Abstract
Aim
To evaluate the Baerveldt glaucoma implant (BGI) in paediatric glaucoma treatment.
Methods
In a retrospective non‐comparative case series 55 eyes of 40 consecutive paediatric patients (⩽16 years) with primary or secondary glaucoma underwent Baerveldt (350 mm2) implantation. Surgical outcome was evaluated by Kaplan‐Meier table analysis.
Results
The overall success rate was 80% at last follow up, with a mean follow up of 32 (range 2–78) months. Cumulative success was 94% at 12 months and 24 months, 85% at 36 months, 78% at 48 months, and 44% at 60 months. 11 eyes (20%) failed postoperatively because of an IOP >21 mm Hg (eight eyes), persistent hypotony (two eyes), and choroidal haemorrhage following cataract surgery (one eye). The most frequent complication needing surgery was tube related (20%). A new observation was mild to moderate dyscoria in 22% of the eyes, all buphthalmic, caused by entrapment of a tuft of peripheral iris in the tube track.
Conclusions
The BGI is effective and safe in the management of primary and secondary glaucoma. When angle surgery has proved to be unsuccessful or inappropriate in paediatric patients, a BGI is a good treatment option. One must be prepared to deal with the tube related problems.
Keywords: glaucoma, Baerveldt implant, congenital glaucoma, paediatrics
Management of primary congenital glaucoma is primarily surgical; goniotomy or trabeculotomy each have success rates of 40–90%.1,2,3,4 In case of secondary glaucomas associated with, for example, aphakia, aniridia, anterior segment dysgenesis, and Sturge‐Weber syndrome, goniotomy or trabeculotomy have poorer success rates.5
When goniotomy or trabeculotomy fail or are inappropriate to control intraocular pressure (IOP) in paediatric patients, alternatives include filtering surgery, cyclodestructive procedures, or drainage implants. Children undergoing filtering surgery do not enjoy the same success rates as older patients. Success rates vary from 37% to 85%, depending on patient population.5,6,7,8,9,10 Additionally, conjunctival scarring from previous ocular surgery may limit the success of repeat surgery. Inhibitors of scar formation such as 5‐fluorouracil and mitomycin C may improve the success rate of filtering surgery in terms of IOP control, but the resultant blebs tend to be thin and avascular and are associated with a high incidence of endophthalmitis.11,12,13,14,15,16,17 Because of the high rate of vision loss and phthisis, cyclodestructive procedures are often reserved for eyes that have failed all other options.18,19,20,21,22,23,24
The use of glaucoma drainage implants is a promising alternative in treating intractable glaucomas.25,26,27,28,29,30,31,32,33,34,35,36,37,38,39 A variety of aqueous shunting devices have been developed, both valved (Ahmed) and non‐valved (Molteno, Baerveldt) designs. In paediatric patients in whom goniotomy and/or trabeculectomy with or without antimetabolites have failed, success rates of 56–95% have been reported with these glaucoma implants.
In this study we retrospectively evaluated a large group of pediatric glaucoma patients with a relatively long follow up after treatment with a Baerveldt glaucoma implant (BGI).
Patients and methods
Paediatric patients (⩽16 years) with primary or secondary glaucoma were enrolled in this retrospective clinical study. Study protocol and informed consent were approved by the institutional review board. All parents gave informed consent. All surgical procedures were performed by one surgeon (PWTdW) at the Rotterdam Eye Hospital, Rotterdam, Netherlands, between January 1998 and December 2003. All Baerveldt implants (AMO Groningen BV, Groningen, Netherlands) had a surface area of 350 mm2.
The sclera in the superior temporal quadrant was exposed with a limbus based conjunctival flap, and the superior and lateral rectus muscles were identified and isolated with muscle hooks. The wings of the implant were positioned under the insertions of the muscles. The plate was secured 1–2 mm posterior to the insertions with two interrupted 9‐0 nylon sutures. The tube was trimmed so that it would extend 1–2 mm beyond the posterior surgical limbus with the bevel towards the corneal endothelium. A stent (3‐0 Prolene), 1–2 mm in length, was passed into and down the tube and positioned about 7 mm behind the limbus. The tube was ligated with 7‐0 Vicryl around the stent. This stent was used for easy constriction of the tube and ensured a watertight closure, preventing early postoperative hypotony. The stent itself hardly affects the flow through the tube (personal measurements with donor eyes). The tube opened after 5–7 weeks in all cases. A paracentesis track through the cornea into the anterior chamber was made to control the IOP and the anterior chamber depth intraoperatively. A 23 gauge needle was used to create an opening for insertion of the tube into the anterior chamber. Glycerin preserved donor sclera was used to cover the tube near the limbus and secured with interrupted 9‐0 Vicryl sutures. Finally Tenon and conjunctiva were reapposed with running 9‐0 Vicryl sutures. A subconjunctival injection of corticosteroids was administered before the eye was patched. Postoperatively, topical steroids were tapered slowly and glaucoma medications were removed or added as IOP and clinical status required.
Preoperative and postoperative examinations included slit lamp examination, ophthalmoscopy, IOP measurement with a pneumotonometer (Model 30 classic, Mentor O&O, Norwell, MA, USA), and measurement of axial length by ultrasound A‐scan (A‐5500 A‐scan system, Sonomed, Lake Success, NY, USA). Most examinations were performed under general anaesthesia in patients younger than 6 years. Older patients were able to undergo examinations in the office. Preoperative IOP was the mean of measurements following the last glaucoma procedure before aqueous shunt implantation.
Success was defined as an IOP <22 mm Hg on the last two follow up evaluations with or without glaucoma medication in eyes with a preoperative IOP >21 mm Hg, or an IOP that was lowered by at least 20% from preoperative values with or without glaucoma medication in eyes with a preoperative IOP <22 mm Hg. Furthermore, requirements for surgical success included no hypotony (IOP ⩽6 mm Hg), no additional glaucoma surgeries or devastating visual complications. In cases of congenital glaucoma, successful cases also showed disappearance of clinical signs of high IOP, including increasing axial length, corneal oedema, or excessive tearing. Surgical outcome was summarised by Kaplan‐Meier life table analysis. Wilcoxon signed rank test was used to test for statistically significant change of preoperative IOP and number of medications.
Results
Fifty five Baerveldt implant procedures were performed on 55 eyes of 40 patients during a 6 year period between January 1998 and December 2003. Table 1 summarises the characteristics of our patients. The 21 eyes that did not have any previous glaucoma surgery were the ones diagnosed as secondary glaucoma or primary congenital glaucoma with such corneal oedema making goniotomy impossible to perform.
Table 1 Demographics of study population.
| No | (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | ||||||||||
| Patients | 40 | |||||||||
| Eyes | 55 | |||||||||
| Age (years) | ||||||||||
| Mean (SD) | 3.3 (3.6) | |||||||||
| Range | 0.2–12 | |||||||||
| <1 | 21 | (38.2) | ||||||||
| 1–6 | 22 | (40.0) | ||||||||
| 7–12 | 12 | (21.8) | ||||||||
| >12 | 0 | |||||||||
| Preoperative intraocular pressure (mm Hg) | ||||||||||
| ⩽21 | 10 | (18.2) | ||||||||
| >21 | 45 | (81.8) | ||||||||
| Diagnosis* | ||||||||||
| Primary congenital glaucoma | 35 | (63.6 ) | ||||||||
| Aphakia after congenital cataract | 7 | (12.7) | ||||||||
| Uveitic glaucoma | 5 | (9.1) | ||||||||
| Sturge‐Weber syndrome | 3 | (5.5) | ||||||||
| Persistent hyperplastic primary vitreous | 2 | (3.6) | ||||||||
| Aniridia | 3 | (5.5) | ||||||||
| Traumatic glaucoma | 1 | (1.8) | ||||||||
| Previous glaucoma surgery* | ||||||||||
| Total (on all eyes) | 57 | |||||||||
| Mean (SD) | 1.0 (1.0) | |||||||||
| Range | 0–4 | |||||||||
| Types previous glaucoma surgery* | ||||||||||
| None | 21 | (38.2) | ||||||||
| Trabeculotomy | 2 | (3.6) | ||||||||
| Goniotomy | ||||||||||
| 1× | 13 | (23.6) | ||||||||
| 2× | 8 | (14.5) | ||||||||
| 3× | 2 | (3.6) | ||||||||
| Trabeculectomy | ||||||||||
| 1× | 3 | (5.5) | ||||||||
| 2× | 3 | (5.5) | ||||||||
| 3× | 1 | (3.8) | ||||||||
| Deep sclerectomy | 2 | (3.6) | ||||||||
| Glaucoma implant | 1 | (1.8) | ||||||||
| Cyclocryocoagulation | 1 | (1.8) | ||||||||
*More than one diagnosis or type of surgery per patient is possible.
The mean length of follow up for all eyes was 31.9 months (range 2–78, table 2). For 11 eyes, the follow up was less than 1 year. Thirty seven eyes were reviewed for 2 years or more, of which 11 were followed up for more than 4 years. Not all eyes were reviewed at every follow up period.
Table 2 Follow up of study population.
| No | (%) | |
|---|---|---|
| Follow up | ||
| Mean (months) (SD) | 31.9 (18.9) | |
| Range | 2–78 | |
| Results at last follow up | ||
| Success | 44 | (80.0) |
| Failure | 11 | (20.0) |
| Postoperative IOP >21 mm Hg | 8 | (72.7) |
| Postoperative hypotony | 3 | (27.3) |
| Complications related to Baerveldt implant | ||
| Entrapment iris in tube track | 12 | (21.8) |
| Motility disturbance | 4 | (7.3) |
| Tube exposure | 3 | (5.5) |
| Tube retraction/anterior migration | 8 | (14.5) |
| Stent in anterior chamber | 1 | (1.8) |
| Persistent shallow anterior chamber | 1 | (1.8) |
| Pupillary block | 1 | (1.8) |
| Persistent hypotony | 2 | (3.6) |
| Cataract | 1 | (1.8) |
| Other complications | ||
| Hypotony after choroidal haemorrhage following cataract surgery | 1 | (1.8) |
Postoperatively, the mean IOP and the mean number of topical medications were significantly lower (Wilcoxon signed rank test) than before the procedure for all follow up periods (table 3). The mean IOP for the total group was 27.2 mm Hg (range 10–50 mm Hg) preoperatively and 15.5 mm Hg (range 0–33 mm Hg) at last follow up, including failures (table 3). The mean reduction in IOP for all eyes, including failures, from the Baerveldt procedure to last follow up was 36.8%.
Table 3 Intraocular pressure (IOP) and number of glaucoma medications.
| Follow up (months) | No | IOP | Number of medications | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | (Range) | p Value* | Mean (SD) | (Range) | p Value* | ||
| Preoperative | 55 | 27.2 (7.9) | (10–50) | 1.6 (1.0) | (0–4) | ||
| Last follow up | 55 | 15.3 (6.6) | (0–33) | <0.001 | 0.6 (0.9) | (0–3) | <0.001 |
| Postoperative | |||||||
| 2–4 | 48 | 10.8 (6.7) | (2–30) | <0.001 | 1.1 (0.9) | (0–3) | 0.013 |
| 5–8 | 37 | 13.0 (6.5) | (4–30) | <0.001 | 0.7 (0.8) | (0–2) | <0.001 |
| 9–15 | 37 | 13.4 (5.5) | (4–30) | <0.001 | 0.6 (0.8) | (0–2) | <0.001 |
| 16–20 | 22 | 12.0 (4.8) | (5–27) | <0.001 | 0.5 (0.7) | (0–2) | <0.001 |
| 21–30 | 29 | 15.0 (5.8) | (7–31) | <0.001 | 0.6 (0.7) | (0–2) | <0.001 |
| 31–48 | 30 | 15.6 (7.2) | (0–38) | <0.001 | 0.4 (0.7) | (0–2) | <0.001 |
| >48 | 15 | 18.8 (7.7) | (10–33) | 0.004 | 0.9 (1.0) | (0–3) | 0.008 |
*Wilcoxon signed rank test, to test for statistically significant change of preoperative intraocular pressure and number of medications.
Preoperatively a mean of 1.6 (range 0–4) topical medications were used by all subjects (table 3). At last follow up, all subjects, including those with eyes that failed, used a mean of 0.9 (40.5%) fewer topical medications (mean 0.6; range 0–3). No medications were needed in 35 (63.6%) eyes at last follow up. There was no change in number of anti‐glaucoma medications for 19 (34.5%) eyes. For three (5.5%) eyes one more topical medication was needed. Two of these eyes were found to be a failure.
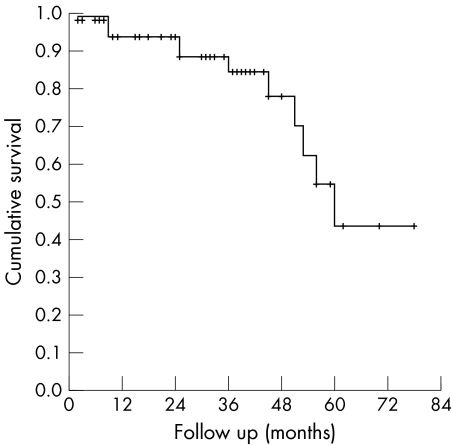
At the time of last follow up, 44 (80.0%) eyes were considered successful. Life table analysis (Kaplan Meier plot, fig 1) revealed an overall mean cumulative probability of success of 0.94 (95% CI: 0.87 to 1.00) at 12 months and 24 months, and 0.85 (95% CI: 0.73 to 0.97) at 36 months. After 36 months the mean cumulative probability of success declined to 0.78 (95% CI: 0.62 to 0.94) at 48 months and 0.44 (95% CI: 0.16 to 0.72) at 60 months. Four eyes lost visual function (no light perception) because of hypotony (two) or uncontrollable high IOP (two). In seven eyes the visual acuity was as low as counting fingers at 50 cm to 1 metre because of hypotony (one), uncontrollable high IOP (three) or deep anisometropic amblyopia (three).
Figure 1 Cumulative probability of success compared with follow up time after Baerveldt implantation (Kaplan‐Meier life table analysis).
Of the 11 eyes that failed, seven eyes were diagnosed as primary congenital glaucoma, two eyes as aphakic glaucoma, two as uveitic glaucoma, and one as glaucoma associated with persistent hyperplastic primary vitreous (PHPV) (tables 2 and 4). We did not find a difference between the failure rates of primary and secondary glaucomas (failure rate of 20% in both groups). One eye failed within 5 months and seven eyes failed after 2 years (at 25, 25, 51, 53, 56, 59, and 60 months) because of an IOP >21 mm Hg.
Table 4 Characteristics of failures.
| Type of glaucoma | Age (years) | Previous glaucoma surgeries | Time of failure (months) | Reason for failure |
|---|---|---|---|---|
| Congenital | 1.2 | 2× goniotomy | 51 | IOP >21 mm Hg |
| Congenital | 0.5 | 2× goniotomy | 9 | IOP >21 mm Hg |
| Congenital | 1.8 | 1× trabeculectomy | 56 | IOP >21 mm Hg |
| 1× deep sclerectomy | ||||
| Congenital | 0.3 | 2× goniotomy | 45 | IOP >21 mm Hg |
| Congenital | 0.7 | 1× goniotomy | 25 | IOP >21 mm Hg |
| Congenital | 0.7 | 1× goniotomy | 25 | IOP >21 mm Hg |
| Congenital | 3.0 | 2× trabeculectomy | 36 | Hypotony |
| Aphakia | 5.0 | none | 2 | Hypotony |
| Aphakia | 10.8 | none | 9 | Hypotony |
| PHPV | 2.0 | 1× goniotomy | 53 | IOP >21 mm Hg |
| Uveitic | 8.4 | none | 60 | IOP >21 mm Hg |
PHPV, persistent hyperplastic primary vitreous.
The remaining three failed because of postoperative hypotony (IOP ⩽6 mm Hg). In one eye, a cyclitic membrane developed 6 months after BGI implantation, which had been combined with an anterior vitrectomy. This resulted in low IOP with choroidal effusion. Although we closed the Baerveldt tube and performed a vitrectomy with membranectomy, the IOP remained <6 mm Hg, resulting in a visual function of counting fingers at 50 cm. In the second eye, closure of the tube was performed 3 months postoperatively because of persistent hypotony. The third eye had congenital glaucoma and had previously undergone a trabeculectomy with adjunctive mitomycin C application, a revision of the trabeculectomy and a deep sclerectomy. Cataract surgery was performed 3 years after Baerveldt implantation, which resulted in a leaking wound with subsequent haemorrhagic choroidal effusion. Surgical occlusion of the Baerveldt implant did not result in higher IOP. The latter two eyes went phthisical and blind. The tubes were closed by a 9.0 nylon suture around the tube at the position of the stent, still being in place in all three cases.
Seventeen eyes (30.9%) required one or more surgical procedures because of postoperative implant related complications (table 2). In 11 eyes (20.0%), tube related complications occurred. Tube exposure occurred in three cases (5.5%), which required recovering of the tube with donor sclera and conjunctiva. No recurrent exposure occurred. In eight eyes (14.5%) reinsertion with or without extension of the tube was performed for tube retraction or anterior migration of the tube. In one eye (1.8%) the Prolene stent dropped into the anterior chamber postoperatively and had to be removed. In 12 eyes (21.8%) mild to moderate dyscoria was seen (fig 2). In all cases gonioscopy revealed a tuft of peripheral iris tissue being trapped in the tube track (fig 3). Mild ocular motility disturbance was found in four cases (7.3%). None of these cases required surgical intervention. Other complications were persistent shallow anterior chamber in one eye (1.8%) which was successfully reformed with viscoelastics, pupillary block in another eye (1.8%) due to posterior synechiae which required a surgical peripheral iridectomy, and cataract in one eye (1.8%).
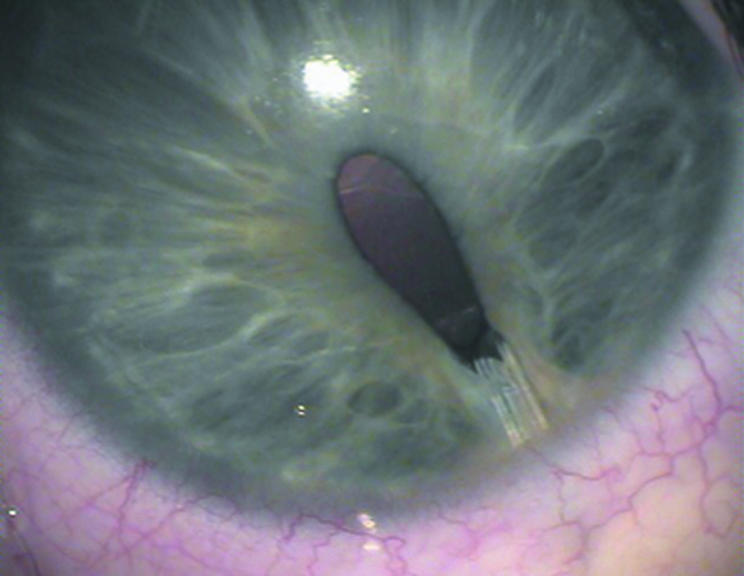
Figure 2 Dyscoria in buphthalmic eye with BGI.

Figure 3 Gonioscopic view of dyscoria of figure 2. Entrapment of peripheral iris in tube track can be seen.
Discussion
The management of paediatric glaucoma is primarily surgical. In the literature goniotomy and trabeculotomy are still the initial surgical procedures of choice for most cases of primary congenital glaucoma. Trabeculotomy has a certain advantage in case of a cloudy cornea. However, even with experience, the procedure remains technically challenging. We do not perform trabeculotomies in our clinic. Furthermore, we only perform goniotomies in previously unoperated eyes with primary congenital glaucoma, the visibility of the chamber angle permitting.
Beck et al25 found that aqueous shunt implantation (Ahmed or Baerveldt) offers a significantly greater chance of successful IOP control compared with trabeculectomy with mitomycin C in infants up to 24 months of age. They found cumulative probabilities of success at 12 months of 87% for the aqueous shunt group and 36% in the trabeculectomy group.
Our mean cumulative probabilities of success at 12, 24, and 36 months of 94%, 94%, and 85% respectively, are comparable to percentages reported recently for the BGI group by Beck et al,25 Rolim de Moura et al,26 and Budenz et al.27 Our findings are also comparable with other previously reported success rates with Baerveldt or Molteno glaucoma drainage implants in paediatric patients.28,29,30,31,32,33,34 Furthermore, our 12 month and 24 month cumulative probabilities of success compare favourably with those reported by investigators who used the Ahmed implants in paediatric patients. They reported success rates ranging from 70% to 93% at 12 months and 58–86% at 24 months.35,36,37,38
In the small group of patients with Sturge‐Weber syndrome in this study, we found no failures after Baerveldt implantation. The follow up times for these eyes were 36 months in two eyes. One eye was lost for follow up after 3 months. Previous studies reported high success rates in controlling IOP of these patients with aqueous drainage implants.35,39
In our study, a decline of success rate occurred after 48 months of follow up (fig 1), which could be attributed to the limited number of patients with a follow up of more than 4 years (15 patients, of which four failed in this follow up period). Figure 1 very closely resembles the cumulative probability of success presented by Rolim de Moura and associates.26 Longer follow up is necessary to examine the long term course of the success rate. However, previous studies suggest that longer follow up almost always leads to decrease of surgical success, whether the surgical procedure is glaucoma implant surgery, trabeculectomy, or cycloablation.14,23,24,25,26,31,35,37
No anti‐glaucoma medications were needed to control IOP in 64% of our patients at last follow up. Previously reported percentages of patients without anti‐glaucoma medication at last follow up vary from 17% to 53%.28,29,30,31,32,35,37,38 We are well aware that study populations may differ considerably, so the figures must be interpreted with care.
The most frequent complication of BGI in this study is tube related. Other studies reported 6.5–39.1% tube related complications.25,26,27 The variability in the published incidence of tube related complications after BGI, may be partly the result of inclusion of differing proportions of buphthalmic eyes in these studies. Hence, it has been reported that the thin elastic sclera in buphthalmic eyes may predispose these eyes to changes in size and shape when the IOP is reduced, leading in turn to tube related complications.26
In 12 cases (21.8%), all buphthalmic eyes, mild to moderate dyscoria was observed shortly after opening of the tube caused by entrapment of a tuft of peripheral iris tissue in the needle‐track (gonioscopy). This observation has not been mentioned before in other studies. The entrapment could be a result of a shallow anterior chamber during a period of hypotony after opening of the tube, in combination with movement of the tube in the needle‐track creating space next to the tube as a result of thin elastic sclera. It seems likely that movement of the tube is induced by movement of the eyelids over a hypotonous eye.
The reported incidence of hypotony and/or shallow anterior chamber in paediatric patients varies from 12% to 23% with the Ahmed implant,35,36,37,38 6% to 11% after Molteno implantation,31,32,33,34 and 0 to 57% after a Baerveldt implant.25,26,27,28,29,30 Nearly all patients with non‐valved glaucoma implant go through a period of hypotony and shallow anterior chamber after tube opening. In our group, IOP normalised and the anterior chamber reformed after 1–4 weeks in all but two patients.
Four patients (7.3%) were found to have mild motility disturbances postoperatively in our study. Strabismus surgery was not required in these patients. Previous studies of paediatric patients with BGIs reported motility disturbances in 0–16% after implantation.25,26,27,28,29,30 Similar findings (0–10%) were noted after implantation of an Ahmed or Molteno implant in paediatric patients.31,32,33,34,35,36,37,38 Motility problems are believed to be secondary to a mass effect from the equatorial filtering bleb, a Faden or posterior fixation effect induced by scarring under the rectus muscle, or fat adherence syndrome.40 Fellenbaum found larger BGIs to have a somewhat greater IOP lowering effect in paediatric patients. However, they did not randomly select the implant surface area.29 Lloyd et al reported similar success with the 350 mm2 and the 500 mm2 implants.41 However, the 500 mm2 implant had a higher complication rate, while requiring fewer glaucoma medications to control IOP. In a more recent study, Britt and colleagues found the 350 mm2 BGI to be more successful than the 500 mm2 implants for overall IOP control.42 We prefer to use a 350 mm2 BGI for IOP control in our paediatric patients.
In conclusion, we think that the BGI is effective and safe in the management of paediatric glaucoma, despite its propensity for tube related postoperative complications. From three other recently published studies25,26,27 and this study the use of the BGI seems a good treatment option when angle surgery has proven to be unsuccessful or inappropriate for paediatric patients.
Abbreviations
BGI - Baerveldt glaucoma implant
IOP - intraocular pressure
Footnotes
Commercial interest: none.
Proprietary interest: none.
References
- 1.Douglas R, Anderson D R. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology 198390805–806. [DOI] [PubMed] [Google Scholar]
- 2.McPherson S D, Jr, Berry D P. Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol 198395427–431. [DOI] [PubMed] [Google Scholar]
- 3.Al‐Hazmi A, Awad A, Zwaan J.et al Correlation between surgical success rate and severity of congenital glaucoma. Br J Ophthalmol 200589449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor R H, Ainsworth J R, Evans A R.et al The epidemiology of pediatric glaucoma: the Toronto experience. J AAPOS 19993308–315. [DOI] [PubMed] [Google Scholar]
- 5.Wallace D K, Plager D A, Snyder S K.et al Surgical results of secondary glaucomas in childhood. Ophthalmology 1998105101–111. [DOI] [PubMed] [Google Scholar]
- 6.Beaucamp G R, Parks M M. Filtering surgery in children:barriers to success. Ophthalmology 197986170–180. [DOI] [PubMed] [Google Scholar]
- 7.Gressel M G, Heuer D K, Parrish RK I I. Trabeculectomy in young patients. Ophthalmology 1984911242–1246. [DOI] [PubMed] [Google Scholar]
- 8.Debnath S C, Teichmann K D, Salamah K. Trabeculectomy versus trabeculotomy in congenital glaucoma. Br J Ophthalmol 198973608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke J P, Bowell R. Primary trabeculectomy in congenital glaucoma. Br J Ophthalmol 198973188–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder M J. Combined trabeculotomy‐trabeculectomy compared with primary trabeculectomy for congenital glaucoma. Br J Ophthalmol 199478745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidoti P A, Belmonte S J, Liebmann J M.et al Trabeculectomy with mitomycin‐C in the treatment of pediatric glaucomas. Ophthalmology 2000107422–429. [DOI] [PubMed] [Google Scholar]
- 12.Freedman S F, McCormick K, Cox T A. Mitomycin C‐augmented trabeculectomy with postoperative wound modulation in pediatric glaucoma. J AAPOS 19993117–124. [DOI] [PubMed] [Google Scholar]
- 13.Al‐Hazmi A, Zwaan J, Awad A.et al Effectiveness and complications of MMC use during pediatric glaucoma surgery. Ophthalmology 19981051915–1920. [DOI] [PubMed] [Google Scholar]
- 14.Susanna R J, Oltrogge E W, Carani C E.et al Mitomycin as adjunct chemotherapy with trabeculectomy in congenital and developmental glaucomas. J Glaucoma 19954151–157. [PubMed] [Google Scholar]
- 15.Mandal A K, Walton D S, John T.et al Mitomycin c‐augmented trabeculectomy in refractory congenital glaucoma. Ophthalmology 1997104996–1003. [DOI] [PubMed] [Google Scholar]
- 16.Sidoti P A, Lopez P F, Michon J.et al Delayed‐onset pneumococcal endophthalmitis after mitomycin‐C trabeculectomy: association with cryptic nasolacrimal obstruction. J Glaucoma 1995411–15. [PubMed] [Google Scholar]
- 17.Waheed M D, Ritterband D C, Greenfeld D S.et al Bleb‐related ocular infection in children after trabeculectomy with mitomycin C. Ophthalmology 19971042117–2120. [DOI] [PubMed] [Google Scholar]
- 18.Autrata R, Rehurek Log‐term results of transscleral cyclophotocoagulation in refractory pediatric glaucoma patients. Ophthalmologica 2003217393–400. [DOI] [PubMed] [Google Scholar]
- 19.Izgi B, Demirci H, Demirci F Y.et al Diode laser cyclophotocoagulation in refractory glaucoma:comparison between pediatric and adult glaucomas. Ophthalmic Surg Lasers 200132100–107. [PubMed] [Google Scholar]
- 20.Aminlari A. Cylocryotherapy in congenital glaucoma. Glaucoma 19813331–332. [Google Scholar]
- 21.Bellows A R, Grant W M. Cyclocryotherapy in advanced inadequately controlled glaucoma. Am J Ophthalmol 197375679. [DOI] [PubMed] [Google Scholar]
- 22.Al Faran M F, Tomey K F, Al Mutlag F A. Cyclocryotherapy in selected cases of congenital glaucoma. Ophthalmic Surg 199021794–798. [PubMed] [Google Scholar]
- 23.Bock C J, Freedman F F, Buckley E G.et al Transscleral diode laser cyclophotocoagulation for refractory pediatric glaucomas. J Pediatr Ophthalmol Strabismus 199734235–239. [DOI] [PubMed] [Google Scholar]
- 24.Wagle N S, Freedman S F, Buckley E G.et al Long‐term outcome of cyclocyotherapy for refractory pediatric glaucoma. Ophthalmology 19981051921–1927. [DOI] [PubMed] [Google Scholar]
- 25.Beck A D, Freedman S, Kammer J.et al Aqueous shunt devices compared with trabeculectomy with mitomycin‐C for children in the first two years of life. Am J Ophthalmol 2003136994–1000. [DOI] [PubMed] [Google Scholar]
- 26.Rolim de Moura C, Fraser‐Bell S, Stout A.et al Experience with the Baerveldt glaucoma implant in the management of pediatric glaucoma. Am J Ophthalmol 2005137847–854. [DOI] [PubMed] [Google Scholar]
- 27.Budenz D L, Gedde S J, Brandt J D.et al Baerveldt glaucoma implant in the management of refractory childhood glaucomas. Ophthalmology 20041112204–2210. [DOI] [PubMed] [Google Scholar]
- 28.Donahue S P, Keech R V, Munden P.et al Baerveldt implant surgery in the treatment of advanced childhood glaucoma. J AAPOS 1997141–45. [DOI] [PubMed] [Google Scholar]
- 29.Fellenbaum P S, Sidoti P A, Heuer D K.et al Experience with the Baerveldt implant in young patients with complicated glaucomas. J Glaucoma 1994491–97. [PubMed] [Google Scholar]
- 30.Netland P A, Walton D S. Glaucoma drainage implants in pediatric patients. Ophthalmic Surg 199324723–729. [PubMed] [Google Scholar]
- 31.Eid T E, Katz L J, Spaeth G L.et al Long‐erm effects of tube‐shunt procedures on management of refractory childhood glaucoma. Ophthalmology 19971041011–1016. [DOI] [PubMed] [Google Scholar]
- 32.Munoz M, Tomey K F, Traverso C.et al Clinical experience with the Molteno implant in advanced infantile glaucoma. J Pediatr Ophthalmol Strabismus 19912868–72. [DOI] [PubMed] [Google Scholar]
- 33.Hill R A, Heuer D K, Baerveldt G.et al Molteno implantation for glaucoma in young patients. Ophthalmology 1991981042–1046. [DOI] [PubMed] [Google Scholar]
- 34.Billson F, Thomas R, Aylward W. The use of two‐stage Molteno implants in developmental glaucoma. J Pediatr Ophthalmol Strabismus 1989263–8. [DOI] [PubMed] [Google Scholar]
- 35.Morad Y, Donaldson C E, Kim Y M.et al The Ahmed drainage implant in the treatment of pediatric glaucoma. Am J Ophthalmol 2003135821–829. [DOI] [PubMed] [Google Scholar]
- 36.Djodeyre M R, Calvo J P, Gomez J A. Clinical evaluation and risk factors of time to failure of Ahmed glaucoma valve implant in pediatric patients. Ophthalmology 2001108614–620. [DOI] [PubMed] [Google Scholar]
- 37.Englert J A, Freedman S F, Cox T A. The Ahmed valve in refratory pediatric glaucoma. Am J Ophthalmol 199912734–42. [DOI] [PubMed] [Google Scholar]
- 38.Coleman A L, Smyth R J, Wilson M R.et al Initial clinical experience with the Ahmed glaucoma valve implant in pediatric patients. Arch Ophthalmol 1997115186–191. [DOI] [PubMed] [Google Scholar]
- 39.Budenz D L, Sakamoto D, Eliezer R.et al Two‐staged Baerveldt glaucoma implant for childhood glaucoma associated with Sturge‐Weber. Ophthalmology 20001072105–2110. [DOI] [PubMed] [Google Scholar]
- 40.Munoz M, Parrish R. Hypertropia after implantation of a Molteno drainage device. Am J Ophthalmol 199211398–100. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd M A, Baerveldt G, Fellenbaum P S.et al Intermediate‐term results of a randomized clinical trial of the 350‐ versus the 500‐mm2 Baerveldt implant. Ophthalmology 19941011456–1464. [DOI] [PubMed] [Google Scholar]
- 42.Britt M T, LaBree L D, Lloyd M A.et al Randomized clinical trial of the 350‐mm2 versus the 500‐mm2 Baerveldt implant: longer term results. Ophthalmology 19991062312–2318. [DOI] [PubMed] [Google Scholar]