. Gastroenterology 19971121648–1655. [DOI] [PubMed] [Google Scholar]
- 6.Delhaye M, Vandermeeren A, Baize M.et al Extracorporeal shock‐wave lithotripsy of pancreatic calculi. Gastroenterology 1992102610–620. [DOI] [PubMed] [Google Scholar]
- 7.Delhaye M, Arvanitakis M, Verset G.et al Long‐term clinical outcome after endoscopic pancreatic ductal drainage for patients with painful chronic pancreatitis. Clin Gastroenterol Hepatol 200421096–1106. [DOI] [PubMed] [Google Scholar]
- 8.Rosch T, Daniel S, Scholz M.et al Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long‐term follow‐up. Endoscopy 200234765–771. [DOI] [PubMed] [Google Scholar]
- 9.Kasmin F E, Siegel J H. Endoscopic therapy for chronic pancreatitis. Surg Clin North Am 200181421–430. [DOI] [PubMed] [Google Scholar]
- 10.Ohara H, Hoshino M, Hayakawa T.et al Single application extracorporeal shock wave lithotripsy is the first choice for patients with pancreatic duct stones. Am J Gastroenterol 1996911388–1394. [PubMed] [Google Scholar]
- 11.Dumonceau J M, Deviere J, Le Moine O.et al Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long‐term results. Gastrointest Endosc 199643547–555. [DOI] [PubMed] [Google Scholar]
- 12.Dumonceau J M, Deviere J, Delhaye M.et al Plastic and metal stents for postoperative benign bile duct strictures: the best and the worst. Gastrointest Endosc 1998478–17. [DOI] [PubMed] [Google Scholar]
- 13.Cappeliez O, Delhaye M, Deviere J.et al Chronic pancreatitis: evaluation of pancreatic exocrine function with MR pancreatography after secretin stimulation. Radiology 2000215358–364. [DOI] [PubMed] [Google Scholar]
- 14.Axon A T, Classen M, Cotton P B.et al Pancreatography in chronic pancreatitis: international definitions. Gut 1984251107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavallini G, Frulloni L, Pederzoli P.et al Long‐term follow‐up of patients with chronic pancreatitis in Italy. Scand J Gastroenterol 199833880–889. [DOI] [PubMed] [Google Scholar]
- 16.Ammann R W, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology 19991161132–1140. [DOI] [PubMed] [Google Scholar]
- 17.Lankisch P G, Lohr‐Happe A, Otto J.et al Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion 199354148–155. [DOI] [PubMed] [Google Scholar]
- 18.Layer P, Yamamoto H, Kalthoff L.et al The different courses of early‐ and late‐onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 19941071481–1487. [DOI] [PubMed] [Google Scholar]
- 19.Miyake H, Harada H, Kunichika K.et al Clinical course and prognosis of chronic pancreatitis. Pancreas 19872378–385. [DOI] [PubMed] [Google Scholar]
- 20.Cotton P B. Outcomes of endoscopy procedures: struggling towards definitions. Gastrointest Endosc 199440514–518. [DOI] [PubMed] [Google Scholar]
- 21.American Gastroenterological Association Medical Position Statement: treatment of pain in chronic pancreatitis. Gastroenterology 1998115763–764. [DOI] [PubMed] [Google Scholar]
- 22.Howard T J, Jones J W, Sherman S.et al Impact of pancreatic head resection on direct medical costs in patients with chronic pancreatitis. Ann Surg 2001234661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotan Y, Cadeddu J A, Roehrborn C G.et al The value of your time: evaluation of effects of changes in medicare reimbursement rates on the practice of urology. J Urol 20041721958–1962. [DOI] [PubMed] [Google Scholar]
- 24.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999319670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farnbacher M J, Schoen C, Rabenstein T.et al Pancreatic duct stones in chronic pancreatitis: criteria for treatment intensity and success. Gastrointest Endosc 200256501–506. [DOI] [PubMed] [Google Scholar]
- 26.Talamini G, Bassi C, Falconi M.et al Pain relapses in the first 10 years of chronic pancreatitis. Am J Surg 1996171565–569. [DOI] [PubMed] [Google Scholar]
- 27.Dite P, Ruzicka M, Zboril V.et al A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy 200335553–558. [DOI] [PubMed] [Google Scholar]
- 28.Izbicki J R, Bloechle C, Broering D C.et al Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus‐preserving pancreatoduodenectomy. Ann Surg 1998228771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izbicki J R, Bloechle C, Knoefel W T.et al Duodenum‐preserving resection of the head of the pancreas in chronic pancreatitis. A prospective, randomized trial. Ann Surg 1995221350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchler M W, Friess H, Muller M W.et al Randomized trial of duodenum‐preserving pancreatic head resection versus pylorus‐preserving Whipple in chronic pancreatitis. Am J Surg 199516965–9, discussion 6970. [DOI] [PubMed] [Google Scholar]
- 31.Gress F, Schmitt C, Sherman S.et al A prospective randomized comparison of endoscopic ultrasound‐ and computed tomography‐guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol 199994900–905. [DOI] [PubMed] [Google Scholar]
- 32.Inui K, Tazuma S, Yamaguchi T.et al Treatment of pancreatic stones with extracorporeal shock wave lithotripsy: results of a multicenter survey. Pancreas 20053026–30. [PubMed] [Google Scholar]
- 33.Conlin M. Complications of extracorporeal shock wave lithotripsy. In: Taneja ss SR, Ehrlich RM, eds. Complications of urologic surgery. Philadelphia: WB Saunders, 2001155–164.
- 34.Wehler M, Reulbach U, Nichterlein R.et al Health‐related quality of life in chronic pancreatitis: a psychometric assessment. Scand J Gastroenterol 2003381083–1089. [DOI] [PubMed] [Google Scholar]
- 35.Pezzilli R, Morselli Labate A M, Ceciliato R.et al Quality of life in patients with chronic pancreatitis. Dig Liver Dis 200537181–189. [DOI] [PubMed] [Google Scholar]
- 36.Fitzsimmons D, Kahl S, Butturini G.et al Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ‐C30 and QLQ‐PAN26. Am J Gastroenterol 2005100918–926. [DOI] [PubMed] [Google Scholar]
- 37.Cahen D L, Gouma D J, Nio Y.et al A prospective randomised trial of endoscopic versus surgical pancreatic duct drainage in chronic pancreatitis; clinical outcome and exo‐ and endocrine function. Gut 200554A39 [Google Scholar]