Abstract
Background/aims
The literature on refractive change in thyroid eye disease (TED) is limited. This study documents the refractive change in patients with TED undergoing orbital decompression. The authors propose possible mechanisms for their acquired refractive error.
Methods
This is a retrospective observational case study of five patients with progressive TED. Their detailed eye examinations including refractive state preoperatively and postoperatively are presented.
Results
An acquired hypermetropic shift with active TED before orbital decompression of up to 3.75D spherical equivalent refraction (SER) is reported in one patient. Post‐orbital decompression, an induced myopic shift of between 1.00–2.50D SER for all patients is observed, noted to range from 1 day following surgery to up to 9 months, dependent on the availability of data. Axial length increased in two cases corresponding to postoperative myopic shift. Magnetic resonance imaging findings of one patient demonstrate flattening of the posterior pole as a cause of the acquired preoperative hypermetropia.
Conclusions
TED has a significant effect on the refractive state of patients. The proposed mechanism of acquired hypermetropia relates to increased volume of orbital contents with flattening of the posterior globe. This is reversed with successful orbital decompression. Documentation of refractive error in all cases of progressive TED is recommended. Progressive acquired hypermetropia may be suggestive of TED activity.
Keywords: thyroid eye disease, refraction, spherical equivalent refraction, visual acuity, orbital decompression
Thyroid eye disease (TED) is a frequent extrathyroidal manifestation of hyperthyroidism, occurring in more than 30% of patients with Graves' disease.1 TED typically presents as an inflammatory orbitopathy with symptoms of ocular irritation, pain, diplopia, photophobia, and blurred vision. The spectrum of clinical signs will depend on the orbital subcompartment involved and can include proptosis, lid oedema and retraction; conjunctival hyperaemia and chemosis; increased extraocular muscle (EOM) bulk with restricted eye movements; secondarily elevated intraocular pressure; choroidal folds, optic nerve compression; corneal tear film deficiencies and exposure keratopathy.2 These symptoms and signs typically peak at approximately six to 24 months following the onset of TED, and have a significant impact on visual function.3,4 We present a series of patients diagnosed with Graves' disease confirmed clinically and serologically by their referring physician. All cases developed TED and demonstrate refractive changes. The current literature on refractive change in TED is limited. We report an observed hypermetropic shift during progressive disease and myopic shift following orbital decompression where no other cause of acquired refractive error could be identified.
Material and methods
This study refers to the positive clinical findings of five patients referred for management with progressive TED. All patients had been treated with carbimazole and systemic steroids. Total thyroidectomy and radioactive iodine were required for cases 2 and 5, respectively. All patients ultimately required orbital decompression. Medical records were studied with patient consent. Ethics approval was not required for this study. Detailed ocular examination included measurement of best corrected visual acuity (BCVA) using a logMAR chart, subjective or automatic refraction, pupil reactions, ocular movements, Hertel's exophthalmometry (HE), slit lamp examination including examination of the tear film, and confrontation visual fields. Magnetic resonance imaging (MRI), axial length, and corneal astigmatism measured by keratometry and corneal topography were assessed in individual patients.
Results
Case 1
A 53 year old man was referred for active TED of over 7 months with progressive hypermetropia, proptosis, chronic irritation, and temporal chemosis. Initial visual acuity (VA) in the left eye was 6/5, without refractive error; VA right eye was 6/36 because of previous trauma which precluded refraction. Fundus examination showed posterior pole elevation, optic disc swelling, and choroidal folds in the left eye. Visual field testing documented an altitudinal inferior field defect in the left eye (Allergan Humphrey/Zeiss). Preoperative proptosis by HE was 23 mm in the left eye. His BCVA in the left eye deteriorated to 6/12 at 3 weeks preoperatively with refraction +3.50/+0.50×45°D; representing a hypermetropic shift of +3.75D SER over approximately 6 months. Primary indication for decompression was compressive optic neuropathy. A left sided two wall orbital decompression was performed without complication. HE decreased by 3 mm postoperatively. A mild worsening of restriction in upgaze was noted postoperatively. Complete VA readings and refractive error during this management episode are reported in table 1. Reduction in hypermetropia was evident within a day of surgery, and 1 month postoperatively his BCVA improved to 6/9 with a refraction of +1.00/+0.50×15°D in the left eye. A 2.50D myopic shift in SER was observed at 1 month.
Table 1 Progress best corrected visual acuity (BCVA), spherical and cylindrical refraction, spherical equivalent refraction (SER), total change in SER, preoperative and postoperative Hertel's exophthalmometry (HE), and change in axial length for cases of progressive TED requiring orbital decompression.
| Case | Eye | Time to follow up | BCVA | Spherical refraction (D) | Cylindrical refraction (D) | SER (D) | Total change in SER | Preop HE (mm) | Postop HE (mm) | Change in axial length (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L | 7.25 months preop | 6/5 | 0.00 | 0.00 | 0.00 | ||||
| 3.25 months preop | 6/9 −3 | +1.25 | +0.50×30° | +1.50 | ||||||
| 3 months preop | 6/9 | +1.75 | +0.50×30° | +2.00 | ||||||
| 1.5 months preop | 6/9 | +2.00 | +0.50×40° | +2.25 | ||||||
| 3 weeks preop | 6/12 | +3.50 | +0.50×45° | +3.75 | +3.75 preop | |||||
| 1 month postop | 6/9 | +1.00 | +0.50×15° | +1.25 | −2.50 postop | 23 | 20 | No data | ||
| 2 | R | Preoperative | 6/5 | 0.00 | +0.50×154° | +0.25 | ||||
| 2 weeks postop | 6/6 | −2.25 | +0.50×90° | −2.00 | −2.25 postop | 28 | 22 | No data | ||
| 3 | L | Immediate preop | 6/6 −1 | −1.50 | +0.50×180° | −1.25 | ||||
| 2.25 months postop | 6/6 −2 | −3.00 | +1.00×64° | −2.50 | −1.25 postop | 24 | 21 | +0.39 | ||
| 4 | R | 1 month preop | 6/18 | +1.25 | +0.25×60° | +1.375 | ||||
| 9 months postop | 6/6 | −0.25 | +0.50×45° | 0.00 | −1.375 postop | 23 | 18 | +0.47 | ||
| L | 1 month preop | 6/9 | +1.00 | +0.25×160° | +1.125 | |||||
| 9 months postop | 6/6 | −0.25 | +0.75×85° | +0.125 | −1.00 postop | 22 | 18 | +0.42 | ||
| 5 | L | 6 weeks preop | 6/6 | +1.50 | +0.50×180° | +1.75 | ||||
| 1 month postop | 6/6 | −1.00 | +0.50×180° | −0.75 | −2.50 postop | No data | No data | No data |
Case 2
A 43 year old woman was referred following a 15 year history of hyperthyroidism. TED was chronic and relatively inactive with visual blurring, diplopia, and proptosis. Preoperative proptosis by HE was 28 mm in the right eye. Preoperatively, BCVA in the right eye was 6/5 with a refraction of 0.00/+0.50×154°D. Indications for decompression were both symptomatic relief and cosmesis. A right sided three wall orbital decompression was performed without complication. HE decreased by 6 mm postoperatively. A marked restriction in upgaze resolved following surgery. Two weeks postoperatively her BCVA was 6/6 with a refraction of −2.25/+0.5×90°D. A 2.25D SER myopic shift was observed 2 weeks postoperatively (table 1).
Case 3
A 34 year old man was referred with rapidly progressive TED over 2 months. TED was active with ocular irritation, epiphora, diplopia and proptosis. Preoperative proptosis by HE was 24 mm in the left eye. Preoperative BCVA in the left eye was 6/6‐1 with a refraction of −1.50/+0.50×180°D. BCVA in the right eye was 6/6 with a refraction of −5.50/+3.25×104°D. Indications for decompression were both symptomatic relief and cosmesis. A left sided two wall orbital decompression was performed without complication. HE decreased by 3 mm postoperatively. Extraocular movements were normal preoperatively and postoperatively. At 2.25 months post‐decompression, BCVA was stable with a refraction of −3.00/+1.00×64°D (table 1). The 1.50D myopic shift in spherical refraction observed postoperatively was found to correspond to an increase in axial length measured with A‐scan (Alcon Biophysic OcuScan Version 3.02). Axial length increased by 0.39 mm from 24.21 mm preoperatively to 24.60 mm postoperatively. There was no significant change in preoperative and postoperative keratometry readings. Corneal topography (Orb Scan II, Version 3.0, Orbtek Inc) was also unchanged.
Case 4
A 58 year old woman was referred with rapidly progressive active TED over 3 months with diplopia, periorbital chemosis, and oedema. BCVA was 6/18 in the right eye with a refraction of +1.25/+0.25×60°D; and BCVA 6/9 in the left eye with a refraction of +1.0/+0.25×160°D, 1 month preoperatively. Preoperative proptosis by HE was 23 mm in the right eye and 22 mm in the left. Computed tomography confirmed apical compression caused by markedly enlarged EOMs. Primary indication for decompression was bilateral compressive optic neuropathy. Bilateral two wall orbital decompressions were performed without complication. HE decreased by 5 mm right eye and 4 mm left eye, postoperatively. Extraocular movements were restricted in all directions preoperatively with marked bilateral restriction in upgaze. Eye movements improved postoperatively, with moderate residual restriction in upgaze bilaterally. A 1.375D SER in the right eye and 1.00D SER in the left myopic shift was noted postoperatively (table 1). The myopic shift in spherical refraction observed postoperatively was found to correspond to an increase in axial length measured with IOL master (Carl Ziess, 2004) in both eyes. Axial length increased by 0.47 mm in the right eye from 21.72 mm to 22.19 mm. Axial length increased by 0.42 mm in the left eyefrom 21.45 mm to 21.87 mm. There was no significant change in preoperatively and postoperative keratometry readings.
Case 5
A 40 year old man was referred following a 6 year history of hyperthyroidism with inactive TED with proptosis and mild diplopia. Preoperative BCVA in the left eye was 6/6 with a refraction of +1.5/+0.5×180°D. Preoperative MRI demonstrates orbital changes consistent with TED, including enlarged EOMs and optic nerve. Flattening of the posterior pole on sagittal view is noted indicated by the arrow (fig 1). Indications for decompression were symptomatic relief and compressive optic neuropathy. A left sided two wall orbital decompression was performed without complication. At 1 month follow up, BCVA remained 6/6 with a refraction of −1.0/+0.5×180°D. A myopic shift of 2.50D SER was observed postoperatively (table 1). A repeat MRI has not been performed.
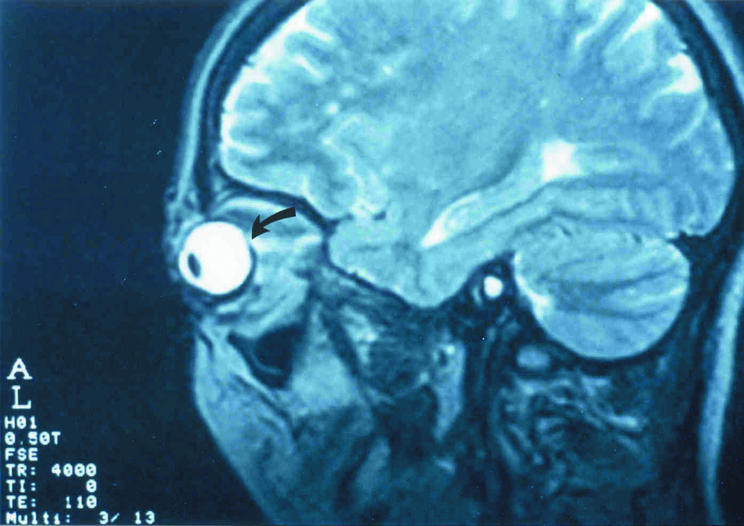
Figure 1 Median parasagittal magnetic resonance imaging scan demonstrating flattening of the posterior pole in association with thyroid eye disease (arrow).
Discussion
We have documented a dramatic acquired hypermetropia during progressive TED with significant myopic shift of 1.00D or more following orbital decompression in a series of five patients. The acquired hypermetropia developed as an acute event, within 6 months, sufficient to reduce VA without correction. The myopic shift following orbital decompression ranged from 1 day following surgery to up to 9 months dependent on the availability of data.
The literature is replete with reports of refractive change in association with other orbital pathologies. An acquired hypermetropia has been documented in association with choroidal folds of varying aetiologies and orbital space occupying lesions (SOLs).5,6,7,8,9 Case 1 only demonstrated choroidal folds as a mechanism of acquired hypermetropia; The literature reports an acquired hypermetropia of up to 6.0D with large choroidal folds.7 Orbital SOLs associated with refractive change include cavernous haemangioma and primary sarcoma of the orbit.10,11,12 In one series, hypermetropic shift was associated with intraconal tumours, and astigmatic shift was associated with extraconal tumours.8
Although there is little reference to refractive change associated with TED reported in the literature, there are three papers that do report other important refractive change. The first describes a large series of patients with Graves' ophthalmopathy demonstrating greater “with the rule” corneal astigmatism, uninfluenced by orbital, strabismus, or eyelid surgery. Soft tissue fibrosis of the superolateral orbit was considered a possible responsible mechanism. Other refractive changes are not described.13 The second describes a steepening of the inferior cornea with flattening of the superior cornea following inferior rectus recession. Further information for the refractive change is not provided.14 The third refers to progressive myopia in two cases of TED, but does not detail astigmatic change. The authors consider infiltration and damage to the ciliary body, related to TED, as a possible mechanism for myopia.15
Our observations of an acquired hypermetropia during active TED (case 1) and myopic shift post‐decompression is consistent with mechanisms involving the posterior pole. We propose that a combination of enlarged EOMs, anterior displacement of the globe, and orbital hypertension related to elevated muscle and fat volumes in TED flatten the posterior pole, which may produce choroidal folds. Multiple choroidal folds were observed at the posterior pole before surgery for case 1; however, for the other cases, choroidal folds did not appear in association with the plausible acquired hypermetropia in eyes subject to similar orbital buckling forces on the posterior pole. MRI findings for case 4 demonstrate compression of the posterior globe during active disease.
Analogous to the effect of a diffuse SOL, we suggest that these buckling forces exert a tangential effect on the posterior pole, decreasing axial length during active disease to explain the observed acquired hypermetropic shift of 3.75D SER. The postoperative increase in axial length (cases 3 and 4), of between 0.39 mm and 0.47 mm, without significant change in keratometry, is consistent with a myopic shift of 1.50D spherical refraction and measurably indicates the effectiveness of orbital decompression.
Missing data are a well recognised limitation of any retrospective clinical case series and this study is not exempt. The relative frequency of refractive change for this series approximates 5.7%. The authors suspect the frequency of refractive change to be higher than that which we have been able to document to date. While this is a small series of five patients, we believe the refractive changes reported are substantially significant in magnitude to account for variation caused by interphysician and intraphysician measurement error which we were also unable to calculate. In addition, refraction was either subjective or automatic, where patients were not assessed at each measure by both methods, possibly reducing the accuracy of our readings.
The recognition of a hypermetropic shift, particularly in patients nearing presbyopic age and afterwards as a cause for decreased near vision, is important to consider. Patients may have limited proptosis, and require careful monitoring for optic neuropathy and the need for decompression. Recognition with careful refraction provides important clinical information. We now monitor refraction preoperatively, on the day of surgery, and during routine follow up at 1 week and 6 weeks as part of our protocol.
Several factors produce significant and varied refractive change in TED. Our findings of an acquired hypermetropia during progressive disease, with reversal following successful decompression share mechanisms in common with orbital SOLs. Given the infrequency of orbital decompression, it is hoped this paper will stimulate further prospective collaborative study.
Acknowledgements
The authors thank the following participating investigators: Dr Peter Martin, Dr Ross Benger, Dr Gina Kourt, and Dr Vidushi Sharma of Sydney Eye Hospital, Sydney, Australia; we also thank Dr Raman Malhotra, Queen Victoria Hospital, East Grinstead, United Kingdom for contributing to this study.
Abbreviations
BCVA - best corrected visual acuity
EOM - extraocular muscle
HE - Hertel's exophthalmometry
MRI - magnetic resonance imaging
SER - spherical equivalent refraction
SOLs - space occupying lesions
TED - thyroid eye disease
Footnotes
Competing interests: none declared
References
- 1.Burch H B, Wartofsky L. Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev 199314747–793. [DOI] [PubMed] [Google Scholar]
- 2.Asman P. Ophthalmological evaluation in thyroid‐associated ophthalmopathy. Acta Ophthalmol Scand 200381437–448. [DOI] [PubMed] [Google Scholar]
- 3.Rundle F F. Eye signs of Graves' disease. In: Pitt‐Rivers R, Trotter WR, eds. The thyroid. Washington DC: Butterworths, 1964171–197.
- 4.Park J J, Sullivan T J, Mortimer R H.et al Assessing quality of life in Australian patients with Graves' ophthalmopathy. Br J Ophthalmol 20048875–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murdoch D, Merriman M. Acquired hypermetropia with choroidal folds. Clin Experiment Ophthalmol 200230292–294. [DOI] [PubMed] [Google Scholar]
- 6.Dailey R A, Mills R P, Stimac G K.et al The natural history and CT appearance of acquired hypermetropia with choroidal folds. Ophthalmology 198693336–342. [DOI] [PubMed] [Google Scholar]
- 7.Kalina R E, Mills R P. Acquired hypermetropia with choroidal folds. Ophthalmology 19808744–50. [DOI] [PubMed] [Google Scholar]
- 8.Friberg T R, Grove A S. Choroidal folds and refractive errors associated with orbital tumors. An analysis. Arch Ophthalmol 1983101598–603. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen D M. Intracranial hypertension and the syndrome of acquired hypermetropia with choroidal folds. J Neuroophthalmol 199515178–185. [PubMed] [Google Scholar]
- 10.Harris G J, Jakobiec F A. Cavernous hemangioma of the orbit. J Neurosurg 197951219–228. [DOI] [PubMed] [Google Scholar]
- 11.D'Hermies F, Elmaleh C, Mourier K.et al Cavernous hemangioma of the orbit. J Fr Opthalmol 199316195–198. [PubMed] [Google Scholar]
- 12.Johnson L N, Sexton F M, Goldberg S H. Poorly differentiated primary orbital sarcoma (presumed malignant rhabdoid tumor). Radiologic and histopathologic correlation. Arch Ophthalmol 19911091275–1278. [DOI] [PubMed] [Google Scholar]
- 13.Mombaerts I, Vandelanotte, Koornneef L. Corneal astigmatism in Graves' ophthalmopathy. Eye (advance online publication, 22 April 2005) [DOI] [PubMed]
- 14.Kwitko S, Feldon S, McDonnell P J. Corneal topographic changes following strabismus surgery in Grave's disease. Cornea 19921136–40. [DOI] [PubMed] [Google Scholar]
- 15.Huismans H. Conspicuous change of refraction in endocrine orbitopathy. Klin Monatsbl Augenheilkd 1991198215–216. [DOI] [PubMed] [Google Scholar]


