Abstract
Background/aim
Malattia leventinese (ML) is an inherited macular degeneration characterised by the presence of small radial drusen. Despite extensive descriptions of this study of the fundus, angiographic features of ML have been inadequately described. The aim is to describe the indocyanine green angiography (ICG) features observed in ML.
Methods
10 eyes from five consecutive ML patients (aged 27–44 years) were prospectively included. A complete ophthalmological examination including colour fundus photographs, autofluorescence, fluorescein angiography (FA), and ICG was performed.
Results
ICG differentiated two types of drusen. Large round aggregated drusen were consistently hypofluorescent in the early phases and presented as hyperfluorescent spots surrounded by halos of hypofluorescence in the late phases. Conversely, small radial drusen were mostly hyperfluorescent in the early phases with decreased fluorescence in the late phases of the ICG sequence. FA also showed differences in staining between the two types of drusen.
Conclusions
ICG angiography revealed marked differences between the large round and small radial drusen observed in ML. The large central drusen presented with an unusual pustuliform feature on the late phases of the ICG sequence. This distinct feature may be useful in the diagnosis of late stage disease when drusen consolidation could obscure the radial drusen.
Keywords: malattia leventinese, drusen, indocyanine, angiography, inherited macular dystrophy
Malattia leventinese (ML) is an inherited autosomal dominant macular degeneration that results in progressive vision loss. In 1925, Vogt described a specific form of familial drusen observed in patients living in the Leventine valley in the Ticino canton of Switzerland.1 These drusen progress to form a mosaic pattern termed Doyne honeycomb retinal dystrophy (DHRD).2 ML is an infrequent disorder but of major interest because of the presence of drusen, a feature shared with age related macular degeneration (AMD), the most common cause of irreversible vision loss in the developed world. Recently, Stone et al identified a single non‐conservative mutation (arg345trp) in the EFEMP1 (EGF containing fibrillin‐like extracellular matrix protein, 2p21–p16) gene in five families affected with ML/DHRD.3,4,5
However, the diagnosis of ML is clinical, based on fundus examination, showing small discrete drusen which radiate into the peripheral retina.6,7 Later, some of the drusen usually become confluent leading to a honeycomb appearance.8 Angiographic description of ML has been limited to fluorescein angiographies (FA) in patients with choroidal neovascularisation (CNV).9,10 Here, we report the ICG features of ML compared with our observations on fundus examination, autofluorescence, and FA in 10 eyes.
Patients and methods
Ten eyes of five consecutive patients affected with ML (four females, one male) were prospectively included. They presented to our department between 1999 and 2004 with complaints of metamorphopsia or loss of vision. The criteria for diagnosis of ML were based on characteristic features on fundus examination, including the drusen with a peculiar distribution in streaks and lines radiating from the centre of the fovea, and diagnosed before the age of 45 years. The age range of our patients at presentation was between 27 and 44, with a mean age of 34 years (table 1). Cases 2 and 3 were sisters; others were unrelated. Patients 1, 4, and 5 originated from North Africa, while patients 2 and 3 originated from the centre of France. No common ancestor could be found by interrogation of these three families. The five study patients showed an autosomal dominant inheritance including parents or siblings affected with ML. No CNV was observed in any of these patients. For each patient, a complete ophthalmological examination including best corrected visual acuity (BCVA), colour fundus photographs of the retina, red free frames, autofluorescence, FA (Zeiss FF450IR, Carl Zeiss Inc, San Leandro, CA, USA or Canon 60 fundus camera, Tokyo, Japan), and ICG (Heidelberg Retina Angiograph Heidelberg Engineering, Heidelberg, Germany) was performed. The observed drusen were defined as small when their size was <63 μm and as large when their size was >125 μm.
Table 1 Visual acuities and ICG features of the malattia leventinese patients.
| Case | Age | Sex | Eye | Metam | Early phase of ICG | Late phase of ICG | |||
|---|---|---|---|---|---|---|---|---|---|
| Visual acuity | Small radial drusen | Large round aggregated drusen | Small radial drusen | Large round aggregated drusen | |||||
| 1 | 38 | F | RE | + | 20/25 | Mildly hyper F | Hypo F | Mildly hyper F | Hyper F/PA |
| LE | − | 20/200 | Hyper F | Hypo F | Non‐detectable | Hyper F/PA | |||
| 2 | 27 | F | RE | + | 20/63 | Non‐detectable | Hypo F | Non‐detectable | Hyper F/PA |
| LE | + | 20/25 | Mildly hyper F | Hypo F | Non‐detectable | Hyper F/PA | |||
| 3 | 33 | F | RE | + | 20/25 | Hyper F | Hypo F | Mildly hyper F | Hyper F/PA |
| LE | + | 20/32 | Mildly hyper F | Hypo F | Non‐detectable | Hyper F/PA | |||
| 4 | 30 | F | RE | + | 20/25 | Hyper F | Hypo F | Mildly Hyper F | Hyper F/PA |
| LE | + | 20/25 | Hyper F | Hypo F | Non‐detectable | Hyper F/PA | |||
| 5 | 44 | M | RE | + | 20/63 | Non‐detectable | Hypo F | Hyper F | Hyper F/PA |
| LE | − | 20/100 | Hyper F | Hypo F | Mildly Hyper F | Hyper F/PA | |||
Hyper F, hyperfluorescence; Hypo F, hypofluorescence; PA, pustuliform aspect. Metam, metamorphopsia.
Results
BCVA ranged from 20/25 to 20/200 (table 1). Metamorphopsia were present in eight of the 10 eyes.
Colour fundus photographs of the retina showed yellow white drusen of different sizes which were located in the macular and peripapillary areas (figs 1A, 2A, 3A). The largest drusen were round, confluent, and located mainly around the macular area; most of these drusen were ill defined with fuzzy borders. The smallest drusen were mainly visible in the peripheral part of the lesion and arranged in a radial distribution. A partial sparing of the central part of the macular area was observed in all the study eyes with varied patterns of sparing. Pigmentary changes including mottling and focal atrophy were observed in the macular area.
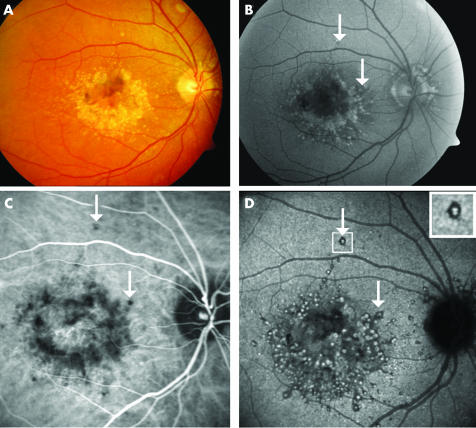
Figure 1 Autofluorescent and indocyanine green angiography features of drusen in malattia leventinese. Case 2, right eye. (A) Colour fundus photograph shows large round drusen aggregated in the paracentral area and small radial drusen located temporally to the macula. (B) Large drusen, in the macular area and around the optic disc, are clearly autofluorescent, whereas small radial drusen are hardly detectable. An appearance of central sparing is observed. (C) In the early phase of the ICG sequence (2 minutes) several dots of hypofluorescence are distributed around the macular area approximating the location of the large round drusen. White arrows indicate some isolated large drusen. (D) In the late phase (30th minute), the large round drusen appear as hyperfluorescent spots surrounded by hypofluorescent halos (white arrows). The small radial drusen cannot be clearly seen in any of the ICG frames.
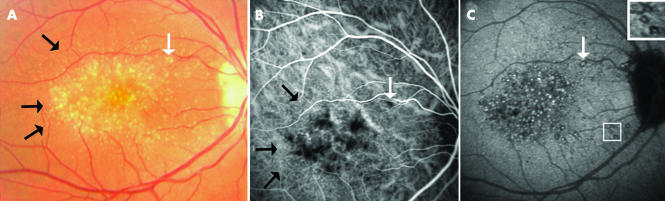
Figure 2 Indocyanine green (ICG) features of malattia leventinese, Case 3, right eye. (A) Colour fundus photograph shows both large round drusen and small radial drusen (black arrows). White arrow indicates the location of one of the large drusen. (B) In the early phase (35 seconds) of the ICG sequence several dots of hypofluorescence are distributed around the macular area approximating the location of the large drusen (white arrow). Pinpoints of hyperfluorescence are seen in the peripheral part of the lesion, some distributed in radial lines (black arrows). These discrete little spots seem to correspond to the small radial drusen. (C) In the late phase (30th minute), the hyperfluorescent spots, surrounded by halos of hypofluorescence, are observed around and inside the macular area without macular sparing. Squares show enlarged views of the peculiar pustuliform pattern of these large drusen.
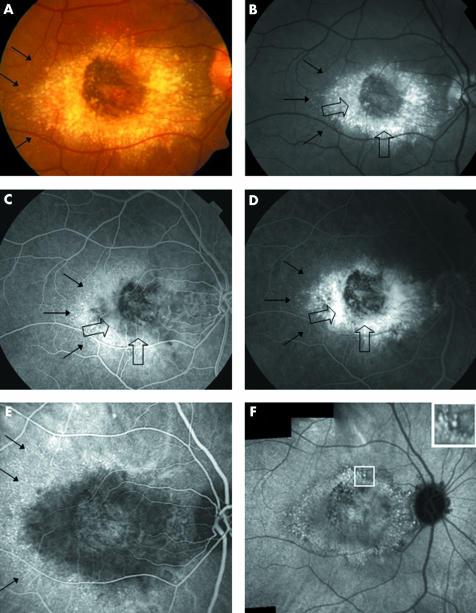
Figure 3 Fluorescein angiography (FA) compared to indocyanine green features in malattia leventinese. Case 4, right eye. (A) Colour fundus photograph shows large drusen aggregated around the macular area. Small radial drusen are indicated by thin black arrows. (B) On the red free frame, the large drusen (indicated by large open arrows) are located around the macular area with a relative sparing of the centre. The large round drusen appear brighter than the small radial ones. The small radial peripheral drusen are in dotted lines (thin black arrows). (C) In the early phase of the FA sequence (30 seconds) we observe, from the centre of the macula to the periphery, a dark area corresponding to the relative central sparing, a grey zone harbouring a buoy pattern (open arrows), surrounded by an ill defined zone of hyperfluorescence and the intense staining of the small radial drusen (small black arrows). (D) At 4 minutes 30 seconds of the FA sequence, the centre of the macular area remains dark and is surrounded by an ill defined zone of hyperfluorescence progressively stained by the dye (open black arrows). The large round drusen are fuzzy and not clearly discernible. The hyperfluorescence of the small radial drusen is still visible although less intense (small black arrows). (E) In the early phase (2 minutes) of the ICG sequence an ovoid hypofluorescent zone surrounds the central macular area. On the periphery of this zone, the small radial drusen appear hyperfluorescent (black arrows). (F) In the 30th minute of the ICG, the composite frame shows aggregation of the hyperfluorescent large drusen which are distributed around the macular area. Most of the hypofluorescent halos are confluent suggesting a confluence of the large drusen. The temporal small drusen are not visible any more. Square shows an enlarged view of the hyperfluorescent dots surrounded by halos of hypofluorescence.
Red free frames showed bright, round drusen contrasting with the grey appearance of the retina (fig 3B). These pictures were comparable with the colour photographs except for accentuating the contrast of the drusen and the pigment changes.
Autofluorescent frames of the posterior pole revealed an intense autofluorescence of the larger drusen located around the macular area as an aggregation of little balls (fig 1B). Autofluorescence was also observed in the drusen located around the optic disc area. Autofluorescence of the small radial drusen was less intense with only the largest of them being detectable.
Fluorescein angiography (FA) showed large paracentral drusen and small radial drusen with different patterns of staining. In the early phases of FA, the large paracentral drusen were indistinct and mainly normal or hypofluorescent (fig 3A). On the contrary, the small peripheral radial drusen were well demarcated and highly hyperfluorescent. In the late phases of FA, the large paramacular drusen were still ill defined but a progressive staining of the juxtamacular area mixed with retinal pigment epithelium (RPE) changes created a blurry zone of hyperfluorescence (fig 3D). On the contrary, the hyperfluorescence of the small radial drusen decreased in the late phases of FA. It is notable that the centre of the macular area appeared relatively hypofluorescent in all the study eyes in all the phases of the FA sequence.
ICG angiography also demonstrated different patterns of staining between the large central drusen and small radial drusen. In the early phases, the large drusen were undetectable (figs 1C, 2B, 3E). A hypofluorescent zone, with ill defined borders and approximating the location of the large paracentral drusen surrounded the macular area (table 1). On the contrary, the small peripheral radial drusen were visible in the early phases of the ICG sequence in eight of the 10 eyes; they appeared as hyperfluorescent points located in the periphery of the lesion. In the late phases, the large drusen appeared as hyperfluorescent spots surrounded by a small halo of hypofluorescence (figs 1D, 2C, 3F). In the macular and juxtamacular area, the hypofluorescent halos were confluent and contained several little white spots that seemed to indicate the location of the large drusen. This peculiar pustuliform‐like feature was found in all the study eyes. The small radial drusen were hardly identifiable in the late stages of the ICG sequence. Around the papillary area, most of the drusen appeared similar to the large central drusen.
Discussion
In malattia leventinese, the fundus features including radial drusen are well described and commonly constitute the basis for the diagnosis of this condition.11 However, the angiographic features of these drusen remain inadequately described probably because of the relative infrequency of this disorder. Here, we analysed the ICG features in 10 eyes affected with ML and compared these with our observations on colour fundus photography, autofluorescence, and FA.
In the early phases of confocal ICG, hypofluorescence was observed around the macular area most probably because of a masking effect of the drusen. Only the small radial drusen, when visible, were hyperfluorescent (figs 2B, 3E). In the late phases of confocal ICG, the large drusen presented as hyperfluorescent spots surrounded by halos of hypofluorescence (figs 1D, 2C, 3F) probably because of a progressive staining of their centre. Aggregation of these drusen gave a peculiar pustuliform‐like aspect observed in all the study patients.
The physical or substructural characteristics of the drusen, which could explain this unusual ICG feature, remains unknown. We hypothesise that this could be the result of a difference in lipid composition between the centre and the edge of the drusen. Such a hypothesis suggests a core‐like structure for the drusen as described by Russell et al in early adult onset drusen.12 Similarly, Curcio et al have demonstrated a cholesterol ester “shell” around a central drusen core in AMD drusen suggesting that a concentric hydrophobic region may surround the central core.13 The hyperfluorescence that we observed in the central part of the drusen could be related to the hyperfluorescence reported in hard drusen in the late phases of the ICG.14 Also, the hypofluorescence observed in the outer part of the drusen could be related to the hypofluorescence observed in soft drusen in the late phases of the ICG. It is remarkable these two opposite patterns of fluorescence were observed in the same drusen in ML. These ICG images were obtained using a confocal HRA angiograph and this specific feature was not investigated with any other ICG angiographs.
In this study, comparison with colour fundus photographs, autofluorescent frames, and FA confirmed the apparent differences between the types of drusen. The small radial drusen were weakly autofluorescent and demonstrated hyperfluorescence that decreased during the FA as well as the ICG sequence (fig 1). The large drusen were strongly autofluorescent and the fluorescence increased during the FA sequence. On the ICG, the large central drusen were hypofluorescent in the early phases but demonstrated a peculiar hyperfluorescent pattern in the late phases. These two types of drusen in ML could be either two distinct entities or represent two different stages in the evolution of the same disorder. The observed differences between these two types of drusen could be related to the findings of Pauleikhoff et al who demonstrated differences in lipid composition between the central and peripheral drusen in AMD.15,16
ICG could contribute to the diagnosis of this disorder in the early stages, advanced stages or unusual cases. ML exhibits features more consistent with AMD than any other inheritable macular dystrophy. The question of the relation between the drusen observed in ML and the drusen in AMD has been raised by several authors.17,18,19,20 Further, the occurrence of CNV, a classic feature in AMD, is also a potential complication in ML.9,10 The specific angiographic features described in this report could be helpful to establish a continuum between ML and some cases of AMD.
Abbreviations
AMD - age related macular degeneration
BCVA - best corrected visual acuity
CNV - choroidal neovascularisation
DHRD - Doyne honeycomb retinal dystrophy
FA - fluorescein angiography
ICG - indocyanine green
ML - malattia leventinese
RPE - retinal pigment epithelium
Footnotes
Competing interests: none declared
References
- 1.Vogt A. Die Ophthalmoskopie im rotfreien Licht. In: Graefe A, Saemisch T, eds. Handbuch der gesammten Augenheilkunde. Untersuchungsmethoden. 3rd ed. Berlin: Leipez, Verlag von Wilhelm Engelman, 19251–118.
- 2.Doyne R W. Peculiar condition of choroiditis occuring in several members of the same family. Trans Ophthalmol Soc UK 18991971 [Google Scholar]
- 3.Heon E, Piguet B, Munier F.et al Linkage of autosomal dominant radial drusen (malattia leventinese) to chromosome 2p16–21. Arch Ophthalmol 1996114193–198. [DOI] [PubMed] [Google Scholar]
- 4.Stone E M, Lotery A J, Munier F L.et al A single EFEMP1 mutation associated with both malattia leventinese and Doyne honeycomb retinal dystrophy. Nat Genet 199922199–202. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto M, Traboulsi E I. Dominant radial drusen and Arg345Trp EFEMP1 mutation. Am J Ophthalmol 2001131810–812. [DOI] [PubMed] [Google Scholar]
- 6.Piguet B, Haimovici R, Bird A C. Dominantly inherited drusen represent more than one disorder: a historical review. Eye 1995934–41. [DOI] [PubMed] [Google Scholar]
- 7.Deutman A F, Jansen L M. Dominantly inherited drusen of Bruch's membrane. Br J Ophthalmol 197054373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zech J C, Zaouche S, Mourier F.et al Macular dystrophy of malattia leventinese. A 25 year follow up. Br J Ophthalmol 1999831195–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pager C K, Sarin L K, Federman J L.et al Malattia leventinese presenting with subretinal neovascular membrane and hemorrhage. Am J Ophthalmol 2001131517–518. [DOI] [PubMed] [Google Scholar]
- 10.Dantas M A, Slakter J S, Negrao S.et al Photodynamic therapy with verteporfin in mallatia leventinese. Ophthalmology 2002109296–301. [DOI] [PubMed] [Google Scholar]
- 11.Evans K, Gregory C Y, Wijesuriya S D.et al Assessment of the phenotypic range seen in Doyne honeycomb retinal dystrophy. Arch Ophthalmol 1997115904–910. [DOI] [PubMed] [Google Scholar]
- 12.Russell S R, Gupta R R, Folk J C.et al Comparison of color to fluorescein angiographic images from patients with early‐adult onset grouped drusen suggests drusen substructure. Am J Ophthalmol 2004137924–930. [DOI] [PubMed] [Google Scholar]
- 13.Curcio C A, Millican C L, Bailey T.et al Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci 200142265–274. [PubMed] [Google Scholar]
- 14.Arnold J J, Quaranta M, Soubrane G.et al Indocyanine green angiography of drusen. Am J Ophthalmol 1997124344–356. [DOI] [PubMed] [Google Scholar]
- 15.Pauleikhoff D, Zuels S, Sheraidah G S.et al Correlation between biochemical composition and fluorescein binding of deposits in Bruch's membrane. Ophthalmology 1992991548–1553. [DOI] [PubMed] [Google Scholar]
- 16.Pauleikhoff D, Harper A, Marshall J.et al Aging changes in Bruch's membrane. Ophthalmology 199097171–178. [PubMed] [Google Scholar]
- 17.Marmorstein L. Association of EFEMP1 with malattia leventinese and age‐related macular degeneration: a mini‐review. Ophthalmic Genet 200425219–226. [DOI] [PubMed] [Google Scholar]
- 18.Stone E M, Braun T A, Russell S R.et al Missense variations in the fibulin 5 gene and age‐related macular degeneration. N Engl J Med 2004351346–353. [DOI] [PubMed] [Google Scholar]
- 19.Gorin M B, Breitner J C, De Jong P T.et al The genetics of age‐related macular degeneration. Mol Vis 1999529. [PubMed] [Google Scholar]
- 20.Zack D J, Dean M, Molday R S.et al What can we learn about age‐related macular degeneration from other retinal diseases? Mol Vis 1999530. [PubMed] [Google Scholar]