Tear cytokine has a major role in various pathophysiological conditions of the ocular surface. So far, studies on tear cytokines have shown significant progress in providing an understanding of ocular surface diseases.1,2,3 The information that could be acquired from each subject, however, until recently has been severely hampered by limited sample volume and assay sensitivity. More importantly, it has become apparent that the relative balance between various cytokines and combinations of cytokines could be more important than absolute concentrations. Previous studies showed that the composition of basic and reflex tears was different, which made it more difficult to understand the ocular surface disorder correctly or to treat the patients suitably.4,5 Cytometric bead array (CBA) is a microparticle based flow cytometric assay that allows us to quantify multiple molecules from a very small sample.3,6,7 Using this method, we evaluated the inflammatory cytokines of basal and reflex tears from a single sample of individual eyes.
Methods
Twenty three normal volunteers (11 males and 12 females, 22–44 years of age, average 28 years) were recruited for this study. None of the subjects had signs of ocular diseases. The study was performed with the approval of the institutional review board. The basal tear samples of 10–15 μl were obtained from each eye by capillary flow, with no nasal stimulation or previous instillation of drugs or vital dyes. Each sample was collected at 5 pm. No anaesthetic drops were instilled. The samples were collected non‐traumatically from the inferior meniscus. Successively, reflex tear samples were collected by inserting application sticks into a participant's nose. The amounts of six inflammatory molecules interleukin (IL)‐1β, IL‐6, IL‐8, IL‐10, IL‐12p70, and tumour necrosis factor α (TNF‐α), were measured by CBA (BD Biosciences, San Diego, CA, USA), according to the manufacturer's instructions. Briefly, for the tear sample and cytokine standard mixture, 10 μl of sample or standard were added to 40 μl sterile purified water, a mixture of 50 μl each of capture Ab‐bead reagent and detector Ab‐phycoerythrin (PE) reagent. The mixture was subsequently incubated for 3 hours at room temperature, and washed to remove any unbound detector Ab‐PE reagent before data acquisition using flow cytometry. A two colour flow cytometric analysis was performed using a FACScan flow cytometer (Beckton Dickinson Immunocytometry Systems). Data were acquired and analysed using BD cytometric bead array software.
Results
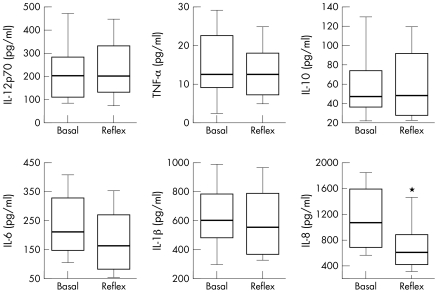
The concentrations of IL‐1β, IL‐6, IL‐10, IL‐12p70, and TNF‐α were not significantly different between basal and reflex tears. In contrast, the concentration of IL‐8 was significantly decreased in reflex tears compared with basal tears in each eye (paired t test, p<0.01, fig 1). In order to illuminate the inter‐relation of each cytokine, the ratio of two different cytokines is shown in table 1.
Figure 1 Change of concentrations of cytokines in basal and reflex tears. The concentration of IL‐1β, IL‐6, IL‐10, IL‐12p70 and TNF‐α are not significantly different between basal and reflex tears. In contrast, IL‐8 is significantly less in reflex tears compared with basal tears in each eye (paired t test, *p<0.01).
Table 1 Ratios of cytokine concentration.
| B | |||||||
|---|---|---|---|---|---|---|---|
| IL‐1b | IL‐6 | TNF‐α | IL‐12p70 | IL‐10 | IL‐8 | ||
| (A) Basal tear | |||||||
| A | IL‐1b | 1 | 3.022 (0.246) | 54.162 (6.549) | 3.322 (0.316) | 12.556 (0.889) | 0.716 (0.086) |
| IL‐6 | 0.386 (0.037) | 1 | 23.568 (5.952) | 1.238 (0.184) | 4.705 (0.553) | 0.246 (0.03) | |
| TNF‐α | 0.022 (0.002) | 0.07 (0.008) | 1 | 0.074 (0.011) | 0.275 (0.028) | 0.017 (0.003) | |
| IL‐12p70 | 0.349 (0.026) | 0.989 (0.071) | 18.269 (2.086) | 1 | 3.981 (0.166) | 0.261 (0.042) | |
| IL‐10 | 0.087 (0.005) | 0.253 (0.02) | 4.518 (0.47) | 0.261 (0.012) | 1 | 0.067 (0.011) | |
| IL‐8 | 2.14 (0.34) | 5.576 (0.694) | 144.913 (45.971) | 7.447 (1.546) | 28.257 (5.078) | 1 | |
| B | |||||||
|---|---|---|---|---|---|---|---|
| IL‐1b | IL‐6 | TNF‐α | IL‐12p70 | IL‐10 | IL‐8 | ||
| (B) Reflex tear | |||||||
| A | IL‐1b | 1 | 3.71 (0.252) | 53.061 (5.46) | 2.918 (0.331) | 12.012 (1.123) | 0.972 (0.094) |
| IL‐6 | 0.304 (0.027) | 1 | 15.807 (1.976) | 0.808 (0.061) | 3.381 (0.274) | 0.276 (0.029) | |
| TNF‐α | 0.021 (0.001) | 0.078 (0.007) | 1 | 0.062 (0.007) | 0.254 (0.026) | 0.02 (0.002) | |
| IL‐12p70 | 0.390 (0.023) | 1.361 (0.082) | 21.357 (3.427) | 1 | 4.231 (0.147) | 0.382 (0.046) | |
| IL‐10 | 0.093 (0.005) | 0.324 (0.018) | 4.837 (0.524) | 0.241 (0.007) | 1 | 0.093 (0.012) | |
| IL‐8 | 1.043 (0.231) | 4.779 (0.665) | 73.465 (13.897) | 4.02 (0.727) | 17.235 (3.251) | 1 | |
Ratio of cytokine concentration was calculated as A/B. Each cell shows the mean (SEM).
Comment
Previously published studies have demonstrated that CBA correlates well with enzyme linked immunosorbent assay (ELISA), but the absolute concentrations obtained from each assay were differed for kits of different manufacturers.7 Indeed, the concentrations of tear cytokines in the present results were almost equal to the previous report using the same kit.3 Nakamura et al performed ELISA for multiple cytokines measuring pooled tears.1 The pooled tears enable measurement of multiple cytokines; however the results can be strongly influenced by samples with high concentrations. Because the absolute concentrations of tear cytokines varied widely, this can have a strong bias. In this study, to our knowledge, we measured the multiple cytokines of basal and reflex tears from a single sample for the first time, which can provide concentration ranges for these cytokines in normal subjects that may prove important for studies of ocular inflammation. Of note, only the concentration of IL‐8 was decreased more significantly in reflex tears than in basal tears. Maitchouk et al showed that there is no distinctive role of major and accessory glands in secreting basal or reflex tears, thus these tears might be produced primarily by the same tissue and differences might be only the result of the secretory rate of reflex tears.8 It was reported that a neuropeptide released from corneal sensory nerves stimulated conjunctival epithelium to secrete IL‐8.9 Because sensory nerves are present in the cornea so abundantly, IL‐8 can be produced constantly on the ocular surface. IL‐8 is a potent pro‐inflammatory cytokine, and has a pivotal role in the host defence system.10 But excessive IL‐8 might be so harmful that constant washout might be helpful for homeostasis of the ocular surface. Indeed, a large amount of IL‐8 was found in the tears of dry eyes.11 Thus, basal tears might be composed of products of the ocular surface including IL‐8 and small amounts of reflex tears that are induced by mild stimulation such as blinks.
In summary, the present study showed that pro‐inflammatory and anti‐inflammatory cytokines/chemokines are present in the ocular surface even in the absence of inflammation and this was detectable from a small sample of single eyes. Stimulating tears, with the exception of IL‐8, has minimal effect on cytokine concentration. We believe the CBA technique can make a valuable contribution in understanding the specific immunopathological mechanisms underlying cytokine interaction with the ocular surface.
References
- 1.Nakamura Y, Sotozono C, Kinoshita S. Inflammatory cytokines in normal human tears. Curr Eye Res 199817673–676. [PubMed] [Google Scholar]
- 2.Lema I, Duran J A. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology 2005112654–659. [DOI] [PubMed] [Google Scholar]
- 3.Cook E B. Tear cytokines in acute and chronic ocular allergic inflammation. Curr Opin Allergy Clin Immunol 20044441–445. [DOI] [PubMed] [Google Scholar]
- 4.Sack R A, Conradi L, Krumholz D.et al Membrane array characterization of 80 chemokines, cytokines, and growth factors in open‐ and closed‐eye tears: angiogenin and other defense system constituents. Invest Ophthalmol Vis Sci 2005461228–1238. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda M, Fullard R J, Willcox M D.et al Fibronectin in the tear film. Invest Ophthalmol Vis Sci 199637459–467. [PubMed] [Google Scholar]
- 6.Chen R, Lowe L, Wilson J D.et al Simultaneous quantification of six human cytokines in a single sample using microparticle‐based flow cytometric technology. Clin Chem 1999451693–1694. [PubMed] [Google Scholar]
- 7.Khan S S, Smith M S, Reda D.et al Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom 20046135–39. [DOI] [PubMed] [Google Scholar]
- 8.Maitchouk D Y, Beuerman R W, Ohta T.et al Tear production after unilateral removal of the main lacrimal gland in squirrel monkeys. Arch Ophthalmol 2000118246–252. [DOI] [PubMed] [Google Scholar]
- 9.Tran M T, Ritchie M H, Lausch R N.et al Calcitonin gene‐related peptide induces IL‐8 synthesis in human corneal epithelial cells. J Immunol 20001644307–4312. [DOI] [PubMed] [Google Scholar]
- 10.Scapini P, Lapinet‐Vera J A, Gasperini S.et al The neutrophil as a cellular source of chemokines. Immunol Rev 2000177195–203. [DOI] [PubMed] [Google Scholar]
- 11.Jones D T, Monroy D, Ji Z.et al Alterations of ocular surface gene expression in Sjogren's syndrome. Adv Exp Med Biol 1998438533–536. [DOI] [PubMed] [Google Scholar]