Abstract
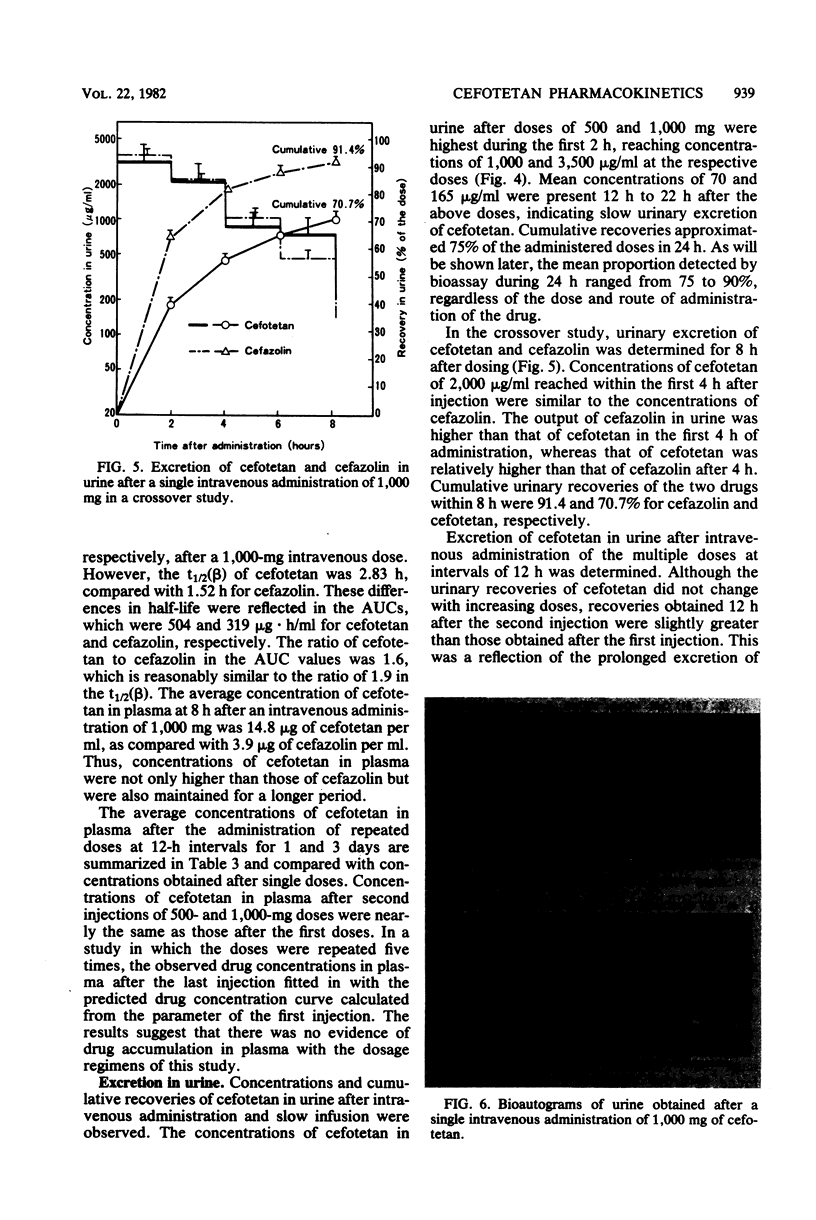
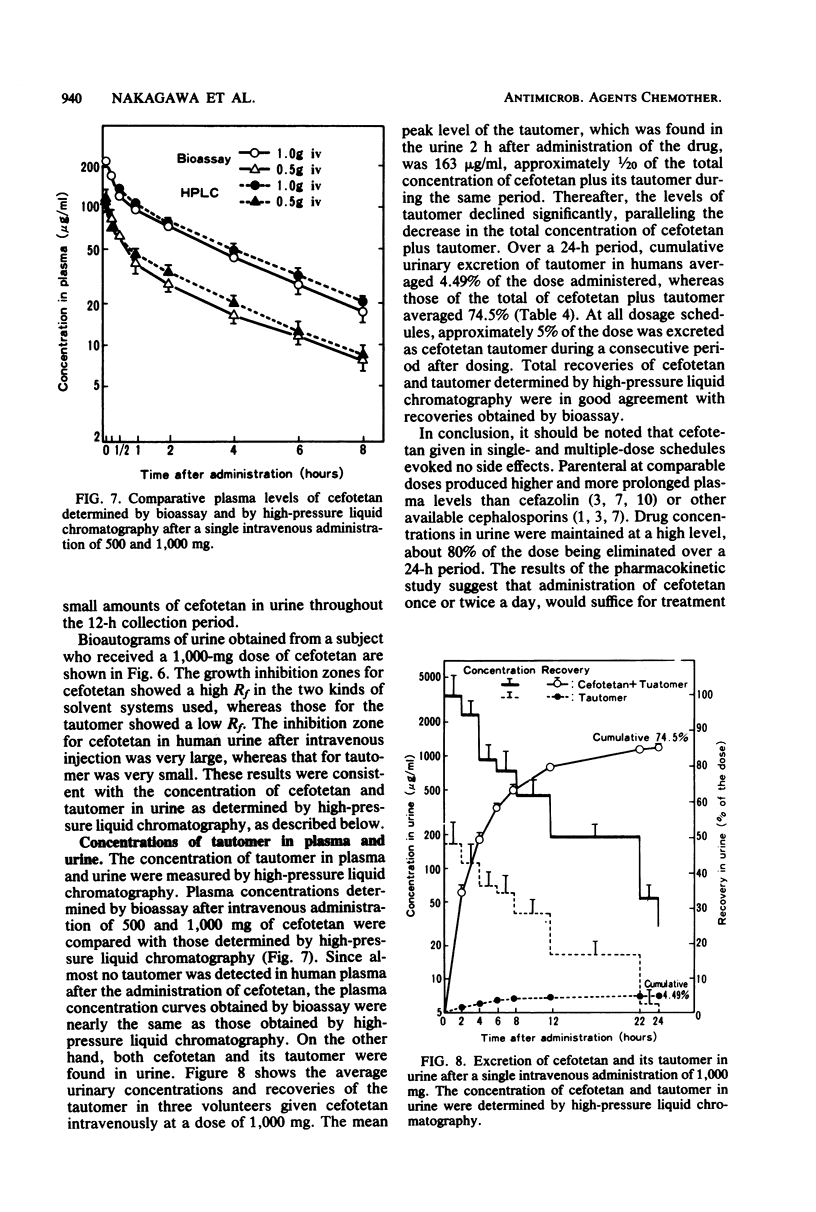
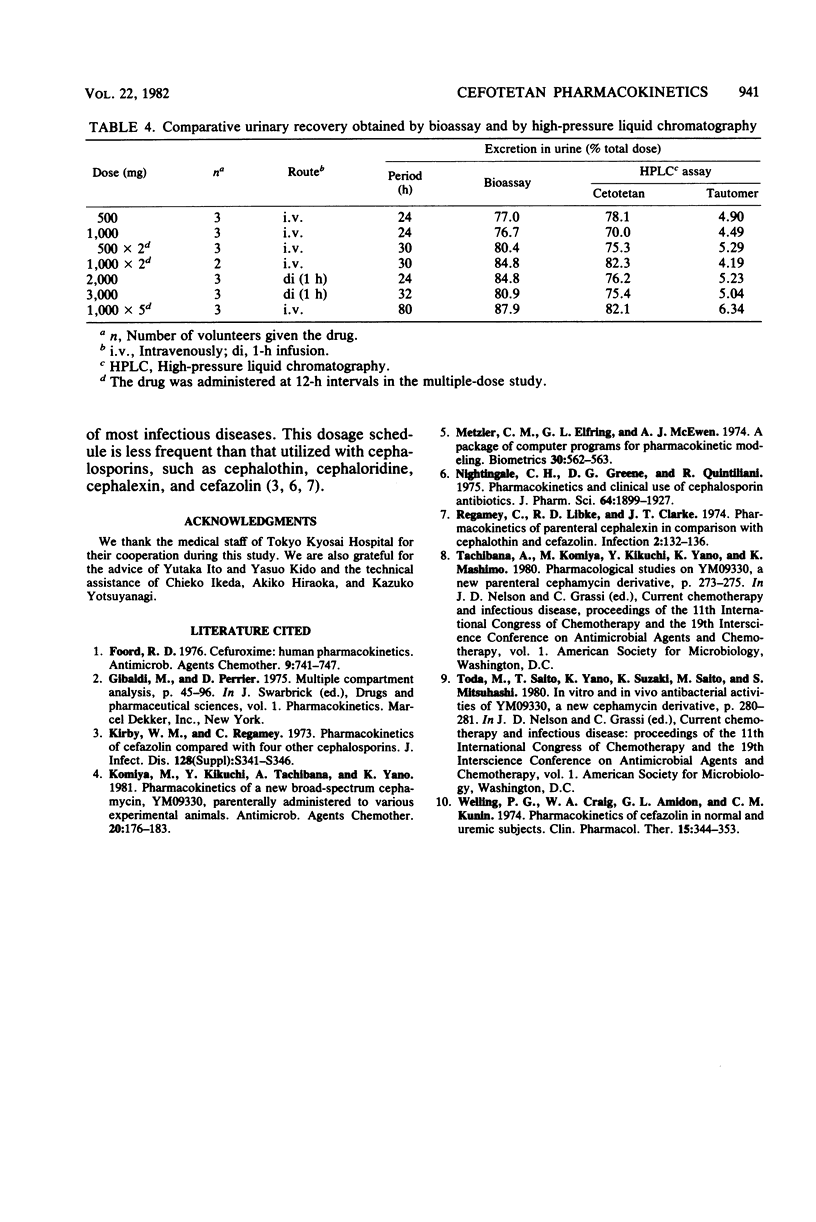
The pharmacokinetics and safety of cefotetan (YM09330) were examined after intravenous administration of single and multiple doses to normal volunteers. Cefotetan was well tolerated in single doses of 500 to 3,000 mg and in multiple doses of 500 and 1,000 mg at 12-h intervals for 1 and 3 days. These doses produced high plasma levels. The half-life (3 h) of cefotetan was longer than that of cefazolin. There was no evidence of drug accumulation in the plasma in the multiple-dose study. Mean recoveries of cefotetan in urine within a 24-h period were 74.5 to 88.4% of the dose, regardless of the route of administration and the dosage. The tautomer of cefotetan accounted for approximately 5% of the dose excreted in the urine. No tautomer was detected in plasma. Concentrations of drug in plasma and urine measured by microbiological assay were in good agreement with those measured by high-pressure liquid chromatography.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Foord R. D. Cefuroxime: human pharmacokinetics.. Antimicrob Agents Chemother. 1976 May;9(5):741–747. doi: 10.1128/aac.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby W. M., Regamey C. Pharmacokinetics of cefazolin compared with four other cephalosporins. J Infect Dis. 1973 Oct;128(Suppl):S341–S346. doi: 10.1093/infdis/128.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- Komiya M., Kikuchi Y., Tachibana A., Yano K. Pharmacokinetics of new broad-spectrum cephamycin, YM09330, parenterally administered to various experimental animals. Antimicrob Agents Chemother. 1981 Aug;20(2):176–183. doi: 10.1128/aac.20.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale C. H., Greene D. S., Quintiliani R. Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci. 1975 Dec;64(12):1899–1926. doi: 10.1002/jps.2600641202. [DOI] [PubMed] [Google Scholar]
- Regamey C., Libke R. D., Clarke J. T., Kirby W. M. Pharmacokinetics of parenteral sodium cephalexin in comparison with cephalothin and cefazolin. Infection. 1974;2(3):132–136. doi: 10.1007/BF01642232. [DOI] [PubMed] [Google Scholar]
- Welling P. G., Craig W. A., Gordon A. L., Kunin C. M. Pharmacokinetics of cefazolin in normal and uremic subjects. Clin Pharmacol Ther. 1974 Apr;15(4):344–353. doi: 10.1002/cpt1974154344. [DOI] [PubMed] [Google Scholar]